Comparing the protective effects of local and remote ischemic preconditioning against ischemia-reperfusion injury in hepatectomy: a systematic review and network meta-analysis
Highlight box
Key findings
• Remote ischemic preconditioning (RIPC) and local ischemic preconditioning (LIPC) could serve as effective strategies in relieving hepatic ischemia-reperfusion injury (HIRI) during hepatectomy. No significant differences were observed between LIPC and RIPC, however, RIPC may become an easily applicable protective strategy to relieve liver injury in hepatectomy.
What is known and what is new?
• HIRI is a major hurdle to the success of hepatectomy. LIPC is known to be a protective strategy but limited in clinical practice due to its poor feasibility and invasiveness in hepatectomy. Growing evidence suggests that RIPC has protective effects against HIRI. Few studies have directly compared the protective effects of the two mechanical preconditioning methods.
• This study provided new evidence that RIPC has protective effects against HIRI as LIPC does.
What is the implication, and what should change now?
• The severity of HIRI is associated with higher rates of postoperative morbidity and mortality.
• More clinical investigations are needed to find more effective strategies to mitigate HIRI in liver resection, especially combined measures.
Introduction
Hepatic resection has been used worldwide for treating both benign and malignant liver masses (1,2). However, intraoperative hepatic bleeding remains a significant challenge (3-5). The Pringle maneuver, involving intermittent inflow occlusion during hepatectomy could effectively reduce blood loss; whereas, it raises concern regarding hepatic ischemia-reperfusion injury (HIRI) during hepatectomy (6), especially when combined with low central venous pressure (7). The severity of HIRI is associated with higher rates of postoperative morbidity and mortality (8,9). Thus, finding effective strategies to mitigate HIRI in liver resection is recognized as a research priority.
Local ischemic preconditioning (LIPC) is a protective strategy that exposes the liver to a temporary period of ischemia before hepatectomy, enabling adaptation to subsequent long-term ischemic insults. Experimental and clinical evidence has revealed that LIPC can ameliorate hepatic injury (10-16). In addition, a recent network meta-analysis (17) demonstrated that LIPC resulted in multiple beneficial clinical endpoints during elective liver resection. Despite these promising results, LIPC has not gained widespread adoption in clinical practice. This may be partly due to the fact that LIPC can induce direct liver ischemia and necessitates additional surgical procedures, thereby increasing the surgical complexity and extending the operative time. Subsequently, remote ischemic preconditioning (RIPC), another mechanical preconditioning strategy, has been recognized for its effectiveness, as numerous studies have demonstrated its benefits within the same organ and in distant organs (18-21). RIPC involves one or more brief cycles achieved by inflating and deflating a standard blood pressure cuff placed on a limb before surgery to play a protective role in organ function. It offers several advantages such as user-friendly control, no need for additional surgical procedures, and no increase in surgical duration. These conveniences have facilitated its translation into clinical settings (22).
However, to our knowledge, no large randomized clinical trials (RCTs) have directly compared the effects of LIPC and RIPC on hepatic function in hepatectomy. Therefore, we performed a network meta-analysis to compare the efficacy of LIPC and RIPC for hepatic injury during liver resection. We present this article in accordance with the PRISMA-NMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-95/rc).
Methods
This meta-analysis was prospectively registered on INPLASY (CRD202370007).
Search strategy
The following databases were searched: Embase, PubMed, Cochrane Library, and China National Knowledge Infrastructure (CNKI) from database inception until January 2023. In addition, meta-analysis and references cited in the included studies were examined. A detailed search strategy is provided in Appendix 1.
Study selection
First, duplicates were removed by Endnote X9. Subsequently, three reviewers (Y.C., J.Y., and K.W.) screened the titles and abstracts of trials independently to select the eligible inclusions based on the inclusion and exclusion criteria. Full-text articles and their relevant references were carefully selected for further assessment. Disagreements were resolved by an independent reviewer (Z.Z.).
Data extraction
Three authors (Y.C., J.Y., and K.W.) extracted data independently from the eligible studies, including study characteristics, participants’ information, preconditioning types, and other interesting outcomes such as liver function and operative outcomes. Disagreements were settled by an independent reviewer (Z.Z.). All data were recorded in Microsoft Excel [2016].
Quality assessment
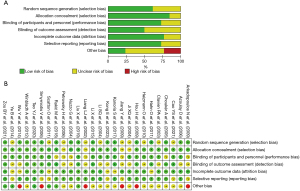
The methodological quality of included trials and risk of bias were evaluated by the Cochrane Collaboration’s tool which includes seven domains: allocation concealment, random sequence generation, incomplete outcome data, selective outcome reporting, blinding of participants and personnel, blinding of outcome assessment, and other biases. The risk of bias was graded as high, unclear, or low. The risk of bias in each trial was evaluated independently by three authors (Y.C., J.Y., and K.W.), and disagreements were discussed with an independent reviewer (Z.Z.) to reach an agreement.
