
A fucoidan plant drink reduces Helicobacter pylori load in the stomach: a real-world study
Highlight box
Key findings
• The fucoidan intervention for 4–8 weeks demonstrated clearance of Helicobacter pylori (Hp).
What is known and what is new?
• Fucoidan has antibacterial, immunomodulatory, and Hp-clearing effects in vitro studies and animal experiments. However, the effectiveness of fucoidan in clearing Hp infection in the human body remains unclear.
• We found that the plant beverage containing fucoidan has a clearing effect on Hp in the human body.
What is the implication, and what should change now?
• Plant beverage containing fucoidan may be a potential method for eradicating Hp infection.
• For individuals with Hp infection for whom antibiotic treatment is ineffective or cannot be used, this could represent a new opportunity.
Introduction
According to epidemiological research data, the global prevalence of Helicobacter pylori (Hp) infection among adults is approximately over 50% with infection rates higher in developing countries (1). The infection rate among children is also considerably high (2). Hp colonizes the gastric mucosa during childhood and persists without successful eradication therapy. Chronic infection can lead to serious complications such as peptic ulcer disease, gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma (3). Hp infection can lead to changes to the delicate balance of the gastric microbiota which has implications in human health and disease (4). Hp infection can induce insulin resistance and predispose individuals to the development of type 2 diabetes mellitus (5). Recent studies have suggested an increased risk of diabetes mellitus and hypertension (6,7). Hp infection is widely recognized as an infectious disease that warrants antimicrobial therapy, with bactericidal eradication being an immediate treatment goal (8). In East Asian countries, Hp eradication therapy has reduced gastric cancer incidence in healthy individuals and patients with gastric tumors. It even appears to lower the mortality associated with gastric cancer (9). However, almost all the currently available treatments for Hp, including the standard triple therapy [STT; a proton pump inhibitor (PPI) plus 2 antibiotics, usually clarithromycin and amoxicillin] and bismuth-free or bismuth-containing quadruple therapy (BQT), are based on a combination of antibiotics and adjuvants. BQT usually consists of a PPI, bismuth, metronidazole and antibiotics (e.g., tetracycline). The recommended STT for eradicating Hp traditionally combines PPI with clarithromycin, amoxicillin, or metronidazole. Failure rate can be up to 20%, predominately due to the increasing antibiotic resistance worldwide (10). In the Asia-Pacific region, the prevalence of Hp resistant to clarithromycin, levofloxacin, and metronidazole has increased to 21%, 27%, and 45%, respectively (11). Patient compliance with STT and BQT is less than satisfactory, with the classical BQT being the least well tolerated: approximately 37% of overall cases had adverse events (AEs), with taste disturbance (7%), diarrhea (7%), nausea (6%), and abdominal pain the most common (12). When used as first-line empirical therapy, the 10-day BQT had a significantly higher eradication rate but lower compliance rate (23.1% vs. 9.1%) than 7-day PPI-clarithromycin containing triple therapy (13). Therefore, Hp eradication remains a significant clinical challenge. The development of effective, less toxic eradication therapies remains a key research topic (14), such as scutellaria baicalensis (15). Recently, the anti-adhesive, antioxidant, antitoxin, immunomodulatory, anti-coagulant, and anti-infective activities of Ascophyllum nodosum (knotted wrack; a type of brown seaweed that contains fucoidan) extracts were extensively studied. It was proposed that fucoidan may be classified as a new drug that could be included in therapeutic regimens for Hp eradication (16). This novel treatment could avoid antibiotic associated side effects and resistance to eradicate HP infection and avoid Hp associated complications (17).
We conducted a real-world clinical study to explore the clinical efficacy of fucoidan (a compounded product) for the clearance of Hp in the stomach. This study was aimed at the assessment whether a drink containing fucoidan was clinically valuable for eradicating Hp and lowering Hp load. We present this article in accordance with the TREND reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-63/rc).
