
Serum zinc concentration as a potential predictor of presarcopenia in patients with chronic liver disease: a preliminary study
Highlight box
Key findings
• Low serum zinc levels may be an independent predictor of presarcopenia in patients with chronic liver disease.
What is known and what is new?
• Presarcopenia is a common complication of chronic liver disease.
• The current study clarifies the relationship between zinc deficiency and presarcopenia.
What is the implication, and what should change now?
• Serum zinc concentration measurements may help identify patients with chronic liver disease who are at a higher risk of presarcopenia.
Introduction
Background
In 1989, Rosenberg defined sarcopenia as an age-related loss of muscle mass (1). Recent reports indicate that the incidence rates of sarcopenia are 1–29% among community residents, 14–33% among long-term care patients, and 10% in patients admitted to acute care hospitals (2).
Based on the etiology, sarcopenia is classified into primary sarcopenia, which is the loss of muscle mass associated with aging, and secondary sarcopenia, which is the loss of muscle mass associated with reduced activity, undernutrition, invasion, or disease. Sarcopenia observed in patients with chronic liver disease is a form of secondary sarcopenia associated with nutritional status and metabolic abnormalities (3). The rate of skeletal muscle loss in Japanese patients with cirrhosis has been reported to be 2.2% per year, and the loss of muscle mass is more pronounced with a worsening hepatic reserve (4).
The frequency of sarcopenia-associated complications in patients with cirrhosis is high, ranging from approximately 40% to 70%, and the mortality rate is reported to be significantly higher than that of patients without sarcopenia (5). Additionally, a skeletal muscle loss rate exceeding 2.4% per year is associated with a poor prognosis (6). Thus, the clinical importance of this disease has recently increased.
The European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS) reported diagnostic algorithms for sarcopenia in 2010 (7) and 2014 (8), respectively. In 2019, the EWGSOP updated the definition based on data accumulated over the past decade and presented a flowchart for diagnosis, which recommended early diagnosis of patients at risk of sarcopenia and timely therapeutic intervention (9). The AWGS revised the diagnostic criteria in 2019, moving from the previous assessment of skeletal muscle mass to placing more emphasis on muscle weakness, thereby allowing earlier interventions for at-risk patients (10).
The Japan Society of Hepatology (JSH) further developed the diagnostic criteria for primary sarcopenia proposed by the AWGS and defined liver disease-related sarcopenia as a condition resulting in decreased muscle mass and muscle weakness (11). Computed tomography (CT) and bioelectrical impedance analysis (BIA) are used to diagnose sarcopenia. The CT area of the third lumbar vertebra (L3) is used to define muscle mass, and muscle strength is defined as grip strength; both of these factors are decreased in sarcopenia.
Rationale and knowledge gap
Recently, for several chronic diseases, including chronic liver disease, decreased serum zinc levels have been reported. Additionally, these patients exhibit various clinical symptoms associated with zinc deficiency (12-16). Zinc is widely distributed in the body, especially in the muscles (57%), bone (29%), skin (6%), and liver (5%), and is reported to be associated with skeletal muscle mass (17).
In this context, it has been reported that serum zinc concentration correlates with liver fibrosis and its severity in patients with chronic liver disease (18), and the usefulness of serum zinc concentration measurement in such patients is being investigated. However, the cutoff values of serum zinc concentration in patients with chronic liver disease have not yet been fully investigated.
Objective
This study aimed to investigate the association between serum zinc concentration and presarcopenia in patients with chronic liver disease. We present this article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-77/rc).
Methods
This retrospective cross-sectional study included 278 patients (133 women and 145 men) with chronic liver disease who underwent abdominal CT and simultaneous serum zinc concentration measurement from October 2015 to December 2019. The inclusion criteria were as follows: (I) patients with liver disease treated at the Saiseikai Niigata Hospital; (II) men and women aged >18 years; and (III) patients not taking zinc preparations. Liver cirrhosis was diagnosed based on physical findings, serum biomarkers, and clinical imaging characteristics. Irregularity and deformity of the shape of the liver was detected imaging modalities [abdominal echography, CT, and magnetic resonance imaging (MRI)]. Zinc deficiency and subclinical zinc deficiency were classified using serum zinc concentration cutoff values of <60 and <80 µg/dL [based on the Japanese Society of Clinical Nutrition (JSCN) guidelines], respectively.