Selection criteria
Studies were selected based on specific inclusion criteria: (I) participants—humans with relevant diseases necessitating hepatectomy, aged over 18 years; (II) interventions—the intervention and comparator should include one of the following: LIPC versus RIPC, LIPC versus no-preconditioning, or RIPC versus no-preconditioning; (III) outcomes—reporting of outcome indicators reflecting liver function, such as aspartate aminotransferase (AST) or alanine aminotransferase (ALT), is required; and (IV) methodological criterion—prospective RCTs.
Exclusion criteria
The following exclusion criteria were used: (I) liver transplantation studies; and (II) cluster or crossover randomized trials.
Statistical analysis
We tried to contact study authors in cases where missing or unclear data were identified. When standard deviations (SDs) were unreported, they were computed from standard errors, P values, t values, confidence intervals (CIs), or graphical representations. Random effects models were used for the network meta-analyses. Summary odds ratios (ORs) for dichotomous outcomes and standardized mean differences (SMD) for continuous outcomes, along with their corresponding 95% CIs, were derived through network meta-analysis. The network meta-analysis was executed using the network meta package within Stata (version 16.1). Prior to conducting the network meta-analysis, an assessment of the transitivity assumption was conducted by scrutinizing the characteristics of the included studies. Statistical heterogeneity was probed via pairwise meta-analyses. Discrepancies between direct and indirect sources of evidence were assessed employing both global and local methodologies. Global inconsistency was evaluated through a design-by-treatment test, while local inconsistency was gauged using a side-splitting approach. Secondary metrics of treatment effect, such as surface under the cumulative rank curve (SUCRA) probabilities and treatment rankings, were also computed. Publication bias was explored by constructing funnel plots and detecting asymmetry. Subgroup analyses were performed between groups with cirrhotic and noncirrhotic livers, groups with major and minor hepatectomies, and groups with different times of vascular exclusion.
Outcomes
Primary outcomes: postoperative serum transaminase level, including AST and ALT on postoperative day 1 (POD1).
Secondary outcomes: other indicators to reflect liver function, like total bilirubin (TBIL) and outcomes presenting surgical process, including operative time, blood loss, and hospital stay.
Results
Study characteristics
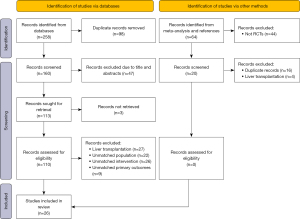
In total, 268 studies were initially identified through the search strategy. After removing duplicates, 160 unique titles and abstracts were screened. Subsequently, 110 articles in the full-text review were assessed. Finally, 26 articles met eligibility for extraction (Table 1). The reasons for exclusion are shown in Figure 1.
Table 1
| ID | Source | Country | Type of preconditioning | Sample sizes, n | M/F, n | Age (years) | Ischemic time (min) | Resected volume | Cirrhosis, n | Steatosis, n |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Choukèr et al. (14) | Germany | LIPC (10+10)×1 | 14 | 7/7 | 58±12 | 32±6.3 | Minor resection | – | – |
| No-preconditioning | 34 | 23/11 | 61±10.8 | 35±11 | – | – | ||||
| 2 | Clavien et al. (23) | Switzerland | LIPC (10+10)×1 | 50 | 22/28 | 54±14 | 36±5.9 | Major resection | – | 13 |
| No-preconditioning | 50 | 25/25 | 57±14 | 35±6.8 | – | |||||
| 3 | Heizmann et al. (24) | Switzerland | LIPC (10+10)×1 | 31 | 19/12 | 55±13 | 33±12 | Minor resection | – | 10 |
| No-preconditioning | 30 | 18/13 | 57±14 | 34±14 | – | 8 | ||||
| 4 | Li et al. (25) | China | LIPC (5+5)×1 | 14 | 12/2 | 50±10.7 | 18.0±3.6 | Minor resection | 13 | – |
| No-preconditioning | 15 | 12/3 | 50±10.3 | 17.4±2.3 | 12 | – | ||||
| 5 | Azoulay et al. (26) | France | LIPC (10+10)×1 | 30 | 18/12 | 58±14.6 | 44.5±9.2 | Major resection | 1 | 4 |