Methods
Compound product
The compounded product was a fucoidan plant drink (FPD) (Lewuyou®). It is categorized as a “food product” and containing the following main ingredients: water, radish seed compounded plant beverage (including water, isomalto-oligosaccharide, glucose, yam, radish seed, and hawthorn), apple juice concentrate, broccoli powder (including maltodextrin and broccoli), fucoidan (200 mg), Lycium ruthenicum Murray powder, instant green tea powder, erythritol, pectin, citric acid, DL-malic acid, sucralose, stevioside, and edible flavors and fragrances. The compound was used due to the following principles: high affinity, the bio-targeted polysaccharide interferes with Hp’s recognition of gastric mucosal epithelial cells and removes Hp by adhesion; potent antioxidants to reduce gastric inflammation and repair gastric mucosa and to improve gastric function.
In China, there is currently no requirement for ethical approval for the use of “food” in research. So, this study did not undergo ethics committee review. All research subjects will be informed of the intervention drink before enrollment. All participants agreed to take the intervention beverage daily as per the study requirements verbally.
Experimental drink
The FPD was presented as a liquid beverage in brown glass vials (50 mL in each vial). The product requires storage at room temperature and protection from light.
Patients and study design
Patient registration
Patients who volunteered to receive the anti-Hp FPD were registered as participants. All participants were treated with FPD. Medical histories and demographic data were collected, including age, gender, medical history, history of smoking, history of alcohol consumption, and history of coffee or tea drinking. Smoking was defined as smoking 1 pack of cigarettes or more per week.
Enrollment
This study is a multicenter real-world investigation. A total of 122 consecutive adult Hp-infected outpatients and health check-up patients aged 20–81 (41.47±12.23) years were enrolled in 7 centers in China (Taian, Taishan, Shanghai, Jinan, Dongying, Xian, Liaocheng) between October 2020 and July 2021. A diagnosis of Hp infection was made according to the following criteria: (I) a recent (within 7 days) positive result of 13C- or 14C-urea breath test (UBT); and/or (II) endoscopy, histologically confirmed Hp infection.
Exclusion criteria
(I) Withdrawal from the study within 1 month (4 weeks); (II) inability to respond to the test request and/or failure to provide UBT results within 5 weeks; (III) methods inconsistent between pre- and post-testing.
Grouping
Real-world design
13C- or 14C-positive Hp carriers were enrolled from large general hospitals or health check-up centers. The changes in 13C or 14C values were compared before and after FPD consumption. The participant activities (including diets) were not restricted during the study period.
FPD consumption
FPD (1 vial each time) was consumed twice daily (after waking and before bedtime) on an empty stomach for 8 consecutive weeks. The drink was shaken well before consumption.
Before and after the therapeutic intervention for Hp eradication, patients (carriers) were asked to return their hospitals at week 2 to receive an assessment of medication adherence and AEs, as specified in the study protocol. UBT was performed at 4 weeks (28±3 days) and 8 weeks (56±3 days) to learn the Hp status and thus assess the therapeutic effectiveness. Effectiveness data: 14C and 13C values were recorded to determine the changes in Hp status. Hp clearance was defined as a negative DOB (delta over baseline) value of UBT after the intervention. The intervention was judged as effective when there was a reduction in the 14C or 13C values.
Primary endpoint
Hp eradication and clearance rates at weeks 4 and 8. The AEs during the treatment period were also analyzed.
Diagnosis of Hp infection and efficacy
Blinding
The staff performing the 13C- or 14C-UBT were blind to the details of this study.
UBT
UBT was performed before enrollment and at weeks 4 and 8 during FPD consumption. If possible, the test must be completed in the same institution using the same method (13C or 14C). If the test was performed using a different method before and after FPD consumption and the data were >0, the data were used for reference only and were not regarded as valid data; these patients were not included in the final analysis.
Evaluation of efficacy
The difference between the baseline and post-intervention values was regarded as the eradication volume of Hp in the stomach after FPD consumption, and the ratio between the eradication volume and baseline value was the eradication rate. The FPD intervention was regarded as effective if the eradication rate was >0, and the Hp status was defined as “cleared” if the eradication rate was 1.