The image analysis software Synapse Vincet V5.3 (Fujifilm Medical Corporation, Tokyo, Japan) was used to evaluate presarcopenia. The measurement method displayed a transverse section at the level of the L3 in a CT image of 5-mm slice thickness, and the area of the skeletal muscles in the displayed section was extracted. CT values of the extracted area were set to −29 to 150 HU, and the area was divided by the square of the height to determine skeletal muscle mass [L3 skeletal muscle index (SMI)] as per the JSH sarcopenia criteria. The cutoff values for defining presarcopenia were 42 cm2/m2 for men and 38 cm2/m2 for women, according to the criteria.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Saiseikai Niigata Hospital (No. E 23-02) and individual consent for this retrospective analysis was waived.
Statistical analysis
For statistical analysis, Wilcoxon’s rank-sum test was used to compare continuous variables. For the comparison of categorical variables, the χ2 test or Fisher’s exact test was used. Based on previous study, age, body mass index (BMI), presence of liver cirrhosis, albumin-bilirubin score (19), and branched-chain amino acid levels were considered as potential factors. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by multivariate analysis using logistic regression with the following confounding variables: internal administration of branched-chain amino acids, sex (5), liver background, presence of hepatocellular carcinoma, and fibrosis-4 index.
When performing the multivariate analysis, the variance inflation factor (VIF) was calculated to check for multicollinearity. The statistical significance level was set at P<0.05. The EZR statistical software (ver. 1.37) was used for the analysis, which extends the functionality of R and R Commander and is distributed free of charge on the website of the Department of Hematology, Saitama Medical Center, Jichi Medical University (20).
Results
Patient backgrounds are shown in Table 1. The median [interquartile range (IQR)] age was 68 [57–76] years. The liver backgrounds were hepatitis B virus for 55 patients, hepatitis C virus for 124 patients, and negative hepatitis B surface antigen and negative anti-hepatitis C virus antibody for 99 patients; 64.4% of patients had viral hepatitis. The median (IQR) BMI was 22.6 (20.3–25.3) kg/m2, median (IQR) serum albumin concentration was 4.2 (3.8–4.4) g/dL, and median (IQR) serum zinc concentration was 73 (64.3–81) µg/dL.
Table 1
| Variables | Value (n=278) |
|---|---|
| Age (years) | 68 [57, 76] |
| Sex (female/male) | 133/145 |
| Etiology (HBV/HCV/NBNC) | 55/124/99 |
| BMI (kg/m2) | 22.6 [20.3, 25.3] |
| Serum albumin (g/dL) | 4.2 [3.8, 4.4] |
| Serum zinc (μg/dL) | 73 [64.3, 81] |
| AST (IU/L) | 23 [19, 31] |
| ALT (IU/L) | 18 [12, 28] |
| T-Bil (mg/dL) | 0.6 [0.5, 0.8] |
| PLT (104/μL) | 20 [16.3, 24.2] |
| Fib-4 index | 2.08 [1.32, 2.74] |
| L3 SMI (cm2/m2) | 40.3 [34.6, 46.6] |
Data are expressed as median [IQR] or n. HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, negative hepatitis B surface antigen and negative anti-hepatitis C virus antibody; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-Bil, total bilirubin; PLT, platelet; Fib-4, fibrosis-4; L3, third lumbar vertebra; SMI, skeletal muscle index; IQR, interquartile range.
A comparison of patients when applying a serum zinc concentration cutoff value of 60 µg/dL is shown in Table 2.
