
Management of irritable bowel syndrome: a narrative review
Introduction
Irritable bowel syndrome (IBS) is a complex gastrointestinal (GI) disorder characterized by a wide array of symptoms, including abdominal discomfort, pain, bloating, and altered bowel habits. According to a 2020 nationwide cross-sectional study, the prevalence of IBS is estimated to be 6.1% in the United States, surpassing previous estimates (1). Beyond significantly impacting quality of life for affected individuals, IBS imposes a substantial healthcare burden, with patients incurring an estimated direct cost ranging from $742 to $7,547 annually (2).
The diagnosis of IBS relies on the Rome criteria, which have undergone periodic updates, with the latest version being Rome IV. IBS is diagnosed when an individual consistently experiences abdominal pain, averaging at least one day per week over the past three months, and associated with two or more of the following factors: (I) related to defecation, (II) associated with a change in the frequency of stool, and (IV) associated with a change in the form (appearance) of stool (3). These criteria must be met for the preceding three months, and the onset of symptoms should be at least six months before the diagnosis (3).
The pathophysiology of IBS remains unclear with present theories encompassing a range of factors including irregularities in motility, visceral sensation, gut-brain interaction, gut dysbiosis, and psychosocial distress (4,5). The multifactorial etiology and diverse clinical presentation of IBS have made its management challenging. However, as the understanding of IBS pathophysiology has evolved, so too has the therapeutic landscape, offering more approaches to alleviate symptoms and improve patients' well-being.
This review paper is dedicated to an in-depth exploration of the diverse therapeutic modalities available for IBS management. It will first encompass a review of the current American College of Gastroenterology (ACG) (6) and American Gastroenterological Association (AGA) (7,8) guidelines on pharmacologic therapies for IBS, while also highlighting clinical trials that have been published after the most recent guidelines. It will then examine literature on dietary modifications, probiotics, fecal microbiota transplant, behavioral therapy, and complementary and alternative medicine approaches to IBS.
The journey towards effective IBS management is multifaceted, hinging on a comprehensive understanding of the patient’s symptoms, lifestyle, and preferences. Despite the numerous therapeutic options for IBS patients, it may still be difficult to find effective treatment strategies since there is much to learn about this condition and objective markers for assessing treatment response are not yet standardized. By discussing the intricacies of IBS therapeutics as we understand them today, we hope to contribute to the improvement of patient care and the overall quality of life for individuals grappling with this complex condition. This article is presented in accordance with the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-96/rc).
Methods
This review utilized information from PubMed/MEDLINE using the key search term “irritable bowel syndrome” as well as the 2020 ACG and 2022 AGA society guidelines on IBS. The search was restricted to articles in the English language only and included peer-reviewed observational studies and randomized controlled trials (RCTs) in adult patients from April 22, 2020 to October 16, 2023. These specific dates were selected because the ACG and AGA guidelines included studies from inception to February 1, 2020 and April 21, 2020 respectively. For literature on therapies recommended in the clinical guidelines but lacking studies within the original search timeframe, the timeframe was broadened to encompass studies published from inception to October 16, 2023. The references of identified studies were examined to further identify relevant studies. Table 1 denotes the search strategy. We omitted articles that did not contain clinical study findings such as clinical trial protocols with no patient enrollment, articles emphasizing non-clinical endpoints, articles studying interventions that have no prior data/validation, articles not primarily focused on IBS, and articles exclusively involving children.
Table 1
| Items | Specification |
|---|---|
| Date of search | October 16, 2023 |
| Databases and other sources searched | PubMed/MEDLINE |
| Search terms used | Irritable bowel syndrome |
| Timeframe | April 22, 2020 to October 16, 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: peer-reviewed observational studies and RCTs in adult patients published in the English language Exclusion criteria: articles that did not contain clinical study findings such as clinical trial protocols with no patient enrollment, articles emphasizing non-clinical endpoints, articles studying interventions that have no prior data/validation, articles not primarily focused on IBS, and articles exclusively involving children |
| Selection process | The study selection was performed by the first author and reviewed by the senior author. References of articles were further reviewed to identify possible sources |
RCTs, randomized controlled trials.