| No-preconditioning | 30 | 16/14 | 61±14 | 47.7±8.3 | 0 | 4 | ||||
| 6 | Winbladh et al. (27) | Sweden | LIPC (10+10)×1 | 16 | 8/8 | 64±14 | 39.5±11 | Major resection | – | 1 |
| No-preconditioning | 16 | 10/6 | 64±9.4 | 44±10 | – | 3 | ||||
| 7 | Hahn et al. (28) | Hungary | LIPC (10+10)×1 | 80 | 42/38 | 57±2.2 | 33±2.9 | Major resection | 30 | 1 |
| No-preconditioning | 80 | 37/43 | 55±1.8 | 28.5±6.2 | 30 | 2 | ||||
| 8 | Scatton et al. (29) | France | LIPC (10+10)×1 | 43 | – | 62±13.6 | 45±19.6 | Major resection | – | – |
| No-preconditioning | 41 | – | 58.2±13 | 52.4±27.7 | – | – | ||||
| 9 | Arkadopoulos et al. (30) | Greece | LIPC (10+15)×1 | 41 | – | – | 42±10 | Major resection | – | – |
| No-preconditioning | 43 | – | – | 42±11 | – | – | ||||
| 10 | Nuzzo et al. (31) | Italy | LIPC (10+10)×1 | 21 | 12/9 | 50±14 | – | Minor resection | – | – |
| No-preconditioning | 21 | 11/10 | 57±11 | – | – | – | ||||
| 11 | Petrowsky et al. (32) | Switzerland | LIPC (10+10)×1 | 36 | 23/13 | 56.5±2.3 | 37.3±1.5 | Major resection | – | 15 |
| No-preconditioning | 37 | 15/22 | 58.9±2.3 | 40.0±2.1 | – | 20 | ||||
| 12 | Ye et al. (33) | China | LIPC (5+5)×1 | 50 | 39/11 | 50±15.3 | – | Minor resection | 33 | – |
| No-preconditioning | 50 | 37/13 | 53±11.4 | – | 34 | – | ||||
| 13 | Smyrniotis et al. (34) | Greece | LIPC (10+10)×1 | 27 | 20/7 | 63±13.75 | 41.4±4.3 | Major resection | – | – |
| No-preconditioning | 27 | 18/9 | 62±14.75 | 42.5±6.3 | – | – | ||||
| 14 | Hou et al. (35) | China | LIPC (5+5)×1 | 24 | – | 47±14 | – | Minor resection | 12 | – |
| No-preconditioning | 24 | – | 48±16 | – | 12 | – | ||||
| 15 | Ji et al. (36) | China | LIPC (5+5)×1 | 18 | 15/3 | 51.6±8.7 | 17.8±2.3 | – | 15 | – |
| No-preconditioning | 16 | 12/4 | 48.6±8.6 | 17.9±1.8 | 13 | – | ||||
| 16 | Liang et al. (37) | China | LIPC (5+5)×1 | 14 | 12/2 | 50±10.7 | 18.0±3.6 | Minor resection | 13 | – |
| No-preconditioning | 15 | 12/3 | 49.5±10.3 | 17.4±2.3 | 12 | – | ||||
| 17 | Jiang et al. (38) | China | LIPC (5+5)×1 | 35 | 25/10 | 47±11.2 | 24.8±9.5 | – | 30 | – |
| No-preconditioning | 25 | 17/8 | 49±10.5 | 22.2±8.5 | 21 | – | ||||
| 18 | Teo et al. (39) | China | RIPC (5+5)×4 | 24 | 20/4 | 64±11.2 | 37±18.7 | Minor resection | 11 | – |
| No-preconditioning | 26 | 19/7 | 67±8.4 | 29±13.5 | 7 | – | ||||
| 19 | Kanoria et al. (40) | England | RIPC (10+10)×3 | 8 | 7/1 | – | – | – | – | – |
| No-preconditioning | 8 | 6/2 | ||||||||
| 20 | Liu et al. (41) | China | RIPC (5+5)×3 | 69 | 59/10 | 51.7±10.6 | 22±4.6 | Minor resection | 56 | – |
| No-preconditioning | 67 | 59/8 | 52.1±10.9 | 22.3±5 | 51 | – | ||||
| 21 | Zou et al. (42) | China | RIPC (5+5)×3 | 20 | 7/13 | 47±11.03 | 49.6±5.14 | Major resection | – | – |
| No-preconditioning | 20 | 9/11 | 52±8.22 | 47.5±3.72 | – | – | ||||
| 22 | Wu et al. (43) | China | RIPC (5+5)×3 | 10 | – | 52±8 | 17±4 | – | – | – |
| No-preconditioning | 10 | – | 49±7 | 17±3 | – | – | – | |||
| 23 | Li et al. (44) | China | RIPC (5+5)×3 | 30 | 17/13 | 39±8.5 | – | – | – | – |
| No-preconditioning | 30 | 16/14 | 40±7.2 | – | – | – | – | |||
| 24 | Cao et al. (45) | China | RIPC (5+5)×3 | 30 | 11/19 | 35±9.1 | 15.4±3.1 | Major resection | – | – |
| No-preconditioning | 30 | 8/22 | 38±11 | 16.2±2.6 | – | – | ||||
| 25 | Kong et al. (46) | China | RIPC (5+5)×3 | 30 | 16/9 | 54±9.77 | 42.2±6.79 | Minor resection | – | – |
| LIPC (5+5)×3 | 30 | 15/13 | 54±10.68 | 39±28.84 | – | – | ||||
| No-preconditioning | 30 | 17/10 | 54±12.13 | 41±7.93 | – | – | ||||
| 26 | Rakić et al. (47) | Croatia | RIPC (5+5)×3 | 20 | – | – | – | Minor resection | – | – |
| LIPC (10+15)×1 | 20 | – | – | – | – | – | ||||
| No-preconditioning | 20 | – | – | – | – | – |
Values are presented as mean ± SD, unless otherwise indicated. Preconditioning types and implementation approaches were recorded. “(5+5)×3” indicates 5 min of ischemia followed by 5 min of reperfusion by three circles. n, number of events; M, male; F, female; LIPC, local ischemic preconditioning; RIPC, remote ischemic preconditioning; SD, standard deviation.