Table 2
| Variables | Low group (<60 μg/dL) (n=37) | High group (≥60 μg/dL) (n=241) | P value |
|---|---|---|---|
| Age (years) | 68.00 (64.00, 76.00) | 68.00 (56.00, 76.00) | 0.47 |
| Sex (male) | 25 (67.6) | 120 (49.8) | 0.05 |
| Etiology (HBV/HCV/NBNC) | 9/18/10 | 46/106/89 | 0.47 |
| BMI (kg/m2) | 22.45 (19.72, 26.58) | 22.68 (20.39, 25.21) | 0.83 |
| Serum zinc level (μg/dL) | 55.00 (50.00, 57.00) | 75.00 (69.00, 83.00) | <0.001 |
| Serum albumin level (g/dL) | 3.70 (3.40, 4.10) | 4.30 (4.00, 4.40) | <0.001 |
| L3 SMI (cm2/m2) | |||
| Male | 42.22 (37.83, 47.36) | 45.89 (42.13, 49.86) | 0.04 |
| Female | 34.63 (29.93, 41.89) | 45.89 (42.13, 49.86) | 0.86 |
| Fib-4 index | 2.59 (2.08, 3.63) | 1.93 (1.23, 2.64) | <0.001 |
| ALBI score | −2.50 (−2.77, −2.04) | −2.93 (−3.09, −2.73) | <0.001 |
| Liver cirrhosis | 19 (51.4) | 48 (19.9) | <0.001 |
| HCC | 20 (54.1) | 36 (14.9) | <0.001 |
| Presarcopenia (JSH) | 21 (56.8) | 109 (45.2) | 0.21 |
| Male | 12/25 (48.0) | 30/120 (25.0) | 0.02 |
| Female | 9/12 (75.0) | 79/121 (65.3) | 0.75 |
| BCAA | 19 (51.4) | 51 (21.2) | <0.001 |
Data are expressed as median (IQR), n (%), n, or n/N (%). HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, negative hepatitis B surface antigen and negative anti-hepatitis C virus antibody; BMI, body mass index; L3, third lumbar vertebra; SMI, skeletal muscle index; Fib-4, fibrosis-4; ALBI, albumin-bilirubin; HCC, hepatocellular carcinoma; JSH, Japan Society of Hepatology; BCAA, branched-chain amino acid; IQR, interquartile range.
There were 37 patients (12 women and 25 men) with low serum zinc concentration (<60 µg/dL; low group) and 241 (121 women and 120 men) patients with higher serum zinc concentration (≥60 µg/dL; high group).
The proportion of men was slightly higher in the low group (67.6%, 25/37); however, the difference was not significant (P=0.05). There were no significant differences in age, liver background, or BMI. The median (IQR) serum albumin levels were significantly higher in the high group [low group, 3.70 g/dL (3.40, 4.10); high group, 4.30 g/dL (4.00, 4.40); P<0.001]. The median (IQR) L3 SMI was significantly higher in men in the high group [low group, 42.22 cm2/m2 (37.83, 47.36); high group, 45.89 cm2/m2 (42.13, 49.86); P=0.04] but was not significantly different in women (P=0.86). Incidence rates of both cirrhosis and liver cancer were significantly lower in the high group [cirrhosis: low group, 51.4% (19/37); high group, 19.9% (48/241); liver cancer: low group, 54.1% (20/37); high group, 14.9% (36/241); P<0.001]. The rate of presarcopenia was significantly higher in men in the low group [low group, 48.0% (12/25); high group, 25.0% (30/120); P=0.02], whereas there was no significant difference between women in the two groups (P=0.75).
A comparison of patients when applying a serum zinc concentration cutoff value of 80 µg/dL is shown in Table 3.
Table 3
| Variables | Sub low group (<80 μg/dL) (n=201) | Sub high group (≥80 μg/dL) (n=77) | P value |
|---|---|---|---|
| Age (years) | 69.00 (58.00, 76.00) | 67.00 (52.00, 72.00) | 0.06 |
| Sex (male) | 100 (49.8) | 45 (58.4) | 0.22 |
| Etiology (HBV/HCV/NBNC) | 38/90/73 | 17/34/26 | 0.83 |
| BMI (kg/m2) | 22.23 (20.02, 24.93) | 23.42 (21.89, 26.31) | 0.01 |
| Serum zinc level (μg/dL) | 69.00 (62.00, 74.00) | 86.00 (83.00, 91.00) | <0.001 |
| Serum albumin level (g/dL) | 4.10 (3.80, 4.30) | 4.40 (4.20, 4.53) | <0.001 |
| L3 SMI (cm2/m2) | |||
| Male | 44.19 (40.17, 48.49) | 47.23 (44.14, 52.11) | 0.001 |
| Female | 34.93 (31.58, 38.95) | 37.73 (30.00, 39.58) | 0.52 |
| Fib-4 index | 2.24 (1.42, 2.89) | 1.52 (0.99, 2.26) | <0.001 |
| ALBI score | −2.80 (−3.02, −2.55) | −3.02 (−3.14, −2.89) | <0.001 |
| Liver cirrhosis | 56 (27.9) | 11 (14.3) | 0.01 |
| HCC | 49 (24.4) | 7 (9.1) | 0.004 |
| Presarcopenia (JSH) | 107 (53.2) | 23 (29.9) | <0.001 |
| Male | 36/100 (36.0) | 6/45 (13.3) | 0.006 |
| Female | 71/101 (70.3) | 17/32 (53.1) | 0.08 |
| BCAA | 62 (30.8) | 8 (10.4) | <0.001 |
Data are expressed as median (IQR), n (%), n, or n/N (%). HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, negative hepatitis B surface antigen and negative anti-hepatitis C virus antibody; BMI, body mass index; L3, third lumbar vertebra; SMI, skeletal muscle index; Fib-4, fibrosis-4; ALBI, albumin-bilirubin; HCC, hepatocellular carcinoma; JSH, Japan Society of Hepatology; BCAA, branched-chain amino acid; IQR, interquartile range.