Therapeutics modalities
Pharmacologic agents for IBS-constipation (IBS-C)
Fiber
Dietary fiber is an inexpensive and frequently utilized therapy for managing symptoms in patients with IBS-C. Fiber types can be categorized based on solubility, viscosity, and resistance to fermentation within the colon (6,9). Soluble fiber has a laxative effect by increasing stool moisture, whereas insoluble fiber adds bulk to stool but does not enhance viscosity or create a laxative effect (10). Furthermore, fibers that undergo fermentation in the colon lose may lead to gas production, potentially worsening symptoms of bloating and flatulence (6,10). Fermentable fibers are targeted in the elimination phase of dietary therapy for IBS, which will be discussed later in this review. Types of soluble fiber include psyllium, betaglucan and galacto-oligosaccharides (GOS) (10). Psyllium is often used for treatment in IBS since it is viscous and not fermentable. Betaglucan, found in oat bran and barley, is viscous and fermentable, and may lead to gas and flatus (10). Galacto-oligosaccharides (GOS), found in beans, is not viscous and does not have water holding capacity but is fermentable, and may also lead to gas and flatus (10). Common sources of insoluble fibers include wheat bran, cellulose found in whole grains and nuts, and lignin found in flax and rye (10). Given its minimal side effects, soluble non-fermentable fiber such as psyllium is considered a reasonable first-line therapy for patients with IBS-C (6,10).
Polyethylene glycol (PEG)
PEG is an accessible and cost-effective over-the-counter osmotic laxative. There is a discrepancy in recommendations regarding PEG, with the ACG advising against its use, while the AGA recommends its use (6,7). Although PEG effectively enhances bowel movements, the disparity in recommendations is rooted in the absence of substantial evidence demonstrating meaningful relief in abdominal pain or overall symptoms for individuals with IBS. It is evident that larger, high-quality studies are essential to comprehensively assess the effectiveness of PEG in patients with IBS-C, particularly when abdominal pain is a predominant symptom. Given its affordability and wide accessibility, it continues to be heavily recommended as a first-line treatment in clinical practice.
Lubiprostone
Lubiprostone is an intestinal secretagogue that activates type-2 chloride channels to accelerate peristalsis. It is recommended by both the ACG and AGA for improving global outcomes and abdominal pain of IBS-C and is tolerated moderately well with nausea and diarrhea being the most common side effects (6,7). Nausea can be mitigated by taking lubiprostone with food, however, diarrhea may lead to discontinuation. There are no recent trials further evaluating lubiprostone.
Linaclotide and plecanatide
Linaclotide and plecanatide are intestinal secretagogues that act on the guanylate cyclase-C receptor, increasing chloride secretion and inhibiting visceral sensory afferent nerves. Both the ACG and AGA endorse these medications for the treatment of IBS, considering them effective, safe, and well-tolerated (6,7,11). Diarrhea is a common side effect in both medications which may lead to discontinuation.
An advantage of linaclotide and plecanatide over lubiprostone is their potential to better alleviate abdominal pain. Although the precise mechanism of linaclotide inhibiting visceral sensory afferent nerves is not fully understood, it may be dependent on the site of linaclotide delivery in the intestine. A recent study by Chey et al. indicates that directing linaclotide delivery to the ileocecal junction and cecum using delayed-release formulations could separate linaclotide’s pain-relief effect from its secretory effects (12). The results of this phase 2b study warrant further investigation into delayed-release formulations of guanylate cyclase-C receptor agonists targeting the ileocecal junction and cecum as an innovative approach for addressing abdominal pain for other IBS subtypes.