Characteristics of the included studies
Among the trials included in this network meta-analysis, 13 studies were conducted in Asia, and 13 were conducted in Europe. These studies were published between 2002 and 2023. The mean age of the participants was 54 (12.7) years, and 484 (62.8%) of the patients were women. Of the 26 studies, six were limited to older adults (aged >60 years) and 10 (38%) enrolled patients undergoing major liver resection.
In ten trials (59%), LIPC was performed through 10 min of inflow occlusion followed by 10 min of reperfusion. In 6 (35%) studies in the LIPC group, the Pringle maneuver was preceded by 5 min of ischemia and 5 min of reperfusion. In 1 (6%) study, LIPC was done by inflow occlusion for 10 min followed by reperfusion for 15 min before continuous hepatic vascular exclusion.
Regarding RIPC, 7 trials (77%) were performed through three cycles of 5 min of inflow occlusion followed by 5 min of reperfusion. In one study, RIPC was conducted by three cycles of 10 min of inflow occlusion followed by 10 min of reperfusion. In another trial, RIPC was performed using four cycles of 5 min of inflow occlusion followed by 5 min of reperfusion.
We found no clear evidence of violations of the transitivity assumption when comparing characteristics of studies across comparisons (Table 1).
Meta-analysis
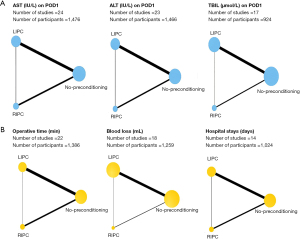
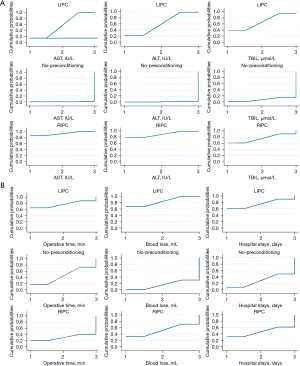
Figure 2 illustrates the network diagram of the total number of patients in each treatment. Figures 3,4 illustrate the network of eligible comparisons for primary and secondary outcomes. Figure 5 illustrates the ranking of treatments based on the SUCRA plot for each outcome.

Primary outcomes
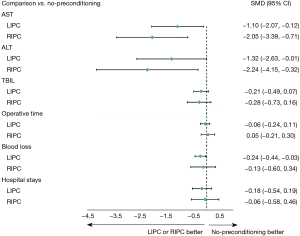
AST: a total of 24 studies with 1,476 participants were included in the network meta-analysis. RIPC and LIPC were more effective in preserving liver function than no-preconditioning (SMD RIPC versus no-preconditioning: −2.05, 95% CI: −3.39, −0.71; SMD LIPC versus no-preconditioning: −1.10, 95% CI: −2.07, −0.12). However, the network meta-analysis of indirect comparisons of RIPC and LIPC suggested no significant differences between RIPC and LIPC (SMD RIPC versus LIPC: −0.95, 95% CI: −2.52, 0.62). Furthermore, RIPC had the highest SUCRA value for AST reduction, followed by LIPC, and no-preconditioning.
ALT: in total, 23 studies with 1,466 participants were included in the network meta-analysis. Compared with no-preconditioning, RIPC (SMD RIPC versus no-preconditioning: −2.24, 95% CI: −4.15, −0.32) and LIPC (SMD LIPC versus no-preconditioning: −1.32, 95% CI: −2.63, −0.01) resulted in a significant reduction in ALT on POD1. However, no significant difference was observed between RIPC and LIPC (SMD RIPC versus LIPC: −0.91, 95% CI: −3.11, 1.28). Similarly, RIPC had the highest SUCRA value for ALT reduction on POD1, followed by LIPC, and no-preconditioning.
Secondary outcomes
TBIL: a total of 17 studies with 924 participants were included in the network meta-analysis. Compared with no-preconditioning, RIPC or LIPC exhibited no protective effect on liver function (SMD RIPC versus no-preconditioning: −0.28, 95% CI: −0.73, 0.16; SMD LIPC versus no-preconditioning: −0.21, 95% CI: −0.49, 0.07). Furthermore, the comparison between RIPC and LIPC revealed no decline in TBIL (SMD RIPC versus LIPC: −0.07, 95% CI: −0.57, 0.42).
Surgical time: 22 studies with 1,386 participants were included in the network meta-analysis. No significant difference was observed between the three pairwise comparisons (SMD RIPC versus LIPC: 0.11, 95% CI: −0.19, 0.42; SMD RIPC versus no-preconditioning: 0.05, 95% CI: −0.21, 0.30; SMD LIPC versus no-preconditioning: −0.06, 95% CI: −0.24, 0.11). LIPC had the highest SUCRA value for reducing surgical time.