There were 201 patients (101 women and 100 men) with a serum zinc concentration <80 µg/dL (sub-low group) and 77 patients (32 women and 45 men) with a serum zinc concentration ≥80 µg/dL (sub-high group). There were no significant differences in age or hepatic background between the groups. The median (IQR) serum albumin levels were significantly higher in the sub-high group [sub-low group: 4.10 g/dL (3.80, 4.30); sub-high group: 4.40 g/dL (4.20, 4.53); P<0.001]. The median (IQR) L3 SMI was significantly higher in men in the sub-high group [sub-low group: 44.19 cm2/m2 (40.17, 48.49); sub-high group: 47.23 cm2/m2 (44.14, 52.11); P=0.001] but not significantly different in women (P=0.52). Incidence rates of both cirrhosis and liver cancer were significantly lower in the sub-high group [cirrhosis: sub-low group: 27.9% (56/201); sub-high group: 14.3% (11/77), P=0.01; liver cancer: sub-low group: 24.4% (49/201); sub-high group: 9.1% (7/77), P=0.004]. The rate of presarcopenia was significantly higher in men in the sub-low group [sub-low group: 36.0% (36/100); sub-high group: 13.3% (6/45); P=0.006] but was not significantly different in women between the two groups (P=0.08).
Results of the univariate analysis related to serum zinc concentration and presarcopenia are shown in Table 4.
Table 4
| Variables | Non-presarcopenia (n=148) | Presarcopenia (n=130) | P value |
|---|---|---|---|
| Age (years) | 66.00 (22.00, 86.00) | 70.50 (34.00, 90.00) | <0.001 |
| Sex (male) | 103 (69.6) | 42 (32.3) | <0.001 |
| Etiology (HBV/HCV/NBNC) | 31/60/57 | 24/64/42 | 0.34 |
| BMI (kg/m2) | 24.89 (18.47, 48.04) | 21.19 (15.11, 29.78) | <0.001 |
| Serum zinc level (μg/dL) | 75.00 (48.00, 109.00) | 71.00 (31.00, 151.00) | 0.005 |
| Serum albumin (g/dL) | 4.30 (2.60, 5.30) | 4.10 (2.70, 4.90) | 0.03 |
| L3 SMI (cm2/m2) | |||
| Male | 47.56 (42.18, 63.9) | 37.91 (23.23, 41.98) | <0.001 |
| Female | 41.64 (38.03, 84.28) | 32.88 (24.05, 37.93) | <0.001 |
| Fib-4 index | 1.71 (0.14, 9.54) | 2.24 (0.50, 10.92) | <0.001 |
| ALBI score | −2.92 (−3.08, −2.68) | −2.87 (−3.04, −2.57) | 0.13 |
| Liver cirrhosis | 32 (21.6) | 35 (26.9) | 0.32 |
| HCC | 29 (19.6) | 27 (20.8) | 0.88 |
| BCAA | 28 (18.9) | 42 (32.3) | 0.01 |
Data are expressed as median (IQR), n (%), or n. HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, negative hepatitis B surface antigen and negative anti-hepatitis C virus antibody; BMI, body mass index; L3, third lumbar vertebra; SMI, skeletal muscle index; Fib-4, fibrosis-4; ALBI, albumin-bilirubin; HCC, hepatocellular carcinoma; BCAA, branched-chain amino acid; IQR, interquartile range.