Tegaserod
Tegaserod is a serotonin type-4 receptor partial agonist which initiates the peristaltic reflex and increases fluid in the GI tract. Initially, the FDA approved tegaserod for short-term treatment of IBS-C in women under 65 years of age. In 2007, it was withdrawn from the market due to a retrospective analysis of clinical trials revealing higher rates of cardiovascular ischemic events compared to placebo. Subsequently, extensive epidemiological studies failed to identify significant differences in cardiovascular events between patients taking tegaserod and those who did not (6,7). These studies indicated that cardiovascular events were more prevalent in patients with a history of, or risk factors for, cardiovascular ischemic events (6,7). As a result, tegaserod was reintroduced to the market in 2019, with a recommendation for use in women under 65 years of age without cardiovascular risk factors such as angina, myocardial infarction, transient ischemic attack, or stroke (13). Common side effects of tegaserod include diarrhea and headaches. There are no recent trials further evaluating tegaserod.
Tenapanor
Tenapanor inhibits intestinal sodium-proton exchanger 3, which leads to decreased intestinal sodium absorption and increased water secretion. A recent study assessing long-term efficacy and safety of tenapanor showed reduction in abdominal pain and global symptoms of IBS over 26 weeks (14). Similar to the other pharmacologic agents for IBS-C, the most common side effect was diarrhea. Tenapanor is recommended by the AGA for IBS-C, but not mentioned in the most recent ACG guidelines (6,7).
Pharmacologic agents for IBS-diarrhea (IBS-D)
Loperamide
Loperamide is a peripheral opioid receptor agonist that inhibits peristalsis and has antisecretory activity in the gut. There is a discrepancy in recommendations regarding loperamide, with the ACG advising against its use, while the AGA recommends its use in patients with IBS-D (6,8). The ACG does not recommend loperamide as first-line therapy because it has not been shown to improve global IBS symptoms (15). The AGA acknowledges this lack of data assessing the effectiveness of loperamide in alleviating abdominal symptoms but continues to recommend its use on the basis that it has been shown to reduce diarrhea (8).
Rifaximin
Rifaximin is a non-absorbed antibiotic that inhibits RNA synthesis and is recommended by the ACG and AGA (6,8). It is approved for treatment of IBS-D over a 2-week period, and also approved for re-treatment if patients experience recurrence of symptoms. Use of rifaximin leads to improvement in abdominal pain, bloating, stool consistency and urgency compared to placebo, and is generally well tolerated (16). Common side effects include nausea and increased incidence of infections such as clostridium difficile, upper respiratory infection, and nasopharyngitis (8).
Eluxadoline
Eluxadoline is a peripherally acting, mixed mu- and kappa-opioid receptor agonist, and delta-opioid receptor antagonist. It has demonstrated effectiveness in improving stool consistency, urgency, and frequency, and is recommended by the ACG and AGA (6,8). The most common side effects include constipation and nausea. Notably, it is crucial to exercise caution when considering Eluxadoline for certain patients. Eluxadoline is contraindicated in patients without a gallbladder, those who consume more than three alcoholic beverages per day, individuals with a history of alcohol abuse, or those with a history of pancreatitis, since it increases the risk of pancreatitis in these specific patient groups.
Alosetron
Alosetron is a selective 5-HT3 antagonist primarily reserved for the management of severe IBS-D. Initially, the FDA granted approval for its use in the treatment of IBS-D in women in 2000. However, the drug was voluntarily withdrawn from the market due to serious adverse events, including ischemic colitis and severe complications related to constipation. In 2002, the FDA authorized the reintroduction of alosetron, but restricted its use to women under a risk management program. It has demonstrated efficacy in improving global symptoms and IBS pain and is recommended by the ACG and AGA for severe IBS-D when other interventions have failed (6,8).
The positive outcomes observed with alosetron in managing IBS-D symptoms have spurred investigations into other medications within the same class, including the widely used drug, ondansetron. Recent studies by Plasse et al. and Gunn et al. indicate that ondansetron is efficacious and safe in treating both men and women with IBS-D, resulting in enhanced stool consistency, although it does not significantly alleviate abdominal pain (17,18). It is not currently recommended by the ACG or AGA and further studies are needed to validate these findings.