Blood loss: 18 studies with 1,259 participants were included in the network meta-analysis. LIPC caused less blood loss compared with no-preconditioning (SMD LIPC versus no-preconditioning: −0.24, 95% CI: −0.44, −0.03). However, the Network meta-analysis of indirect comparisons of RIPC and LIPC also suggested no difference in bleeding (SMD RIPC versus LIPC: 0.10, 95% CI: −0.39, 0.60). LIPC had the highest SUCRA value, followed by RIPC, and no-preconditioning.
Hospital stays: This network meta-analysis analyzed 14 studies with 1,024 participants. No statistical difference was observed between LIPC and no-preconditioning or RIPC and no-preconditioning or LIPC and RIPC (SMD LIPC versus no-preconditioning: −0.18, 95% CI: −0.54, 0.19; SMD RIPC versus no-preconditioning: −0.06, 95% CI: −0.58, 0.46; SMD RIPC versus LIPC: 0.12, 95% CI: −0.49, 0.72). However, our results showed that LIPC had the highest SUCRA value, followed by RIPC, and no-preconditioning.
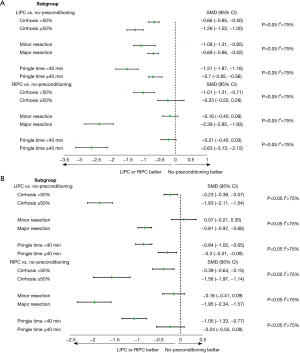
No inconsistency was found in our study, and the evaluation of local inconsistency for each primary outcome was presented in Appendix 2. Moreover, the ranking of treatments based on the SUCRA plot for each outcome was shown in Figure 5. Subsequently, we conducted subgroup analyses to explore cirrhosis, resected liver volume, and inflow occlusion time (Figure 6). These results did not substantially differ from those of the primary analyses for most of the comparisons. RIPC demonstrated protective effects in terms of AST and ALT levels on POD1, especially in terms of patients who had cirrhosis or undergone major resection (Figure 6). The Cochrane Collaborative bias risk tool was used to assess the risk of bias of the included studies. Figure 7 shows the bias assessment of each methodological component of the eligible studies.
Discussion
To our knowledge, this is the first meta-analysis study to directly compare the efficiency of LIPC and RIPC in patients undergoing hepatectomy. We found that LIPC and RIPC were superior to no-preconditioning in alleviating liver injury. Moreover, no significant difference was observed between the effect of LIPC and RIPC. Furthermore, in subgroup analysis, RIPC demonstrated potential protective effects on liver function after major liver resection or when patients diagnosed with cirrhosis. These findings provided evidence that RIPC had the potential to reduce HIRI during liver resection as well as LIPC did.
LIPC has been confirmed to have beneficial effects in relieving HIRI in animals and humans over the past three decades (48-51). Our study also found that LIPC offers protection in liver function which is consistent with the findings of the latest meta-analysis (52). This protection could be further enhanced by manipulation of apoptotic pathways, activating related signaling pathways or inhibiting hepatocyte apoptosis (53). While laboratory and experimental evidence is favorable, a major limitation to clinical application of LIPC is the potential to damage to the portal vein and its small branches. Furthermore, prior studies have not reached a consensus on the standardization of ischemia and reperfusion durations for LIPC. Most studies have followed a standardized protocol of 10 min of ischemia followed by 10 min of reperfusion; however, the protocol remains controversial. Therefore, it is necessary to discover better ways to mitigate liver damage.
RIPC, as another form of mechanical preconditioning, can be achieved noninvasively by simple inflating and deflating a standard blood pressure cuff placed on a limb, which facilitates RIPC translation into the clinical settings. Initially demonstrated in the canine heart (54), its protective effect on heart was later confirmed in humans. Subsequent studies have shown that RIPC protects muscle flaps, brain, kidneys, and heart from ischemic injury (55-62). In recent years, numerous animal experiments have shown the protective effects of RIPC on the liver (63,64). Several small clinical trials have presented evidence supporting the potential benefit of RIPC during hepatectomy (40,43), but two trials with small samples failed to demonstrate liver protection with RIPC (41,65). Thus, the role of RIPC in liver protection remains controversial. Interesting, our study provides new insight into the potential effectiveness of RIPC in liver resection. This may be linked to the ability of RIPC to promote the regeneration of marginal liver remnants, leading to improved survival after extended hepatectomy in a vascular endothelial growth factor (VEGF) dependent manner (66).
Our study demonstrated that no significance difference was observed between LIPC and RIPC. Currently, there are only two RCTs directly comparing RIPC and LIPC. Our result aligns with the latest clinical trial designed by Kong et al. (46), while it contradicts to the findings of the other study (47). We speculate that this may be related to several possible reasons. Firstly, anesthesia may be a confounding factor in the role of RIPC in liver surgery. Many studies supported that volatile but not propofol was a positive influencing factor in RIPC for myocardial protection (67-69). Similar results were confirmed in kidney protection during cardiac surgery (55,70). Unfortunately, due to a lack of sufficient data, subgroup analysis of anesthetic methods was not conducted in our study. Nevertheless, five eligible studies in our study indicated the beneficial impact of RIPC on liver function, all of which utilized propofol. Therefore, propofol may enhance the effects of RIPC. As Kong’ trial, patients in RIPC group received invasive anesthesia by propofol which had lower AST and ALT levels, while the anesthesia mode in another RCT directly comparing RIPC and LIPC was not clear. Future research is needed to confirm the impact of anesthesia on RIPC in liver resection. Secondly, different ischemic preconditioning protocols may influence the effects of these two strategies, including variations in the site, duration, intensity (71). Further research is needed to confirm the relationship between these factors and the effectiveness of LIPC and RIPC. Moreover, whether participants have other complications, the severity of diseases, and other factors can be crucial. Thus, individual differences may have an impact on the therapeutic efficacy.