The following factors were associated with the presence of presarcopenia in the univariate analysis: age [non-presarcopenia: 66.00 years (22.00, 86.00); presarcopenia: 70.50 years (34.00, 90.00); P<0.001], male sex [non-presarcopenia: 69.6% (103/148); presarcopenia: 32.3% (42/130); P<0.001], BMI [non-presarcopenia: 24.89 kg/m2 (18.47, 48.04), presarcopenia: 21.19 kg/m2 (15.11, 29.78); P<0.001], serum zinc concentration [non-presarcopenia: 75.00 µg/dL (48.00, 109.00); presarcopenia: 71.00 µg/dL (31.00, 151.00); P=0.005], fibrosis-4 index [non-presarcopenia: 1.71 (0.14, 9.54); presarcopenia: 2.24 (0.50, 10.92); P<0.001], and serum albumin concentration [non-presarcopenia: 4.30 g/dL (2.60, 5.30); presarcopenia: 4.10 g/dL (2.70, 4.90), P=0.03].
The L3 SMI was significantly higher in the non-presarcopenia group for both men and women [men, non-presarcopenia: 47.56 cm2/m2 (42.18, 63.90); presarcopenia: 37.91 cm2/m2 (23.23, 41.98); women, non-presarcopenia: 41.64 cm2/m2 (38.03, 84.28); presarcopenia: 32.88 cm2/m2 (24.05, 37.93); P<0.001 for both]. There were no significant differences in the rates of cirrhosis or liver cancer between the two groups.
Based on the univariate analysis results, multivariate analysis was performed using logistic regression for factors associated with presarcopenia. The results of the multivariate analysis are presented in Table 5.
Table 5
| Variables | Multivariate | |||
|---|---|---|---|---|
| OR | 95% CI | P value | VIF | |
| Age (years) | 1.030 | 0.997–1.060 | 0.08 | 1.41 |
| Sex (male) | 0.194 | 0.089–0.419 | <0.001 | 1.24 |
| Etiology (virus) | 0.805 | 0.316–2.050 | 0.65 | 1.14 |
| BMI (kg/m2) | 0.666 | 0.582–0.761 | <0.001 | 1.14 |
| HCC (present) | 1.160 | 0.359–3.740 | 0.80 | 1.80 |
| LC (present) | 1.280 | 0.405–4.070 | 0.67 | 1.72 |
| ALBI score | 1.150 | 0.595–2.210 | 0.68 | 1.15 |
| Fib-4 index | 0.854 | 0.635–1.150 | 0.30 | 1.74 |
| Zinc level (<60 μg/dL) | 5.930 | 1.480–23.80 | 0.01 | 1.61 |
| Zinc level (60 μg/dL ≤ Zn <80 μg/dL) | 1.910 | 0.824–4.420 | 0.13 | 1.37 |
| BCAA (intake) | 1.540 | 0.527–4.490 | 0.43 | 1.49 |
Model fitting criteria: Hosmer-Lemeshow test for logistic regression gave P=0.85. OR, odds ratio; CI, confidential interval; VIF, variance inflation factor; BMI, body mass index; HCC, hepatocellular carcinoma; LC, liver cirrhosis; ALBI, albumin-bilirubin; Fib-4, fibrosis-4; BCAA, branched-chain amino acid.
The results of the analysis showed that male sex (OR, 0.194; 95% CI: 0.089–0.419; P<0.001), BMI (OR, 0.666; 95% CI: 0.582–0.761; P<0.001), and serum zinc concentration <60 µg/dL (OR, 5.930; 95% CI: 1.480–23.80; P=0.01), were significantly associated with presarcopenia. The OR for serum zinc concentrations between 60 and 80 µg/dL was 1.910 (95% CI: 0.824–4.420; P=0.13).
The incidences of presarcopenia according to serum zinc concentration [deficient group (<60 µg/dL), subclinical deficient group (≥60 but <80 µg/dL), normal group (≥80 µg/dL)] are shown in Figure 1.