Pharmacologic agents for global symptoms
Antispasmodics
Antispasmodics continue to be a commonly employed therapy for IBS, primarily working by relaxing intestinal smooth muscle to reduce muscle contractions. However, it is important to note the significant variability that exists among clinical trials investigating antispasmodics, leading to divergent recommendations from the ACG and AGA. The ACG recommends against the use of antispasmodics to treat global symptoms due to low quality and varying evidence (6). Conversely, the AGA suggests conditional use of antispasmodics, though with caution in IBS-C due to potential anticholinergic effects (8). To gain a clearer understanding of the efficacy of antispasmodics in IBS treatment, there is a pressing need for new high-quality trials.
Selective serotonin reuptake inhibitors (SSRI)
SSRIs are often considered for IBS treatment because of their centrally mediated influence on the gut-brain axis. Nevertheless, in several trials, they have failed to demonstrate a significant impact on visceral sensation and have not proven effective in alleviating global symptoms or abdominal pain in IBS patients (8). They are not recommended by the AGA and are not discussed in the most recent ACG guidelines (6,8).
Tricyclic antidepressants (TCA)
TCAs are central and peripheral neuromodulators that affect motility, secretion, and sensation. They have been shown to be effective in improving overall IBS symptoms and abdominal pain, and their use is supported by both the ACG and AGA (6,8). To maximize the benefits of TCAs in managing IBS symptoms, it is advisable to initiate treatment at low doses and gradually increase as necessary over several weeks. Common side effects include drowsiness and dry mouth, which can be managed by starting at a low dose and gradually adjusting as needed until the lowest therapeutic dose is reached for symptom control. Utilizing secondary amines such as nortriptyline or desipramine is often preferable over tertiary amines like amitriptyline to mitigate side effects.
Dietary modification
Dietary therapy is a cornerstone in treating patients with IBS. The most validated dietary therapy is a limited trial of a diet low in dietary fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) (6,19-22). This complex diet consists of three phases and is most effective when overseen by a trained GI dietitian to prevent overly restrictive diets. The first phase of the diet involves substituting high FODMAPs foods with low FODMAPs alternatives, while the second phase entails a gradual reintroduction of foods with a careful assessment of symptoms. The third phase customizes the diet to avoid trigger foods. Recent research indicates that individuals with more severe symptoms may experience more favorable outcomes with the low FODMAPs diet (19,21). Notably, there have been significant methodological challenges in evaluating this diet since most studies have been small and single-blinded, therefore yielding low quality of evidence.
A gluten free diet is another common dietary approach for IBS treatment. While this approach has shown symptom improvement in observational studies, there have been mixed results in RCTs (23).
Another approach that has been explored is a reduced starch and sucrose-reduced diet. However, there is insufficient data to confirm its clinical efficacy (24,25). Further research and direct comparisons with the low FODMAPs diet are necessary before it can be implemented in clinical practice.
Probiotics
Probiotics have been the subject of increasing research interest in the treatment of IBS. However, these studies have exhibited substantial variability in terms of the specific microbial strains utilized, dosages administered, and the methodologies employed for research. Multiple studies have concluded that multi-strain probiotics have some effect on IBS symptoms, but there is a general lack of knowledge of which strain, or combinations, are most promising (26,27). Consequently, the AGA refrains from making definitive recommendations regarding the use of probiotics for IBS treatment, and the ACG advises against the use of probiotics for alleviating global IBS symptoms (6,28). Both organizations advocate for the initiation of more rigorous trials aligned with endpoints established by the U.S. Food and Drug Administration (FDA) to provide clearer insights into the effectiveness of probiotics in IBS treatment. At present, the most substantial body of data supports the potential benefits of Lactobacillus and Bifidobacterium strains in ameliorating IBS symptoms (26,29-35).
Fecal microbiota transplant
Given the association between IBS and microbial dysbiosis, fecal microbiota transplantation (FMT) has been hypothesized to have a positive effect in the treatment of IBS. Over the past few years, numerous small-scale studies have been conducted, yielding varying outcomes. Several of these studies indicate that FMT can be an effective treatment for IBS patients for a period of up to three months, emphasizing the importance of selecting a donor with a normal dysbiosis index (36,37). However, long-term efficacy seems to be limited, with only transient symptom relief initially, followed by a return of symptoms within 3- to 12-month (38,39). Conversely, some studies have failed to demonstrate any clinically significant improvements in abdominal pain, stool frequency, or stool consistency (40). Given these conflicting results, it is evident that additional research is necessary to comprehensively evaluate the effectiveness of FMT in managing IBS.