AEs and adherence during the follow-up period
Safety data (if any)
The type, severity, and duration of AEs, serious adverse events (SAEs) and, in particular, adverse drug reactions (ADRs) were recorded in detail at each visit. AEs that affected the patient quality of life including abdominal pain, diarrhea, constipation, dizziness, dysgeusia, headache, anorexia, nausea, vomiting and rash were also recorded.
Adherence
“Adherence” was defined according to whether FPD was consumed in keeping with the trial protocol and when and why it was discontinued. Poor adherence was defined if the FPD was consumed less than 80% of the set amount or if more than 50% of doses were missed.
Statistical analysis
Treatment outcomes and main factors were compared using the software SPSS 10.1 (IBM Corp., Chicago, IL, USA). The chi-square test, with or without Yates’ continuity correction and Fisher’s exact test, was performed when appropriate.
Results
Participant characteristics
A total of 122 patients with Hp infection were enrolled in this study. All participants were asked to consume FPD, and the effects of the drink on 13C- or 14C-UBT results were analyzed. A total of 85 patients [37 males and 48 females aged 20–81 years (mean: 41.5±12.2 years)] completed the first phase of the study (17 in the 13C group and 68 in the 14C group). Among the 85 patients, 41 patients (5 in the 13C group and 36 in the 14C group) completed the second phase of the study and were considered eligible cases. Among the 41 patients who completed the 8-week intervention, the data of 7 patients were collected 2 weeks after cessation of the intervention.
Hp eradication rate
After 4 weeks of FPD consumption, the Hp eradication rate was 77.6% (66/85) and the clearance (negative conversion) rate was 20.0% (17/85); after 8 weeks of administration, the Hp eradication rate was 80.5% (33/41) and the clearance rate was 26.8 (11/41) .
The actual 13C/14C values were compared before (at day 0) and after (4 weeks later, n=85; at 8 weeks later, n=41) FPD consumption. A line graph visualizing the overall decreasing trend and the number of cases reaching point 0 is displayed in Figure 1. We used scatter plots to illustrate the declining trend of Hp infection in patients at baseline, 4 weeks, and 8 weeks post-intervention. We set UBT value =4 as the cutoff for negative/positive classification (13C); and UBT value =99 as the cutoff for negative/positive classification (14C) (Figure 2).
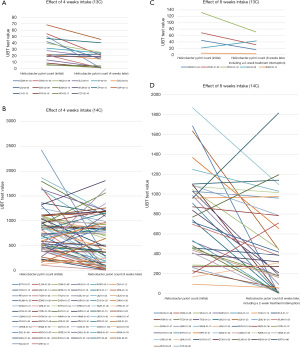
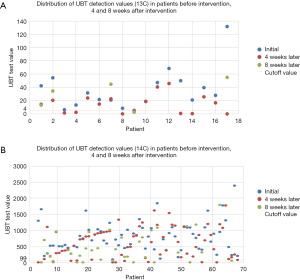
Symptom improvement
Symptoms, including abdominal distension, nausea, belching, and acid reflux were improved in some patients. In a post-bone marrow transplant, for a patient with intestinal rejection combined with cytomegalovirus (CMV) infection who had been producing 30 bloody, watery stools per day, the frequency of defecation decreased to 4 times/day after FPD consumption.
AEs and complications
AEs were investigated among all participants. No AE occurred in all 122 participants from the first day of administration to week 8. The medication compliance rate was 69.7% in the first month and 33.6% in the second.
Discussion
We conducted a real-world clinical study to explore the clinical efficacy of fucoidan (a compounded product) for the clearance of Hp in the stomach. It was found in this small-sample clinical observation study that fucoidan was clinically valuable for eradicating Hp in the stomach and lowering Hp load, as validated by the DOB values of 13C- or 14C-UBT.
The infection rate of Hp is extremely high worldwide, with the highest prevalence in developing countries (1). Current guidelines recommend using triple or quadruple therapy as the treatment of choice for Hp infection, but the failure rate exceeds 20%. The rapid rise of antibiotic resistance, poor compliance and disruption of the gut microbiota has stimulated interest in alternative therapies (18). Among them, marine biologics are particularly promising. For example, epinecidin-1, a multifunctional antimicrobial peptide produced by Epinephelus coioides, was shown to have the potential to replace antibiotics with its significant efficacy in fighting against Staphylococcus aureus (S. aureus) and Hp (19). Epinecidin-1 (Epi-1) was found to have potent bactericidal activity against Hp in vitro and modulated Hp-induced host immune responses in a mouse model (20).
