Laparoscopic versus robotic cholecystectomy: a systematic review with meta-analysis to differentiate between postoperative outcomes and cost-effectiveness
Highlight box
Key findings
• Robotic cholecystectomy (RC) failed to prove any clinical advantage over laparoscopic cholecystectomy (LC) for postoperative outcomes including longer duration of operation; moreover LC was more cost effective.
What is known and what is new?
• The cost of robotic surgeries has always been a topic of concerns for surgeons and RC is no different.
• The single-site RC can be a viable replacement for conventional LC, but at present this is an expensive approach.
What is the implication, and what should change now?
• This will help surgeons in making a choice between RC or LC in a patient.
Introduction
One of the most common causes of surgical admissions is due to cholecystitis in any hospital. Acute cholecystitis is defined as an inflammation of the gall bladder usually due to obstruction of the biliary tree or incomplete emptying of the gall bladder (1). The annual incidence of acute cholecystitis in the USA alone is 200,000/year (2). The global prevalence of gallstones is around 10–15% and 10–15% of these patients primarily present with acute cholecystitis (3). There is also a substantial overall mortality (0.9%) and morbidity (17.8%) linked to cholecystectomy (4).
Acute cholecystitis is that stage in cholecystitis which can last up to 10 days, usually followed by sub-acute cholecystitis and chronic cholecystitis. Primary surgical treatment for acute cholecystitis can be subcategorized as an emergency or hot cholecystectomy or an elective or delayed cholecystectomy (5). Laparoscopic cholecystectomy (LC) is a minimally invasive procedure, involving the removal of diseased gall bladder (6). This technique has replaced open cholecystectomy as a part of routine surgical practice since the 1990s. The laparoscopic approach is superior to the open approach and has shown significant improvement in terms of morbidity, mortality and hospital stay (7). Further advancement in surgical techniques led to the use of the robotic approach in surgical practice, with the earliest use documented in the early 2000s (8). Recently a novel approach with single-site robotic cholecystectomy (SSRC) based on the da Vinci platform has been developed (9-11). This could be a viable successor for LC. Robotic surgery is much more minimally invasive when compared with laparoscopic surgery due to tissue trauma and inflammation being less.
The cost of robotic surgeries has always been a topic of concern for surgeons and robotic cholecystectomy (RC) is no different. Breitenstein et al. concluded that the cost of RC is high and is not justifiable with the added benefits (12). In comparison, Salman et al. demonstrated that when comparing the overall cost of RC, which includes the length of stay, it is as cost-effective as the non-robotic approach (13).
This systematic review will compare the cost of RC with LC and then we will also be comparing the post-operative outcomes of RC with LC. This will help surgeons in making a choice between RC or LC in a patient. We present this article in accordance with the PRISMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-56/rc).
Methods
Data sources and literature search technique
The systematic review has been registered in Research Registry (https://www.researchregistry.com/) with the registration number Reviewregistry1720. Research exploration was done in a carefully planned and systematic fashion from electronic databases like EMBASE, MEDLINE, Cochrane Library and PubMed. MeSH search terms were used to identify the relevant articles. Boolean operators (AND, OR, NOT) were used for refining the search and narrowing down the results. The titles were carefully screened for the study selection process. Additionally, careful screening of references was done from the selected articles which were further analysed to identify any additional relevant trials that could be included.
Trial selection
The inclusion criteria were the comparative trials comparing RC with the conventional LC. For cost analysis, trial selection was done primarily with a cost comparison of SSRC with LC, all the available trials were included.
Data collection and management
Reported data was collected from the included studies by independent researchers on a standard data extraction sheet. The collected dataset was compared among the involved researchers and was found to be in satisfactory inter-researcher agreement. The extracted data consisted of a list of the authors, title of the published study, journal of publication, country/region and year of the publication, testing sample size, and the number of patients in each group of LC and RC cohort. Following data extraction, the researchers went through discussing their individual results and a consensus of mutual agreement was reached in case of discrepancies.
Endpoint
Postoperative complications or absence of postoperative complications was considered as the principal endpoint in the meta-analysis for comparison of outcomes while cost incurred during the operation was the end point when comparing the cost.
Quality of analysis
The methodological quality of the included trials was initially assessed using published guidelines of Jadad et al., Chalmers et al. and Rangel et al. (14-16). A comprehensive table for the assessment of quality among the included trials is given in Tables 1,2.