There were some limitations that must be considered. First, laboratory indicators such as AST and ALT are commonly used as primary outcomes in clinical trials; however, they may not provide a comprehensive assessment of short-term recovery and clinical outcomes following liver surgery. Therefore, lacking sensitive and specific indicators reflecting liver function remains a significant concern in clinical studies, which needs to attract enough attention in future investigations. Additionally, given differences between eligible studies, clinical and statistical heterogeneity may exist. While SUCRA plots were utilized to determine the ranking of relative outcomes, caution is warranted in interpreting their values, as the comparisons of SMD were not significant for most outcomes. Finally, due to insufficient data, the role of anesthetic methods as a confounding factor in the efficacy of RIPC and LIPC was not analyzed in our study.
Conclusions
RIPC and LIPC could serve as effective strategies in relieving HIRI during hepatectomy. No significant differences were observed between LIPC and RIPC, however, RIPC may become an easily applicable protective strategy to relieve liver injury in hepatectomy. More large-scale clinical trials are needed in the future to confirm the application of LIPC and RIPC in hepatectomy.
Acknowledgments
Thanks to Dr. Lijun Shang and Dr. Bairen Wang for the language revision and other suggestions on the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-NMA reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-95/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-95/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-95/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl 2002;84:371-80. [Crossref] [PubMed]
- Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. Biosci Trends 2022;16:178-88. [Crossref] [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Effect of perioperative blood transfusion on clinical outcomes in hepatic surgery for cancer. World J Gastroenterol 2009;15:3976-83. [Crossref] [PubMed]
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-9; discussion 869-70. [Crossref] [PubMed]
- Bahde R, Spiegel HU. Hepatic ischaemia-reperfusion injury from bench to bedside. Br J Surg 2010;97:1461-75. [Crossref] [PubMed]
- Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg 1998;85:1058-60. [Crossref] [PubMed]
- Cannistrà M, Ruggiero M, Zullo A, et al. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg 2016;33:S57-70. [Crossref] [PubMed]
- Franken C, Lau B, Putchakayala K, et al. Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg 2014;149:941-6. [Crossref] [PubMed]
- Banga NR, Homer-Vanniasinkam S, Graham A, et al. Ischaemic preconditioning in transplantation and major resection of the liver. Br J Surg 2005;92:528-38. [Crossref] [PubMed]
- Desai KK, Dikdan GS, Shareef A, et al. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl 2008;14:1569-77. [Crossref] [PubMed]
- Gurusamy KS, Kumar Y, Pamecha V, et al. Ischaemic pre-conditioning for elective liver resections performed under vascular occlusion. Cochrane Database Syst Rev 2009;CD007629. [Crossref] [PubMed]
- O'Neill S, Leuschner S, McNally SJ, et al. Meta-analysis of ischaemic preconditioning for liver resections. Br J Surg 2013;100:1689-700. [Crossref] [PubMed]
- Choukèr A, Martignoni A, Schauer R, et al. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg 2005;140:129-36. [Crossref] [PubMed]
- Prieto I, Monsalve M. ROS homeostasis, a key determinant in liver ischemic-preconditioning. Redox Biol 2017;12:1020-5. [Crossref] [PubMed]
- Schulz R, Walz MK, Behrends M, et al. Minimal protection of the liver by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol 2001;280:H198-207. [Crossref] [PubMed]
- Simillis C, Robertson FP, Afxentiou T, et al. A network meta-analysis comparing perioperative outcomes of interventions aiming to decrease ischemia reperfusion injury during elective liver resection. Surgery 2016;159:1157-69. [Crossref] [PubMed]
- Moskowitz MA, Waeber C. Remote ischemic preconditioning: making the brain more tolerant, safely and inexpensively. Circulation 2011;123:709-11. [Crossref] [PubMed]
- Pan JS, Sheikh-Hamad D. Remote ischemic preconditioning for kidney protection. JAMA 2015;313:2124-5. [Crossref] [PubMed]
- Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 2017;469:159-81. [Crossref] [PubMed]
- Heusch G, Bøtker HE, Przyklenk K, et al. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177-95. [Crossref] [PubMed]