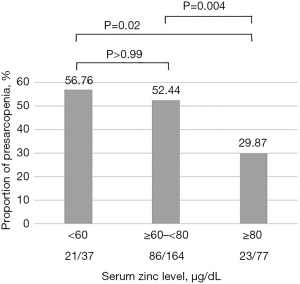
The proportions of patients with presarcopenia were 56.76% (21/37), 52.44% (86/164), and 29.87% (23/77) in the deficient, subclinical deficient, and normal groups, respectively. The normal serum zinc group had a significantly lower rate of presarcopenia than the zinc-deficient and subclinical deficient groups (normal vs. deficient group, P=0.02; normal vs. subclinical deficient group, P=0.004; deficient vs. subclinical deficient group, P>0.99).
Discussion
Key findings
Herein, we investigated the association between serum zinc concentration and skeletal muscle mass (L3 SMI) and found that low serum zinc concentration (<60 µg/dL) was an independent predictor of presarcopenia in patients with chronic liver disease.
Strengths and limitations
This study has some limitations. First, sarcopenia was previously defined as only a decrease in muscle mass associated with aging; however, after periodic revisions, muscle mass loss, muscle weakness, and physical functional decline were also taken into consideration (7). Herein, we only evaluated skeletal muscle mass and did not consider muscle weakness or physical dysfunction; therefore, the results cannot be directly applied to patients with other complications of sarcopenia, and caution must be exercised when interpreting the data. Second, this was a single-center, retrospective, observational study including Japanese participants without non-patients with chronic liver disease as a control group. Third, bias may have been introduced because concomitant medications such as diuretics and angiotensin-converting enzyme inhibitors that may affect serum zinc concentrations, dietary intake, and serum ammonia levels, were not evaluated. Fourth, factors related to lifestyle were not examined. Nonetheless, we were able to examine multiple factors, including laboratory values, muscle mass, and medications.
Comparison with similar research
Zinc is an important trace element in patients with chronic liver disease, and its various clinical benefits have been reported (12,15,18). There are also reports that skeletal muscle mass affects mortality in patients with chronic liver disease (21). In this context, there are a few studies that have discussed serum zinc concentration and sarcopenia in patients with chronic liver disease (19,22).
In a previous report, Horiguchi et al. compared L3 SMI before zinc drug loading with a cut-off serum zinc concentration of 60 µg/dL and reported no significant difference between the two groups (P=0.61) (23), which is similar to our findings.
Nishikawa et al. reported low zinc concentration as an independent predictor related to the presence of sarcopenia in patients with chronic liver disease (19). Furthermore, serum zinc concentration is a significant predictor of frailty in patients with chronic liver disease (24). Murata et al. also reported that low serum zinc concentration and aging are independent factors associated with the development of sarcopenia in patients with cirrhosis who have serum zinc concentration <80 µg/dL, and that serum zinc concentration is an independent predictor of sarcopenia and decreased grip strength (22). However, we were unable to demonstrate a relationship between cirrhosis and presarcopenia, as well as a relationship with severity of cirrhosis (Table S1, Figure S1). On the other hand, the rate of presarcopenia was significantly higher in the elderly (≥65 years), indicating a relationship between age and skeletal muscle mass (Table S2). The JSCN guidelines also recommend zinc administration in the presence of zinc deficiency or subclinical zinc deficiency.
In summary, further prospective studies are needed to determine whether interventions such as zinc supplementation for subclinical zinc deficiency reduce the risk of developing sarcopenia in patients with chronic liver disease.
Furthermore, the observations suggest an association between normal serum zinc levels and skeletal muscle mass in patients with chronic liver disease as defined by the JSCN guidelines.
However, Japanese gastroenterology-related societies have not yet provided clear guidelines on the relationship between serum zinc concentration and liver disease as defined by the JSCN guidelines.
Appropriate screening is necessary because the loss of skeletal muscle mass and strength in patients with chronic liver disease can lead to sarcopenia, which can further progress to frailty through weight loss and decreased activity.
Explanations of findings
In the present study, multivariate analysis revealed a serum zinc concentration <60 µg/dL to be a factor significantly associated with presarcopenia, suggesting the importance of zinc supplementation as a nutritional therapy for patients with chronic liver diseases. Given that a decrease in skeletal muscle mass has been reported to be associated with increased mortality (25), controlling sarcopenia is an important issue in aging patients with chronic liver disease, which may be also true for presarcopenia, which is evaluated only by skeletal muscle mass.