Table 1
| Study | Randomization technique | Concealment | Blinding | Intention to treat analysis | Ethical approval | Registration number | Power calculation |
|---|---|---|---|---|---|---|---|
| Grochola, 2019 (17) | Electronic | Not reported | Single | Reported | Declared | NCT02485392 | Reported |
| Kudsi, 2017 (18) | Manual | Sealed envelope | Absent | Not reported | Declared | NCT01932216 | Reported |
| Pietrabissa, 2016 (19) | Electronic | Sealed envelope | Double | Reported | Declared | ACTRN12614000119695 | Reported |
Table 2
| Quality variables | Hagen, 2018 (20) | Li, 2017 (21) | Su, 2017 (22) |
|---|---|---|---|
| Inclusion criteria | 1 | 1 | 1 |
| Exclusion criteria | 0 | 1 | 1 |
| Demographics comparable? | 1 | 1 | 1 |
| Can the number of participating centres be determined | 1 | 1 | 1 |
| Can the number of surgeons who participated be determined | 1 | 1 | 1 |
| Can the reader determine where the authors are on the learning curve for the reported procedure | 1 | 1 | 1 |
| Are diagnostic criteria clearly stated for clinical outcomes if required | 1 | 1 | 1 |
| Is the surgical technique adequately described | 1 | 1 | 1 |
| Is there any way that they have tried to standardize the operative technique | 1 | 1 | 1 |
| Is there any way that they have tried to standardize perioperative care | 1 | 1 | 1 |
| Is the age and range given for patients in the laparoscopic group | 1 | 1 | 1 |
| Do authors address whether there is any missing data | 0 | 1 | 1 |
| Is the age and range given for patients in the robotic group | 1 | 1 | 1 |
| Were patients in each group treated along similar timelines | 1 | 1 | 1 |
| Did all the patients asked to enter the study take part | 1 | 1 | 0 |
| Dropout rates stated | 0 | 0 | 0 |
| Outcomes clearly defined? | 1 | 1 | 1 |
| Blind assessors | 0 | 0 | 0 |
| Standardised assessment tools? | 1 | 0 | 1 |
| Analysis by intention to treat | 0 | 0 | 0 |
| Score | 15 | 16 | 16 |
Statistical analysis
Statistical analysis was performed using RevMan 5.4 (Review Manager 5.4, The Nordic Cochrane Centre, Copenhagen, Denmark) (23). For comparing the continuous variables, the standardised mean difference (cost comparison) or mean difference (operative time) and for comparing dichotomous variables, odds ratio (bile leakage, conversion rate and post operative complications) with a confidence interval (CI) of 95% were used under the random-effects model analysis (24,25). A forest plot was used to calculate the heterogeneity by computing the χ2 test, with significance set at P<0.05 as well as using the I2 test with a maximum value of 30% identifying low heterogeneity (26). For the calculation of the standardised mean difference, the inverse-variance method was used under the random effect model (27) analysis. If no event happened in the treatment and the control group, 0.5 was added to the cell frequency in the sensitivity analysis, according to the method recommended by Deeks et al. (28). In the event of unavailable standard deviation, the guidelines provided by the Cochrane Collaboration were used for the risk of bias calculation (24). The criteria used as per the guidelines assumed that variance was the same in both the groups since this might not be true in all the cases, and if this is the case then variance was estimated either from range or P value. The estimate of the difference between both techniques was pooled, depending upon the effect weights in results determined by each trial estimate variance. Graphical displays of the results were represented by using a forest plot. The square around the estimate represented the accuracy of estimation (sample size) while the horizontal line represented 95% CI.
Results
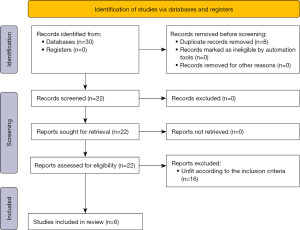
The initial database search generated 30 studies. After assessment of the studies for duplication, study type and inclusion criteria, 24 were excluded. Three RCTs and 3 comparative trials were included for comparing postoperative outcomes and 1 RCT and 3 comparative studies were included for cost comparison in the final meta-analysis (Figure 1).
Characteristics and demographics of included studies
Three RCTs and three comparative trials on 1,013 patients were included to study for postoperative outcomes and cost comparison in the meta-analysis and principles advised by the Cochrane Collaboration were used in this analysis. The PRISMA flow chart which was used in the selection of trial is given in Figure 1. The trials included were conducted in Switzerland (17,20), Taiwan (21,22), USA (18), and Italy (19). Primary demographic characteristics of the studies included are specified in Table 3 and the protocol used in the treatment for each study is given in Table 4.