- Kloner RA. Clinical application of remote ischemic preconditioning. Circulation 2009;119:776-8. [Crossref] [PubMed]
- Clavien PA, Selzner M, Rüdiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg 2003;238:843-50; discussion 851-2. [Crossref] [PubMed]
- Heizmann O, Meimarakis G, Volk A, et al. Ischemic preconditioning-induced hyperperfusion correlates with hepatoprotection after liver resection. World J Gastroenterol 2010;16:1871-8. [Crossref] [PubMed]
- Li SQ, Liang LJ, Huang JF, et al. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol 2004;10:2580-4. [Crossref] [PubMed]
- Azoulay D, Lucidi V, Andreani P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg 2006;202:203-11. [Crossref] [PubMed]
- Winbladh A, Björnsson B, Trulsson L, et al. Ischemic preconditioning prior to intermittent Pringle maneuver in liver resections. J Hepatobiliary Pancreat Sci 2012;19:159-70. [Crossref] [PubMed]
- Hahn O, Blázovics A, Váli L, et al. The effect of ischemic preconditioning on redox status during liver resections--randomized controlled trial. J Surg Oncol 2011;104:647-53. [Crossref] [PubMed]
- Scatton O, Zalinski S, Jegou D, et al. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg 2011;98:1236-43. [Crossref] [PubMed]
- Arkadopoulos N, Kostopanagiotou G, Theodoraki K, et al. Ischemic preconditioning confers antiapoptotic protection during major hepatectomies performed under combined inflow and outflow exclusion of the liver. A randomized clinical trial. World J Surg 2009;33:1909-15. [Crossref] [PubMed]
- Nuzzo G, Giuliante F, Vellone M, et al. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl 2004;10:S53-7. [Crossref] [PubMed]
- Petrowsky H, McCormack L, Trujillo M, et al. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg 2006;244:921-8; discussion 928-30. [Crossref] [PubMed]
- Ye B, Zhao H, Hou H, et al. Ischemic preconditioning provides no additive clinical value in liver resection of cirrhotic and non-cirrhotic patients under portal triad clamping: a prospective randomized controlled trial. Clin Res Hepatol Gastroenterol 2014;38:467-74. [Crossref] [PubMed]
- Smyrniotis V, Theodoraki K, Arkadopoulos N, et al. Ischemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. Am J Surg 2006;192:669-74. [Crossref] [PubMed]
- Hou H, Geng XP, Zhu LX, et al. The value of hepatic ischemic preconditioning in hepatectomy with a prospective randomized controlled study. Zhonghua Wai Ke Za Zhi 2009;47:586-9. [PubMed]
- Ji XQ, Li CL, Yang JC, et al. Application of ischemic preconditioning before hepatic vascular exclusion for resection of hepatocellular carcinoma. Di Yi Jun Yi Da Xue Xue Bao 2004;24:66-8, 71. [PubMed]
- Liang L, Li S, Huang J. The protective effect and mechanism of ischemic preconditioning for hepatic resection under hepatic blood inflow occlusion in hepatocellular carcinoma patients with cirrhosis. Zhonghua Wai Ke Za Zhi 2002;40:265-7. [PubMed]
- Jiang Y, Wu BQ, Qin XH, et al. Effect of ischemic preconditioning on hepatic cancer in perioperation. Journal of Hepatopancreatobiliary Surgery 2009;21:347-9.
- Teo JY, Ho AFW, Bulluck H, et al. Effect of remote ischemic preConditioning on liver injury in patients undergoing liver resection: the ERIC-LIVER trial. HPB (Oxford) 2020;22:1250-7. [Crossref] [PubMed]
- Kanoria S, Robertson FP, Mehta NN, et al. Effect of Remote Ischaemic Preconditioning on Liver Injury in Patients Undergoing Major Hepatectomy for Colorectal Liver Metastasis: A Pilot Randomised Controlled Feasibility Trial. World J Surg 2017;41:1322-30. [Crossref] [PubMed]
- Liu X, Cao L, Zhang T, et al. Effect of Remote Ischemic Preconditioning in Patients Undergoing Hepatectomy With Portal Triad Clamping: A Randomized Controlled Trial. Anesth Analg 2019;129:1742-8. [Crossref] [PubMed]
- Zou BY. Effect of dexmedetomidine combined with remote limb ischmic preconditioning on hepatic ischemia reperfusion injury in patients undergoing liver resection. Changsha: Hunan Normal University; 2021. doi:
10.27137/d.cnki.ghusu.2021.002020 .10.27137/d.cnki.ghusu.2021.002020 - Wu Y, Zhang Y, Hu XW, et al. Effect of remote ischemic preconditioning on post-operative liver function of patients undergoing hemihepatectomy. Acta Universitatis Medicinalis Anhui 2014;49:1472-4, 1475.
- Li X, Long XJ, Hu YH, et al. Effect of limb remote ischemic preconditioning on levels of serum TNF-αand HMGB1 in liver operation. Journal of Clinical Anesthesiology 2015;31:1193-5.