Behavioral therapies
Some patients can experience significant benefits from brain-gut psychotherapies provided by mental health professionals who specialize in GI psychology. While cognitive-behavioral therapy (CBT) has been widely studied and demonstrated effectiveness in managing IBS (41,42), it is often hampered by limited accessibility. Recent research by Kikuchi et al. indicates that group CBT represents a promising and more accessible treatment option than individual therapy (43). A recent proof-of-concept study by Murray et al. suggests that CBT delivered by nurse practitioners may also be effective, and there is an upcoming RCT aiming at further investigating this approach (44). Additionally, preliminary findings by Owusu et al. suggest the potential efficacy of web-based CBT, warranting a larger trial for a more comprehensive evaluation (45).
The other behavioral therapy that has been increasingly studied and recommended by the ACG is gut directed hypnosis (6). This technique encompasses patient education about the digestive system, the induction of a hypnotic state, and the utilization of personalized imagery to help normalize GI functioning. Recent findings from a RCT by Hasan et al. have demonstrated that as few as six sessions of gut-directed hypnotherapy have led to notable improvements in IBS symptoms, non-colonic symptoms, anxiety, depression, and overall quality of life. Furthermore, these results indicate that the effectiveness of the six-session regimen is non-inferior to the previously established 12-session protocol (46).
Complementary and alternative medicine (CAM)
The most prevalent CAM therapies for patients with IBS include herbs and supplements, mind-body therapies, and manipulative-type therapies (47). Patients turn to CAM for various reasons, including a perception of safety and naturalness, dissatisfaction with conventional treatments, and the potential for placebo effects (47). Nevertheless, the scientific research on the safety and effectiveness of CAM therapies for IBS remains limited, and as a result, evidence-based guidelines generally do not widely endorse their use. However, certain CAM therapies including the dietary modifications and behavior health techniques discussed earlier, as well as peppermint oil and exercise, are gaining acceptance among gastroenterologists as more evidence emerges.
Peppermint oil is commonly employed to address IBS symptoms and is recommended by the ACG guidelines (6); however, results have been inconsistent when compared to placebo particularly in more recent studies by Cash et al. and Nee et al. (48,49). Furthermore, it is important to note that peppermint relaxes the lower esophageal sphincter and can worsen reflux in patients with hiatal hernia and gastroesophageal reflux disease. It should therefore be avoided in patients with reflux. However, for patients without reflux, peppermint oil, which is an affordable treatment, may be justified by the potential for even modest symptom relief (50).
Some studies suggest that practices like yoga and locomotor activity, such as increased walking, can have a positive impact on IBS symptoms (51-53). More research is needed to evaluate the efficacy of other CAM therapies, including acupuncture, chiropractic care, massage, herbal supplements, etc.
Integrated care
It is imperative to adopt an integrative model that utilizes a multimodal therapeutic approach to provide optimal care for patients with IBS (54,55). Traditionally, care for IBS patients has predominantly been pharmacotherapy centered, yet this approach has yielded symptom improvement for only a minority of patients (56). In contrast, integrated multidisciplinary care models involving GI dieticians, GI psychologists, and CAM therapies alongside gastroenterologist management have shown better results in symptom management, psychological well-being, quality of life, and overall cost of care compared to gastroenterologist-only care (54,56). In fact, it has been suggested that it is not possible to evaluate pharmaceutical or dietary interventions in IBS without considering mental disorders since any improvement in GI symptoms may influence mood (57). Overall, a comprehensive approach offers the greatest likelihood of success in managing patients with IBS (Figure 1).