Table 3
| Study | Surgery | Country/region | Type | Age (years) | Gender (men), percentage | Sample size |
|---|---|---|---|---|---|---|
| Grochola, 2019 (17) | Laparoscopic | Switzerland | RCT | 51.5±11.5* | 46.67 | 30 |
| Robotic | 52.4±13.0* | 33.33 | 30 | |||
| Hagen, 2018 (20) | Laparoscopic | Switzerland | Retrospective study | 47±14 | 27.27 | 99 |
| Robotic | 47.4±12.6 | 27.27 | 99 | |||
| Li, 2017 (21) | Laparoscopic | Taiwan | Retrospective study | 51.44±14.11* | 43.9 | 367 |
| Robotic | 59.69±13.35* | 48.3 | 78 | |||
| Su, 2017 (22) | Laparoscopic | Taiwan | Retrospective study | 50.94±13.79 | 36.51 | 63 |
| Robotic | 53.64±15.54 | 35.29 | 51 | |||
| Kudsi, 2017 (18) | Laparoscopic | USA | RCT | 46.5±17.3 | 7 | 53 |
| Robotic | 46.8±15.5 | 21 | 83 | |||
| Pietrabissa, 2016 (19) | Laparoscopic | Italy | RCT | NR | NR | 30 |
| Robotic | NR | NR | 30 |
*, Bing AI was used to calculate mean ± SD from the available data. NR, not reported; RCT, randomised control trial; AI, artificial intelligence; SD, standard deviation.
Table 4
| Study | Laparoscopic technique | Robotic technique |
|---|---|---|
| Grochola, 2019 (17) | SILC—12 mm multiple lumen access device | SSRC—multiple lumen single site port (one 8.5-mm port, two 4-mm ports and one 5-mm port) |
| Diagnosis—cholecystolithiasis (96.67 %) and GB polyps (3.3%) | Diagnosis—cholecystolithiasis (96.67%) and GB polyps (3.3%) | |
| Hagen, 2018 (20) | MPLC—one 12-mm periumbilical port (open technique), two 5-mm ports: right flank and the sub-xiphoidal area, one 12-mm port: left upper hemi-abdomen, cholangiogram used | SSRC—detail not available; cholangiogram not used |
| Diagnosis—cholecystolithiasis and acute cholecystitis | Diagnosis—cholecystolithiasis and acute cholecystitis | |
| Li, 2017 (21) | MPLC—detail not available | SSRC—detail not available |
| Diagnosis—GB stone (64%), GB stone with AC (24.8%) and GB polyp (11.2%) | Diagnosis—GB stone (68%), GB stone with AC (21.8%) and GB polyp (10.3%) | |
| Su, 2017 (22) | SILC—Lagiport system, including two 5-mm seals and two 12-mm seals | SSRC—da Vinci single-site multichannel port |
| Diagnosis—GB stones and GB polyps | Diagnosis—GB stones and GB polyps | |
| Kudsi, 2017 (18) | MPLC—one umbilical port and three 5-mm ports (one in xiphoid and two in right upper quadrant) | SSRC—da Vinci single-site instrumentation and accessories |
| Diagnosis—chronic cholecystitis (26.4%), cholelithiasis (62.3%), others (13.2%) | Diagnosis—chronic cholecystitis (30.1%), cholelithiasis (53%), others (16.8%) | |
| Pietrabissa, 2016 (19) | MPLC—one 12-mm umbilical port, three 5-mm ports at left subcoastal, right subcoastal and right iliac fossa | SSRC—daVinci single site surgical system |
| Diagnosis—GB stone and GB polyp | Diagnosis—GB stone and GB polyp |
SILC, single incision laparoscopic cholecystectomy; SSRC, single site robotic cholecystectomy; GB, gall bladder; MPLC, multiport laparoscopic cholecystectomy; AC, acute cholecystitis.
Methodological quality of included studies
The methodological quality of RCTs included and comparative trials are concise in Tables 1,2. The Mantel Haenszel model for random effects was used to calculate the strength and weakness to any outlier from the included trials. The randomization in RCTs was done electronically (17,19), and the concealment was done using sealed envelopes (18,19). Single blinding (17), double blinding (19) and no blinding (18) were adopted in the included randomized trials. The quality of the included three retrospective comparison trials was analysed by using the principles of the Scottish Intercollegiate Guidelines Network and Dudgeon et al. (16), and all three included studies were found to have good quality (20-22).