Fucoidan (also known as fucoidin or sulfated fucan), first extracted from seaweed in 1913, is a negatively charged, highly hygroscopic polysaccharide soluble in water and acid solutions (21). It contains L-fucose and sulfate groups and is mainly derived from brown algae, red algae, and some marine invertebrates. It is well known amongst the food and pharmaceutical industry, due to its good therapeutic effects on some specific diseases. Fucoidan is not a single-structural compound, but contains xylose, mannose, galactose, arabinose, and glucuronic acid. Its biological functions are ascribed to its unique biological structure. Classical bioactivities associated with fucoidan include antioxidant, anti-tumor, anti-coagulant, anti-thrombotic, immunoregulatory, anti-viral and anti-inflammatory effects (22). A study assessing the bioactive antimicrobial capability of fucoidan (“Generally Recognized as Safe” approval—European Commission December 2017) from different species of Phaeophyceae algae (Fucus vesiculosus, Undaria pinnatifida, Macrocystis pyrifera) against Hp, all the studied fucoidans showed bacteriostatic and bactericidal effects at the studied concentrations (5–100 µg/mL) and exposure time (0–7 days) (23).
The exact mechanism by which fucoidan eradicates Hp remains unclear. Fucoidan is believed to interfere with the linkage/attachment of Hp to gastric mucosal cells. Shibata et al. found that fucoidan could block the attachment of Hp to gastric cells (24). An in vitro experiment also confirmed that Cladosiphon fucoidan inhibited the Hp attachment to porcine gastric mucin at pH 2.0 and 4.0 (17). Based on this property, genipin-crosslinked low molecular weight fucoidan/chitosan-N-arginine nanogels (FCSA) were prepared for targeted delivery of amoxicillin to the site of Hp-infected human gastric adenocarcinoma epithelial (AGS), cells and the negatively charged nanogels (n-FCSA) adhered to Hp and exhibited pH-responsive drug release property to reduce cytotoxic effects in Hp-infected AGS cells (25). In the study conducted by Chua et al., adding fucoidan polysaccharide formulation (1,000 µg/mL) to the culture medium did not inhibit the growth of Hp. However, adding different fucoidan salt formulations (1,000 µg/mL) to the tissue culture medium exhibited toxicity towards AGS cells, reducing the number of viable AGS cells. Furthermore, a fucoidan polysaccharide formulation at a concentration of 100 µg/mL significantly decreased the adhesion of HP, suggesting a potential mechanism of fucoidan treatment for HP infection involving AGS cell toxicity and reduction of HP adhesion (26).
Furthermore, it was demonstrated in Cai et al. research that a combination of fucoidan polysaccharides and evening primrose extract (FEMY-R7), exhibited complete inhibition of Hp in vitro at a concentration of 100 µg/mL. Animal experiments revealed that FEMY-R7 cleared gastric mucosal infection by direct killing the bacteria and preventing their adhesion and invasion (27). In a clinical study, humans confirmed to be infected with Hp were orally administered twice daily with a capsule containing 150 mg FEMY-R7 for 8 weeks. FEMY-R7 significantly decreased the value in UBT and the serum pepsinogens I and II levels. The results indicate that FEMY-R7 eliminates Hp from animal and human gastric mucosa and improves gastric function (28).
Additionally, the observations on healthy volunteers indicate that fucoidan can be absorbed and metabolized by the human body, with absorption being correlated to the frequency of Hp infection and seaweed product usage (29,30). The ingestion of fucoidan may also exert antimicrobial, antiviral, and even anti-tumor effects by improving the body’s immune status.