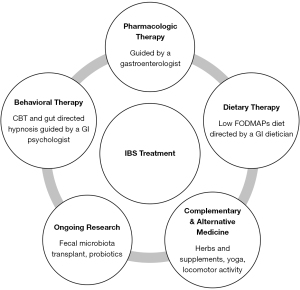
Limitations
While this review provides a comprehensive overview of therapeutic modalities for managing IBS, there are several limitations that should be considered. The review mentions the difficulty in finding effective treatment strategies for IBS due to the lack of standardized objective markers for assessing treatment response. This limitation highlights a broader challenge in the field of IBS and emphasizes the need for more robust outcome measures in clinical trials. Additionally, the search strategy is based on a single key term (“irritable bowel syndrome”) and the guidelines from two gastroenterological societies. This approach may result in the omission of studies that use different terminology or focus on IBS from diverse perspectives. Finally, this review excluded non-clinical studies, proof-of-concept articles with no patient enrollment, and studies on interventions that do not have prior data/validation. While this criterion aims to ensure high-quality evidence, it may lead to the exclusion of valuable information, especially in areas with limited research.
Conclusions
The management of IBS is a multifaceted endeavor, driven by an evolving understanding of the condition’s complex pathophysiology and the development of a range of therapeutic modalities. This review has shed light on the diverse approaches that are currently available for IBS management, including pharmacologic agents, dietary modifications, probiotics, FMT, behavioral therapies, and CAM therapies. It is evident that IBS management has moved beyond a one-size-fits-all approach and is increasingly focused on tailoring treatment to individual patient needs. Despite the many treatment options that are available, there is a great need for further research to gain a better understanding of the pathophysiology of IBS. As the field of IBS management continues to evolve, it is essential for healthcare professionals to remain informed and open to the array of therapeutic options to ultimately offer patients the most effective and personalized care.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-96/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-96/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-96/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Almario CV, Sharabi E, Chey WD, et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology 2023;165:1475-87. [Crossref] [PubMed]
- Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 2014;40:1023-34. [Crossref] [PubMed]
- Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016; Epub ahead of print. [Crossref] [PubMed]
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949-58. [Crossref] [PubMed]
- Shrestha B, Patel D, Shah H, et al. The Role of Gut-Microbiota in the Pathophysiology and Therapy of Irritable Bowel Syndrome: A Systematic Review. Cureus 2022;14:e28064. [Crossref] [PubMed]
- Lacy BE, Pimentel M, Brenner DM, et al. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol 2021;116:17-44. [Crossref] [PubMed]
- Chang L, Sultan S, Lembo A, et al. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology 2022;163:118-36. [Crossref] [PubMed]
- Lembo A, Sultan S, Chang L, et al. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2022;163:137-51. [Crossref] [PubMed]
- El-Salhy M, Ystad SO, Mazzawi T, et al. Dietary fiber in irritable bowel syndrome Int J Mol Med 2017;40:607-13. (Review). [Crossref] [PubMed]
- Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol 2013;108:718-27. [Crossref] [PubMed]
- Menees SB, Franklin H, Chey WD. Evaluation of Plecanatide for the Treatment of Chronic Idiopathic Constipation and Irritable Bowel Syndrome With Constipation in Patients 65 Years or Older. Clin Ther 2020;42:1406-1414.e4. [Crossref] [PubMed]
- Chey WD, Sayuk GS, Bartolini W, et al. Randomized Trial of 2 Delayed-Release Formulations of Linaclotide in Patients With Irritable Bowel Syndrome With Constipation. Am J Gastroenterol 2021;116:354-61. [Crossref] [PubMed]
- Shah ED, Lacy BE, Chey WD, et al. Tegaserod for Irritable Bowel Syndrome With Constipation in Women Younger Than 65 Years Without Cardiovascular Disease: Pooled Analyses of 4 Controlled Trials. Am J Gastroenterol 2021;116:1601-11. [Crossref] [PubMed]