Outcome of the primary variable
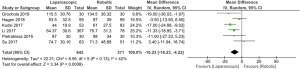
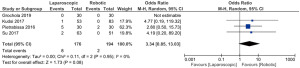
In postoperative outcome comparison, random effect model analysis was used, the duration of operation (mean difference: −10.23, 95% CI: −16.23 to −4.22, Z=3.34, P=0.0008) was shorter in the LC cohort. Moderate heterogeneity was seen (Tau2=22.21; Chi2=8.56, df=5, P=0.13, I2=42%) between included RCTs (Figure 2). For the complication of bile leak (odds ratio: 3.34, 95% CI: 0.85 to 13.03, Z=1.73, P=0.08) without heterogeneity (Tau2=0.00, Chi2=0.11, df=2, P=0.95, I2=0%) (Figure 3) and postoperative complications (odds ratio: 1.49, 95% CI: 0.50 to 4.46, Z=0.72, P=0.47) with moderate heterogeneity (Tau2=0.82; Chi2=8.96, df=4, P=0.06, I2=55%) (Figure 4), both were statistically similar between RC and LC groups. When cost comparison random effect model was used again, LC was associated with the reduced cost (standardised mean difference: −7.42, 95% CI: −13.10 to −1.74, Z=2.56, P=0.01) compared to RC. However, there was noteworthy heterogeneity (Tau2=24.85, Chi2=629.70, df=3, P<0.00001, I2=100%) (Figure 5) among the studies included.


Discussion
Key findings
Three RCTs and three comparative trials on 1,013 (642 patients in LC group and 371 patients in RC group) patients were used in post operative outcomes and four comparative studies (1 RCT and 3 retrospective studies) on 817 patients undergoing RC versus LC were found suitable for cost comparison. RC failed to prove any clinical advantage over LC for postoperative outcomes including longer duration of operation; moreover, the LC seems to be more cost effective compared to RC in terms of procedural cost. These findings are insufficient to draw a stronger conclusion due to the paucity of RCTs and statistically significant heterogeneity among included studies; therefore, a major multicentre RCT is required to strengthen and validate the existing evidence.
Comparison with existing literature
Sun et al. did a meta-analysis comparing post operative outcome between SSRC and multiport laparoscopic cholecystectomy (MPLC) (29). It concluded that SSRC is having higher risk of incisional hernia. Meta-analysis done by Han et al., and Huang et al., also conclude that RC has similar perioperative outcomes (30,31). Hoffman et al. have published a major comparative trial concluding that patient with robot-assisted cholecystectomy in New York State had a higher complication rate (32). We did a thorough literature review and there is no reported systematic review in comparing the cost effectiveness of SSRC vs. LC.
Strength and limitations
Two of the trials used (17,22) were in between single incision LC and SSRC, while rest (18-21) were between MPLC and SSRC. One of the RCTs used (18) had manual randomization that had no blinding and no reported intention-to-treat analysis was reported in this trial. Also, there was no reported blinding in one of the RCT (17). The aim of the systematic review was to provide a concise review comparing the cost as well as perioperative complication of this approach, and therefore some studies are comparing SSRC with MPLC and some with SILC.
The heterogeneity among the included trials was not there, and the RCTs used in the systematic review despite their shortcomings were of good strength.
Implications
The SSRC can be a viable replacement for conventional LC, but at present this is an expensive approach. Also, there is no added benefits of SSRC over MPLC as shown in this systematic review and by Sun et al. (29), moreover there is a higher risk of incisional hernia.
Conclusions
RC failed to prove any clinical advantage over LC for postoperative outcomes including longer duration of operation; moreover, LC was more cost effective. To further confirm the findings of the systematic review, a major multicentre RCT is needed. Also, it might be possible to perform the comparison of cost on a greater cohort of the patient and this might be imperative to confirm the cost-effectiveness of SSRC.