A study on mice (Tomori et al.) revealed that when different doses of fucoidan were administered orally continuously for 6 weeks, immune cell proliferation, interleukin (IL)-2, macrophage phagocytes, and serum antibodies (IgM, -G, -A) increased significantly. Still, the levels of IL-4, -5, and IgE decreased significantly. These results indicated that fucoidan modulated cellular and humoral immunity (31). When cells were incubated with fucoidan in the presence of a viral mimic, fucoidan inhibited the production of some cytokines, chemokines, and prostaglandin E2. Additionally, fucoidan may help alleviate airway inflammation caused by viral infections (32). A recent study showed that fucoidan had a significant antiviral activity at 3.90–500 µg/mL concentrations, even for treating and preventing the coronavirus disease 2019 (COVID-19) (33). Ishikawa et al. investigated the anti-primary effusion lymphoma (PEL) effects of fucoidan obtained from Cladosiphon okamuranus Tokida, cultivated in Okinawa, Japan. Fucoidan dose-dependently inhibited the proliferation of KSHV-infected PEL cell lines, highlighting the anti-PEL actions of fucoidan, and the mechanism is believed to be associated with its immunomodulatory effects (34).
The compounded fucoidan product in our present study effectively reduced the Hp load in the stomach of participants (effective rate: 77.6% at 1 month and 80.5% at 2 months). It achieved Hp clearance in some carriers (clearance rate: 20.0% at 1 month and 26.8 % at 2 months), showing promising clinical efficacy in treating Hp.
Some studies implemented in China have investigated the clinical effectiveness of both STT and quadruple therapy. For the STT, the combination of pantoprazole, clarithromycin, and amoxicillin resulted in an eradication rate of 68.8% (55/80) (35) after 7 consecutive days and 80.8% (80/99) after 10 days (36); the combination of omeprazole, levofloxacin, and amoxicillin for 7 consecutive days, followed by 2 weeks of omeprazole, achieved an eradication rate of 80.2% or 83.5% when omeprazole was replaced by ecabet sodium (37). For the quadruple therapy, the combination of rabeprazole, colloidal bismuth, clarithromycin, and amoxicillin achieved an eradication rate of 92.00% (46/50); the eradication rate was 84% (42/50) when amoxicillin was replaced by metronidazole (38) or 83.67% (41/49) when rabeprazole was replaced by omeprazole (39). In contrast, the clearance rate of Hp was slightly lower after 8 weeks of FPD consumption in our current study, and a variety of factors might explain. Participant diets were not restricted during the study period, and frequent re-infections were possible. Unlike antibiotic treatment, which has a half-life in the body and still has an antibacterial effect in the event of re-infection, FPD only has a physical adherence effect and/or an immunomodulatory activity. Since fucoidan is not an antibiotic, it is difficult to eliminate new infections between FPD consumption intervals.
As to other limitations of the study, the vast majority of the participants were asymptomatic, and some of them lacked knowledge and awareness of Hp infection, which increased the difficulty in project management and resulted in poor compliance. In addition, the custom of eating together among Chinese populations increases the risk of Hp persistence or infection. As a result, the dropout rate was high during the implementation of our study. No control group with standard practice was organized to fully compare intervention effectiveness. The follow up limited to 8 weeks.
Conclusions
In summary, FPD consumption was safe and effective at reducing Hp load on the gastric mucosa, with favorable eradication rates. More research is required to determine the long-term eradication rates, impact on Hp associated diseases and random controlled trials comparing with current standard therapy.