- Chey WD, Lembo AJ, Yang Y, et al. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome With Constipation: A 26-Week, Placebo-Controlled Phase 3 Trial (T3MPO-2). Am J Gastroenterol 2021;116:1294-303. [Crossref] [PubMed]
- Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am J Gastroenterol 2018;113:1-18. [Crossref] [PubMed]
- Lacy BE, Chang L, Rao SSC, et al. Rifaximin Treatment for Individual and Multiple Symptoms of Irritable Bowel Syndrome With Diarrhea: An Analysis Using New End Points. Clin Ther 2023;45:198-209. [Crossref] [PubMed]
- Plasse TF, Barton G, Davidson E, et al. Bimodal Release Ondansetron Improves Stool Consistency and Symptomatology in Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Trial. Am J Gastroenterol 2020;115:1466-73. [Crossref] [PubMed]
- Gunn D, Topan R, Barnard L, et al. Randomised, placebo-controlled trial and meta-analysis show benefit of ondansetron for irritable bowel syndrome with diarrhoea: The TRITON trial. Aliment Pharmacol Ther 2023;57:1258-71. [Crossref] [PubMed]
- Zhang Y, Feng L, Wang X, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: a parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am J Clin Nutr 2021;113:1531-45. [Crossref] [PubMed]
- Nordin E, Brunius C, Landberg R, Hellström PM. Fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs), but not gluten, elicit modest symptoms of irritable bowel syndrome: a double-blind, placebo-controlled, randomized three-way crossover trial. Am J Clin Nutr 2022;115:344-52. [Crossref] [PubMed]
- Algera JP, Demir D, Törnblom H, et al. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: A randomized crossover trial. Clin Nutr 2022;41:2792-800. [Crossref] [PubMed]
- Goyal O, Batta S, Nohria S, et al. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: A prospective, randomized trial. J Gastroenterol Hepatol 2021;36:2107-15. [Crossref] [PubMed]
- Chey WD, Hashash JG, Manning L, et al. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology 2022;162:1737-1745.e5. [Crossref] [PubMed]
- Nilholm C, Larsson E, Sonestedt E, et al. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021;13:416. [Crossref] [PubMed]
- Saidi K, Nilholm C, Roth B, et al. A carbohydrate-restricted diet for patients with irritable bowel syndrome lowers serum C-peptide, insulin, and leptin without any correlation with symptom reduction. Nutr Res 2021;86:23-36. [Crossref] [PubMed]
- Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, et al. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 2021;13:756. [Crossref] [PubMed]
- McFarland LV, Karakan T, Karatas A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021;41:101154. [Crossref] [PubMed]
- Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020;159:697-705. [Crossref] [PubMed]
- Andresen V, Gschossmann J, Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol Hepatol 2020;5:658-66. [Crossref] [PubMed]
- Oh JH, Jang YS, Kang D, et al. Efficacy of a Synbiotic Containing Lactobacillus paracasei DKGF1 and Opuntia humifusa in Elderly Patients with Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Gut Liver 2023;17:100-7. [Crossref] [PubMed]
- Barraza-Ortiz DA, Pérez-López N, Medina-López VM, et al. Combination of a Probiotic and an Antispasmodic Increases Quality of Life and Reduces Symptoms in Patients with Irritable Bowel Syndrome: A Pilot Study. Dig Dis 2021;39:294-300. [Crossref] [PubMed]
- Sabaté JM, Iglicki F. Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome. World J Gastroenterol 2022;28:732-44. [Crossref] [PubMed]
- Sadrin S, Sennoune S, Gout B, et al. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig Liver Dis 2020;52:534-40. [Crossref] [PubMed]
- Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, et al. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients 2020;12:1999. [Crossref] [PubMed]
- Yang B, Yue Y, Chen Y, et al. Lactobacillus plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front Immunol 2021;12:746585. [Crossref] [PubMed]
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020;69:859-67. [Crossref] [PubMed]