Acknowledgments
The provisional abstract of this systematic review has been presented as poster at annual conference of “Association of Surgeons of Great Britan and Ireland” on 17th – 19th May at Harrogate, United Kingdom and has been published in British Journal of Surgery as abstract.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-56/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-56/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jones MW, Genova R, O'Rourke MC. Acute Cholecystitis. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Gallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA 2022;327:965-75. [Crossref] [PubMed]
- Pisano M, Allievi N, Gurusamy K, et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J Emerg Surg 2020;15:61. [Crossref] [PubMed]
- Papi C, Catarci M, D'Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol 2004;99:147-55. [Crossref] [PubMed]
- Adachi T, Eguchi S, Muto Y. Pathophysiology and pathology of acute cholecystitis: A secondary publication of the Japanese version from 1992. J Hepatobiliary Pancreat Sci 2022;29:212-6. [Crossref] [PubMed]
- Hassler KR, Collins JT, Philip K, et al. Laparoscopic Cholecystectomy. 2023 Jan 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Coccolini F, Catena F, Pisano M, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg 2015;18:196-204. [Crossref] [PubMed]
- Hockstein NG, Gourin CG, Faust RA, et al. A history of robots: from science fiction to surgical robotics. J Robot Surg 2007;1:113-8. [Crossref] [PubMed]
- Morel P, Pugin F, Bucher P, et al. Robotic single-incision laparoscopic cholecystectomy. J Robot Surg 2012;6:273-4. [Crossref] [PubMed]
- Wren SM, Curet MJ. Single-port robotic cholecystectomy: results from a first human use clinical study of the new da Vinci single-site surgical platform. Arch Surg 2011;146:1122-7. [Crossref] [PubMed]
- Balaphas A, Hagen ME, Buchs NC, et al. Robotic laparoendoscopy single site surgery: a transdisciplinary review. Int J Med Robot 2013;9:1-11. [Crossref] [PubMed]
- Breitenstein S, Nocito A, Puhan M, et al. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Ann Surg 2008;247:987-93. [Crossref] [PubMed]
- Salman M, Bell T, Martin J, et al. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. Am Surg 2013;79:553-60. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Chalmers TC, Smith H Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49. [Crossref] [PubMed]
- Rangel SJ, Kelsey J, Colby CE, et al. Development of a quality assessment scale for retrospective clinical studies in pediatric surgery. J Pediatr Surg 2003;38:390-6; discussion 390-6. [Crossref] [PubMed]
- Grochola LF, Soll C, Zehnder A, et al. Robot-assisted versus laparoscopic single-incision cholecystectomy: results of a randomized controlled trial. Surg Endosc 2019;33:1482-90. [Crossref] [PubMed]
- Kudsi OY, Castellanos A, Kaza S, et al. Cosmesis, patient satisfaction, and quality of life after da Vinci Single-Site cholecystectomy and multiport laparoscopic cholecystectomy: short-term results from a prospective, multicenter, randomized, controlled trial. Surg Endosc 2017;31:3242-50. [Crossref] [PubMed]
- Pietrabissa A, Pugliese L, Vinci A, et al. Short-term outcomes of single-site robotic cholecystectomy versus four-port laparoscopic cholecystectomy: a prospective, randomized, double-blind trial. Surg Endosc 2016;30:3089-97. [Crossref] [PubMed]
- Hagen ME, Balaphas A, Podetta M, et al. Robotic single-site versus multiport laparoscopic cholecystectomy: a case-matched analysis of short- and long-term costs. Surg Endosc 2018;32:1550-5. [Crossref] [PubMed]
- Li YP, Wang SN, Lee KT. Robotic versus conventional laparoscopic cholecystectomy: A comparative study of medical resource utilization and clinical outcomes. Kaohsiung J Med Sci 2017;33:201-6. [Crossref] [PubMed]
- Su WL, Huang JW, Wang SN, et al. Comparison study of clinical outcomes between single-site robotic cholecystectomy and single incision laparoscopic cholecystectomy. Asian J Surg 2017;40:424-8. [Crossref] [PubMed]
- Review Manager (RevMan) [Computer program]. Version 5.4. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, 2008. Available online: 10/03/2018http://tech.cochrane.org/revman/download/windows64
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Matthias E, Davey SG, Altman DG. editors. Systematic reviews in health care: meta-analysis in context. 2nd edition. London: BMJ Publishing Group, 2001;487.
- Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG. editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd ed. London: John Wiley & Sons, Ltd, 2001:285-312.
- Sun N, Zhang J, Zhang C, et al. Single-site robotic cholecystectomy versus multi-port laparoscopic cholecystectomy: A systematic review and meta-analysis. Am J Surg 2018;216:1205-11. [Crossref] [PubMed]
- Han C, Shan X, Yao L, et al. Robotic-assisted versus laparoscopic cholecystectomy for benign gallbladder diseases: a systematic review and meta-analysis. Surg Endosc 2018;32:4377-92. [Crossref] [PubMed]
- Huang Y, Chua TC, Maddern GJ, et al. Robotic cholecystectomy versus conventional laparoscopic cholecystectomy: A meta-analysis. Surgery 2017;161:628-36. [Crossref] [PubMed]
- Hoffman AB, Myneni AA, Towle-Miller LM, et al. The Early (2009-2017) Experience With Robot-assisted Cholecystectomy in New York State. Ann Surg 2021;274:e245-52. [Crossref] [PubMed]
Cite this article as: Singh A, Kaur M, Swaminathan C, Siby J, Singh KK, Sajid MS. Laparoscopic versus robotic cholecystectomy: a systematic review with meta-analysis to differentiate between postoperative outcomes and cost-effectiveness. Transl Gastroenterol Hepatol 2024;9:3.