Acknowledgments
We thank Taian City Central Hospital, Shandong Provincial Taishan Hospital, Shanghai East Hospital, Dongying Kanghui Health Center, Xijing Hospital, Liaocheng Cancer Hospital, Jinan People’s Hospital, for their support of the research. Thanks to the enrolled research subjects for their contribution to the study, and Blue Regale Clinical Nutrition Technology Co., Ltd. for providing product support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-63/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-63/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-63/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-63/coif). All authors report that Blue Regale Clinical Nutrition Technology Co., Ltd. provides product support for this study. QHP is from Blue Regale Clinical Nutrition Technology Co., Ltd., Kunshan, China. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In China, there is currently no requirement for ethical approval for the use of “food” in research. So, this study did not undergo ethics committee review. All research subjects will be informed of the intervention drink before enrollment. All participants agreed to take the intervention beverage daily as per the study requirements verbally.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li Y, Choi H, Leung K, et al. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2023;8:553-64. [Crossref] [PubMed]
- Mezmale L, Coelho LG, Bordin D, et al. Review: Epidemiology of Helicobacter pylori. Helicobacter 2020;25:e12734. [Crossref] [PubMed]
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Lopetuso LR, Napoli M, Rizzatti G, et al. Considering gut microbiota disturbance in the management of Helicobacter pylori infection. Expert Rev Gastroenterol Hepatol 2018;12:899-906. [Crossref] [PubMed]
- Yang YJ, Sheu BS. Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota. Microorganisms 2016;4:15. [Crossref] [PubMed]
- Man S, Ma Y, Jin C, et al. Association between Helicobacter pylori Infection and Diabetes: A Cross-Sectional Study in China. J Diabetes Res 2020;7201379. [Crossref] [PubMed]
- Xiong X, Chen J, He M, et al. Helicobacter pylori infection and the prevalence of hypertension in Chinese adults: The Dongfeng-Tongji cohort. J Clin Hypertens (Greenwich) 2020;22:1389-95. [Crossref] [PubMed]
- Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2007;56:772-81. [Crossref] [PubMed]
- Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113-21. [Crossref] [PubMed]
- Guevara B, Cogdill AG. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig Dis Sci 2020;65:1917-31. [Crossref] [PubMed]
- Liou JM, Lee YC, Wu MS. Treatment of Helicobacter pylori infection and its long-term impacts on gut microbiota. J Gastroenterol Hepatol 2020;35:1107-16. [Crossref] [PubMed]
- Nyssen OP, Perez-Aisa A, Tepes B, et al. Adverse Event Profile During the Treatment of Helicobacter pylori: A Real-World Experience of 22,000 Patients From the European Registry on H. pylori Management (Hp-EuReg). Am J Gastroenterol 2021;116:1220-9. [Crossref] [PubMed]
- Kim YI, Lee JY, Kim CG, et al. Ten-day bismuth-containing quadruple therapy versus 7-day proton pump inhibitor-clarithromycin containing triple therapy as first-line empirical therapy for the Helicobacter pylori infection in Korea: a randomized open-label trial. BMC Gastroenterol 2021;21:95. [Crossref] [PubMed]
- He XJ, Zeng XP, Jiang CS, et al. Antofloxacin-based bismuth quadruple therapy is safe and effective in Helicobacter pylori eradication: A prospective, open-label, randomized trial. Arab J Gastroenterol 2021;22:47-51. [Crossref] [PubMed]
- Dmitrieva A, Kozlova O, et al. Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori. Int J Mol Sci. 2023;24:11906. [Crossref] [PubMed]
- Besednova NN, Zaporozhets TS, Somova LM, et al. Review: prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter 2015;20:89-97. [Crossref] [PubMed]
- Shibata H, Iimuro M, Uchiya N, et al. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2003;8:59-65. [Crossref] [PubMed]
- Lionetti E, Francavilla R, Castellazzi AM, et al. Probiotics and Helicobacter pylori infection in children. J Biol Regul Homeost Agents 2012;26:S69-76. [PubMed]