- Cao YS. Effect of limb remote ischemic preconditioning on liver function in patients with hepatic hydatid disease after hepatectomy at high altitude. Xining: Qinghai University; 2021. doi:
10.27740/d.cnki.gqhdx.2021.000050 .10.27740/d.cnki.gqhdx.2021.000050 - Kong E, Yuan C, Li Y, et al. Protective Efficiency Comparison of Direct and Remote Ischemic Preconditioning on Ischemia Reperfusion Injury of the Liver in Patients Undergoing Partial Hepatectomy. Biomed Res Int 2023;2023:2763320. [Crossref] [PubMed]
- Rakić M, Patrlj L, Amić F, et al. Comparison of hepatoprotective effect from ischemia-reperfusion injury of remote ischemic preconditioning of the liver vs local ischemic preconditioning of the liver during human liver resections. Int J Surg 2018;54:248-53. [Crossref] [PubMed]
- Rüdiger HA, Kang KJ, Sindram D, et al. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg 2002;235:400-7. [Crossref] [PubMed]
- Peralta C, Fernández L, Panés J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology 2001;33:100-13. [Crossref] [PubMed]
- Pasupathy S, Homer-Vanniasinkam S. Surgical implications of ischemic preconditioning. Arch Surg 2005;140:405-9; discussion 410. [Crossref] [PubMed]
- Compagnon P, Lindell S, Ametani MS, et al. Ischemic preconditioning and liver tolerance to warm or cold ischemia: experimental studies in large animals. Transplantation 2005;79:1393-400. [Crossref] [PubMed]
- de Oliveira GC, de Oliveira WK, Yoshida WB, et al. Impacts of ischemic preconditioning in liver resection: systematic review with meta-analysis. Int J Surg 2023;109:1720-7. [Crossref] [PubMed]
- Jiménez-Castro MB, Cornide-Petronio ME, Gracia-Sancho J, et al. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019;8:1131. [Crossref] [PubMed]
- Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893-9. [Crossref] [PubMed]
- Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015;313:2133-41. [Crossref] [PubMed]
- Endre ZH. Renal ischemic preconditioning: finally some good news for prevention of acute kidney injury. Kidney Int 2011;80:796-8. [Crossref] [PubMed]
- Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke 2014;45:159-67. [Crossref] [PubMed]
- Haapanen H, Herajärvi J, Arvola O, et al. Remote ischemic preconditioning protects the spinal cord against ischemic insult: An experimental study in a porcine model. J Thorac Cardiovasc Surg 2016;151:777-85. [Crossref] [PubMed]
- Addison PD, Neligan PC, Ashrafpour H, et al. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol 2003;285:H1435-43. [Crossref] [PubMed]
- Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013;382:597-604. [Crossref] [PubMed]
- Kleinbongard P, Peters J, Jakob H, et al. Persistent Survival Benefit From Remote Ischemic Pre-Conditioning in Patients Undergoing Coronary Artery Bypass Surgery. J Am Coll Cardiol 2018;71:252-4. [Crossref] [PubMed]
- Hausenloy DJ, Candilio L, Evans R, et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med 2015;373:1408-17. [Crossref] [PubMed]
- Limani P, Linecker M, Oberkofler CE, et al. Remote Ischemic Preconditioning: A Novel Strategy in Rescuing Older Livers From Ischemia-reperfusion Injury in a Rodent Model. Ann Surg 2016;264:797-803. [Crossref] [PubMed]
- Oberkofler CE, Limani P, Jang JH, et al. Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology 2014;60:1409-17. [Crossref] [PubMed]
- Jung KW, Kang J, Kwon HM, et al. Effect of Remote Ischemic Preconditioning Conducted in Living Liver Donors on Postoperative Liver Function in Donors and Recipients Following Liver Transplantation: A Randomized Clinical Trial. Ann Surg 2020;271:646-53. [Crossref] [PubMed]
- Kambakamba P, Linecker M, Schneider M, et al. Novel Benefits of Remote Ischemic Preconditioning Through VEGF-dependent Protection From Resection-induced Liver Failure in the Mouse. Ann Surg 2018;268:885-93. [Crossref] [PubMed]
- Zangrillo A, Musu M, Greco T, et al. Additive Effect on Survival of Anaesthetic Cardiac Protection and Remote Ischemic Preconditioning in Cardiac Surgery: A Bayesian Network Meta-Analysis of Randomized Trials. PLoS One 2015;10:e0134264. [Crossref] [PubMed]
- Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand 2012;56:30-8. [Crossref] [PubMed]
- Pierce B, Bole I, Patel V, et al. Clinical Outcomes of Remote Ischemic Preconditioning Prior to Cardiac Surgery: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2017;6:e004666. [Crossref] [PubMed]
- Zhou H, Yang L, Wang G, et al. Remote Ischemic Preconditioning Prevents Postoperative Acute Kidney Injury After Open Total Aortic Arch Replacement: A Double-Blind, Randomized, Sham-Controlled Trial. Anesth Analg 2019;129:287-93. [Crossref] [PubMed]
- Peters J. Remote ischaemic preconditioning of the heart: remote questions, remote importance, or remote preconditions? Basic Res Cardiol 2011;106:507-9. [Crossref] [PubMed]
Cite this article as: Chen Y, Yan J, Wang K, Zhu Z. Comparing the protective effects of local and remote ischemic preconditioning against ischemia-reperfusion injury in hepatectomy: a systematic review and network meta-analysis. Transl Gastroenterol Hepatol 2024;9:13.