- Guo Q, Lin H, Chen P, et al. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered 2021;12:11885-97. [Crossref] [PubMed]
- Holvoet T, Joossens M, Vázquez-Castellanos JF, et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021;160:145-157.e8. [Crossref] [PubMed]
- Lahtinen P, Jalanka J, Hartikainen A, et al. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther 2020;51:1321-31. [Crossref] [PubMed]
- Madsen AMA, Halkjær SI, Christensen AH, et al. The effect of faecal microbiota transplantation on abdominal pain, stool frequency, and stool form in patients with moderate-to-severe irritable bowel syndrome: results from a randomised, double-blind, placebo-controlled study. Scand J Gastroenterol 2021;56:761-9. [Crossref] [PubMed]
- Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag 2017;10:231-7. [Crossref] [PubMed]
- Laird KT, Tanner-Smith EE, Russell AC, et al. Short-term and Long-term Efficacy of Psychological Therapies for Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:937-947.e4. [Crossref] [PubMed]
- Kikuchi S, Oe Y, Ito Y, et al. Group Cognitive-Behavioral Therapy With Interoceptive Exposure for Drug-Refractory Irritable Bowel Syndrome: A Randomized Controlled Trial. Am J Gastroenterol 2022;117:668-77. [Crossref] [PubMed]
- Burton Murray H, Weeks I, Thurler A, et al. Nurse practitioner-delivered cognitive-behavioral treatment as a novel implementation route for irritable bowel syndrome: A proof of concept. Neurogastroenterol Motil 2023;35:e14526. [Crossref] [PubMed]
- Owusu JT, Sibelli A, Moss-Morris R, et al. A pilot feasibility study of an unguided, internet-delivered cognitive behavioral therapy program for irritable bowel syndrome. Neurogastroenterol Motil 2021;33:e14108. [Crossref] [PubMed]
- Hasan SS, Whorwell PJ, Miller V, et al. Six vs 12 Sessions of Gut-focused Hypnotherapy for Irritable Bowel Syndrome: A Randomized Trial. Gastroenterology 2021;160:2605-2607.e3. [Crossref] [PubMed]
- Nguyen L. Complementary and Alternative Medicine for the Management of Irritable Bowel Syndrome. Gastroenterol Hepatol (N Y) 2018;14:536-8. [PubMed]
- Cash B. Novel Peppermint Oil Formulation for Dietary Management of Irritable Bowel Syndrome. Gastroenterol Hepatol (N Y) 2015;11:631-3. [PubMed]
- Nee J, Ballou S, Kelley JM, et al. Peppermint Oil Treatment for Irritable Bowel Syndrome: A Randomized Placebo-Controlled Trial. Am J Gastroenterol 2021;116:2279-85. [Crossref] [PubMed]
- Weerts ZZRM, Essers BAB, Jonkers DMAE, et al. A trial-based economic evaluation of peppermint oil for the treatment of irritable bowel syndrome. United European Gastroenterol J 2021;9:997-1006. [Crossref] [PubMed]
- Shahabi L, Naliboff BD, Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychol Health Med 2016;21:176-88. [Crossref] [PubMed]
- D'Silva A, Marshall DA, Vallance JK, et al. Meditation and Yoga for Irritable Bowel Syndrome: A Randomized Clinical Trial. Am J Gastroenterol 2023;118:329-37. [Crossref] [PubMed]
- Hamaguchi T, Tayama J, Suzuki M, et al. The effects of locomotor activity on gastrointestinal symptoms of irritable bowel syndrome among younger people: An observational study. PLoS One 2020;15:e0234089. [Crossref] [PubMed]
- Berry SK, Chey WD. Integrated Care for Irritable Bowel Syndrome: The Future Is Now. Gastroenterol Clin North Am 2021;50:713-20. [Crossref] [PubMed]
- Chey WD, Keefer L, Whelan K, et al. Behavioral and Diet Therapies in Integrated Care for Patients With Irritable Bowel Syndrome. Gastroenterology 2021;160:47-62. [Crossref] [PubMed]
- Basnayake C, Kamm MA, Stanley A, et al. Standard gastroenterologist versus multidisciplinary treatment for functional gastrointestinal disorders (MANTRA): an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:890-9. [Crossref] [PubMed]
- Staudacher HM, Mikocka-Walus A, Ford AC. Common mental disorders in irritable bowel syndrome: pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol Hepatol 2021;6:401-10. [Crossref] [PubMed]
Cite this article as: Tetali B, Suresh S. Management of irritable bowel syndrome: a narrative review. Transl Gastroenterol Hepatol 2024;9:26.