- Neshani A, Zare H, Akbari Eidgahi MR, et al. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol Toxicol 2019;20:33. [Crossref] [PubMed]
- Narayana JL, Huang HN, Wu CJ, et al. Epinecidin-1 antimicrobial activity: In vitro membrane lysis and In vivo efficacy against Helicobacter pylori infection in a mouse model. Biomaterials 2015;61:41-51. [Crossref] [PubMed]
- Cui K, Tai W, Shan X, et al. Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens. Int J Biol Macromol 2018;120:1817-22. [Crossref] [PubMed]
- Luthuli S, Wu S, Cheng Y, et al. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar Drugs 2019;17:487. [Crossref] [PubMed]
- Palacios-Gorba C, Pina R, Tortajada-Girbés M, et al. Caenorhabditis elegans as an in vivo model to assess fucoidan bioactivity preventing Helicobacter pylori infection. Food Funct 2020;11:4525-34. [Crossref] [PubMed]
- Shibata H. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobacter pylori to human gastric cells. J Nutr Sci Vitaminol (Tokyo) 1999;45:325-36. [Crossref] [PubMed]
- Lin YH, Lu KY, Tseng CL, et al. Development of genipin-crosslinked fucoidan/chitosan-N-arginine nanogels for preventing Helicobacter infection. Nanomedicine (Lond) 2017;12:1491-510. [Crossref] [PubMed]
- Chua EG, Verbrugghe P, Perkins TT, et al. Fucoidans Disrupt Adherence of Helicobacter pylori to AGS Cells In Vitro. Evid Based Complement Alternat Med 2015;2015:120981. [Crossref] [PubMed]
- Cai J, Kim TS, Jang JY, et al. In vitro and in vivo anti-Helicobacter pylori activities of FEMY-R7 composed of fucoidan and evening primrose extract. Lab Anim Res 2014;30:28-34. [Crossref] [PubMed]
- Kim TS, Choi EK, Kim J, et al. Anti-Helicobacter pylori activities of FEMY-R7 composed of fucoidan and evening primrose extract in mice and humans. Lab Anim Res 2014;30:131-5. [Crossref] [PubMed]
- Tokita Y, Nakajima K, Mochida H, et al. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci Biotechnol Biochem 2010;74:350-7. [Crossref] [PubMed]
- Tomori M, Nagamine T, Iha M. Are Helicobacter pylori Infection and Fucoidan Consumption Associated with Fucoidan Absorption? Mar Drugs 2020;18:235. [Crossref] [PubMed]
- Tomori M, Nagamine T, Miyamoto T, et al. Evaluation of the Immunomodulatory Effects of Fucoidan Derived from Cladosiphon Okamuranus Tokida in Mice. Mar Drugs 2019;17:547. [Crossref] [PubMed]
- Dutot M, Grassin-Delyle S, Salvator H, et al. A marine-sourced fucoidan solution inhibits Toll-like-receptor-3-induced cytokine release by human bronchial epithelial cells. Int J Biol Macromol 2019;130:429-36. [Crossref] [PubMed]
- Song S, Peng H, Wang Q, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct 2020;11:7415-20. [Crossref] [PubMed]
- Ishikawa C, Mori N. In vitro and in vivo anti-primary effusion lymphoma activities of fucoidan extracted from Cladosiphon okamuranus Tokida. Oncol Rep 2017;38:3197-204. [Crossref] [PubMed]
- Zhang L. Clinical Effect Comparison of Sequential Therapy and Triple Therapy in Eradication of Helicobacter pylori infection. Chinese Journal of Clinical Gastroenterology 2015;27:262-5.
- Sui SJ, Yao HJ. Observation on the Efficacy of Quadruple Therapy in Eradication of Helicobacter pylori. Chinese Coumunity Docotors 2012;14:127-8.
- Peng XJ, Wu ZX. Observation on the treatment efficacy of Pantoprazole Levofloxacin and Amoxicillin Thriple Therapy of Helicobacter pylori. Chinese Journal of General Practice 2015;13:681-2.
- Yang X, Lyu YN. Quadruple drugs therapy in treatment of chronic gastritis with Helicobacter pylori infection: an analysis of safety and efficacy. Journal of Huaihai Medicine 2017;35:272-3.
- Cai YG, Bai L, Dong KB, et al. Efficacy of High-dose Amoxicillin Combined with Esomeprazole in the Treatment of Patients with Helicobacter pylori Infection. China Practical Medicine 2021;16:98-101.
Cite this article as: Teng QL, Sui SJ, Zhu Z, Gao Q, Ge H, Wang KQ, Lino-Silva LS, Sigal M, White JR, Peng QH, Wei YZ. A fucoidan plant drink reduces Helicobacter pylori load in the stomach: a real-world study. Transl Gastroenterol Hepatol 2023;8:34.