This study also revealed that sex differences should be considered when examining presarcopenia. In the examination of L3 SMI between the sexes, it was observed to be significantly higher in men with higher serum zinc concentrations (both >60 and >80 µg/dL), while no differences were observed in women. In men, there was a significant difference in the incidence of presarcopenia between the two groups at both cut-off values, indicating an association between serum zinc concentration and presarcopenia. In contrast, in women, there was no difference in the incidence of presarcopenia between the two groups at any cutoff values, and both groups showed a high incidence of presarcopenia.
The median age of patients in the presarcopenia group was 70.50 years, with women accounting for 68% of all patients. Furthermore, the results of multivariate analysis confirmed a statistically significant difference with odds of 0.194 for men.
These sex differences may be explained by the fact that women on average have less skeletal muscle mass than men (26,27).
This trend is most pronounced after puberty and persists until old age. In women, skeletal muscle loss accelerates after menopause, and the rate of muscle loss is reportedly lower in men (27).
In addition, previous studies have reported a higher proportion of women in the presarcopenia group, with female sex being an independent predictor of presarcopenia (28); a trend toward a higher prevalence of sarcopenia in women of older age has also been reported (29). However, there are also reports of higher incidences of sarcopenia in men with liver cirrhosis (30,31), Although there are trends in the target groups, no consensus has been reached. In this study, male sex was observed to be a predictor in the multivariate analysis. Additionally, no difference in skeletal muscle mass in women was observed; however, a difference in skeletal muscle mass was observed in men. It may be necessary to consider sex differences in the relationship between serum zinc concentration and skeletal muscle mass in intervention studies.
In general, body composition changes significantly with age, including a decline in hormones.
Age-related testosterone decline is associated with the development of sarcopenia (32,33) and, testosterone replacement therapy has been reported to increase muscle strength and lean body mass (34,35). In addition, randomized controlled trials in patients with liver cirrhosis have demonstrated the benefit of testosterone administration (36). Testosterone is a hormone that increases muscle protein synthesis at rest and inhibits muscle protein catabolism during disuse (27). It may be a treatment of interest in patients with chronic liver disease who frequently suffer from a protein-energy hyponutrient status. However, sex differences have not been investigated and its widespread use is limited by safety issues such as the increased incidence of cardiovascular events (36).
Implications and actions needed
We demonstrated an association between serum zinc concentration and presarcopenia. Our results may help identify patients with chronic liver diseases who are at higher risk of presarcopenia. Validation of these results and collection of additional data are needed in future multi-center prospective studies.
Conclusions
The results of this present study showed that serum zinc concentrations <60 µg/dL were associated with presarcopenia in patients with chronic liver disease. Furthermore, we reported that gender differences need to be taken into account.
However, it is not clear from this study whether maintaining normal serum zinc concentrations is linked to skeletal muscle loss, and intervention studies with zinc supplementation, including sex differences, are needed. furthermore, various factors may be involved in the mechanism of sarcopenia in patients with chronic liver disease, further data collection and analysis, including evaluation of muscle strength, are needed for future therapeutic interventions while continuing to measure skeletal muscle mass in daily medical care.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-77/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-77/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-77/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-77/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Saiseikai Niigata Hospital (No. E 23-02) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr 1989;50:1121-235. [PubMed]
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748-59. [Crossref] [PubMed]
- Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol 2014;20:8061-71. [Crossref] [PubMed]
- Hanai T, Shiraki M, Ohnishi S, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 2016;46:743-51. [Crossref] [PubMed]
- Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015;31:193-9. [Crossref] [PubMed]
- Jeong JY, Lim S, Sohn JH, et al. Presence of Sarcopenia and Its Rate of Change Are Independently Associated with Long-term Mortality in Patients with Liver Cirrhosis. J Korean Med Sci 2018;33:e299. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951-63.
- Fukasawa H, Furuya R, Kaneko M, et al. Clinical Significance of Trace Element Zinc in Patients with Chronic Kidney Disease. J Clin Med 2023;12:1667. [Crossref] [PubMed]
- Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract 2015;30:371-82. [Crossref] [PubMed]
- Jeng SS, Chen YH. Association of Zinc with Anemia. Nutrients 2022;14:4918. [Crossref] [PubMed]
- Farooq M. Zinc Deficiency is Associated with Poor Glycemic Control. J Coll Physicians Surg Pak 2019;29:253-7. [Crossref] [PubMed]
- Nakanishi K, Toyoshima M, Ichikawa G, et al. Zinc deficiency is associated with gynecologic cancer recurrence. Front Oncol 2022;12:1025060. [Crossref] [PubMed]
- Santos HO, Teixeira FJ, Schoenfeld BJ. Dietary vs. pharmacological doses of zinc: A clinical review. Clin Nutr 2020;39:1345-53. [Crossref] [PubMed]
- Kim MC, Lee JI, Kim JH, et al. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One 2020;15:e0240195. [Crossref] [PubMed]
- Nishikawa H, Enomoto H, Yoh K, et al. Serum Zinc Concentration and Sarcopenia: A Close Linkage in Chronic Liver Diseases. J Clin Med 2019;8:336. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Welch N, Dasarathy J, Runkana A, et al. Continued muscle loss increases mortality in cirrhosis: Impact of aetiology of liver disease. Liver Int 2020;40:1178-88. [Crossref] [PubMed]
- Murata K, Namisaki T, Fujimoto Y, et al. Clinical Significance of Serum Zinc Levels on the Development of Sarcopenia in Cirrhotic Patients. Cancer Diagn Progn 2022;2:184-93. [Crossref] [PubMed]
- Horiguchi S, Naganuma A, Tateyama Y, et al. Efficacy of Zinc Acetate Treatment for Patients with Decompensated Liver Cirrhosis Complicated by Hypozincemia. Biol Trace Elem Res 2022;200:497-504. [Crossref] [PubMed]
- Nishikawa H, Yoh K, Enomoto H, et al. Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases. J Clin Med 2020;9:1570. [Crossref] [PubMed]
- Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;76:588-99. [Crossref] [PubMed]
- Lee SJ, Janssen I, Heymsfield SB, et al. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr 2004;80:1215-21. [Crossref] [PubMed]
- Smith GI, Mittendorfer B. Sexual dimorphism in skeletal muscle protein turnover. J Appl Physiol (1985) 2016;120:674-82. [Crossref] [PubMed]
- Ohashi K, Ishikawa T, Imai M, et al. Relationship between pre-sarcopenia and quality of life in patients with chronic liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol 2019;31:1408-13. [Crossref] [PubMed]
- Kitamura A, Seino S, Abe T, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle 2021;12:30-8. [Crossref] [PubMed]
- Anand A, Mohta S, Agarwal S, et al. European Working Group on Sarcopenia in Older People (EWGSOP2) Criteria With Population-Based Skeletal Muscle Index Best Predicts Mortality in Asians With Cirrhosis. J Clin Exp Hepatol 2022;12:52-60. [Crossref] [PubMed]
- Lowe R, Hey P, Sinclair M. The sex-specific prognostic utility of sarcopenia in cirrhosis. J Cachexia Sarcopenia Muscle 2022;13:2608-15. [Crossref] [PubMed]
- Chrysavgis L, Adamantou M, Angelousi A, et al. The association of testosterone with sarcopenia and frailty in chronic liver disease. Eur J Clin Invest 2024;54:e14108. [Crossref] [PubMed]
- Tian X, Lou S, Shi R. From mitochondria to sarcopenia: role of 17β-estradiol and testosterone. Front Endocrinol (Lausanne) 2023;14:1156583. [Crossref] [PubMed]
- Lee TW, Kao PY, Chen YC, et al. Effects of Testosterone Replacement Therapy on Muscle Strength in Older Men with Low to Low-Normal Testosterone Levels: A Systematic Review and Meta-Analysis. Gerontology 2023;69:1157-66. [Crossref] [PubMed]
- Manestar D, Malvic G, Velepic M, et al. Perioperative substitution testosterone therapy in patients with advanced head and neck squamous cell carcinoma. Crit Rev Oncol Hematol 2023;188:104062. [Crossref] [PubMed]
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010;363:109-22. [Crossref] [PubMed]
Cite this article as: Suzuki M, Ishikawa T, Ohashi K, Hoshii A, Hirosawa H, Noguchi H, Honma T. Serum zinc concentration as a potential predictor of presarcopenia in patients with chronic liver disease: a preliminary study. Transl Gastroenterol Hepatol 2024;9:20.


