Arterial resection during operative management of pancreatic head and uncinate process adenocarcinoma: a systematic review
Highlight box
Key findings
• Arterial resection can be performed with an acceptable peri-operative morbidity and mortality during resections for adenocarcinoma of the pancreatic head and uncinate process.
• However, data are still conflicting with regards to any long-term oncological benefit.
What is known and what is new?
• Surgical resection after neoadjuvant treatment is indicated where possible for the management of pancreatic cancer with arterial involvement.
• Resectability depends on various factors, including anatomical variants and disease distribution, as well as surgical experience and expertise.
• Arterial resection in this context is however not widely supported due to the paucity and diversity of the reported evidence in the literature.
What is the implication, and what should change now?
• Better patient and disease biology selection may improve long term outcomes from aggressive management of patients with pancreatic cancer with arterial involvement.
• Future research should concentrate in this field.
Introduction
Pancreatic cancer is associated with poor prognosis; one of the key contributors to this is that it is frequently diagnosed at an advanced stage (1). In recent years, with advances in surgical technique, alongside the advent of neoadjuvant therapies, the definition of an operable pancreatic malignancy has evolved. Historically local disease involving the regional arteries [hepatic artery (HA), superior mesenteric artery (SMA) and coeliac axis (CA)] was considered inoperable (2). However, arterial involvement is no longer an absolute contraindication to surgical resection after patient and disease biology selection with neoadjuvant treatment (3). Peri-adventitial dissection is preferable, however if this is not possible intra-operatively, arterial resection can be considered in select cases. There is a paucity of evidence in the literature in this area (4), especially in the context of proximal pancreatic tumors, whereas the data on distal pancreatectomies with resection of the CA are more widely reported. This systematic review therefore aims to assess the evidence on the perioperative and long-term outcomes related to arterial resection for proximal pancreatic tumours. We present this article in accordance with the PRISMA reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-33/rc) (5).
Methods
Literature search
A systematic literature search was conducted. Three databases (PubMed, MEDLINE and the Cochrane Library) were searched for by two independent reviewers (Dermanis AA and Halle-Smith J) to identify potential papers for inclusion. The following MESH terms were used in this search in conjunction with Boolean operators “AND” and “OR”: “Pancreaticoduodenectomy”, “Pancreatomy”, “Pancreatectomy”, “Whipple”, “Pancreatic cancer”, “Pancreatic adenocarcinoma”, “Arterial resection”, “Vascular resection”. This search was performed in December 2022 and included any studies published after January 2000. The search terms were applied to titles and abstracts and was limited to English language articles. Following the removal of duplicates two independent reviewers (Dermanis AA and Halle-Smith J) screened by title and abstract and then by full text review. Articles were considered appropriate if they reported outcomes following resection of a tumour of the pancreatic head/uncinate process involving the SMA, CHA and/or CA by means of pancreaticoduodenectomy (PD) or total pancreatectomy (TP). The exclusion criteria were: animal studies, conference abstracts, literature and/or systematic reviews, video articles, technical and case reports, as well as small case series (<10 patients). Studies that presented a mixture of patients where data for arterial resections relating to proximal tumours only could not be extracted were also excluded. Any disputes were resolved by the senior author (Chatzizacharias N).
Data extraction
Two independent reviewers (Dermanis AA and Halle-Smith J) extracted data from each included study. Information was extracted from the included full texts and supplementary materials on the number of arterial resections, age, gender, preoperative radiological investigations and findings, adjuvant and neoadjuvant chemotherapy, location of the tumour, perioperative outcomes, disease free survival (DFS), overall survival (OS) and survival at 1, 2, 3 and 5 years. Intraoperative details, namely, the artery resected, the type of reconstruction performed if relevant and any arterial graft used were extracted. The perioperative outcomes included: intraoperative blood loss, length of operation, complications, Clavien-Dindo grade ≥3, re-operation rates, morbidity rates and mortality rates. Due to the small number of studies with expected high heterogeneity, median values were calculated in the place of means in order to minimise the influence of outliers. Consequently, no statistical analysis was performed in this review.
Results
Search results
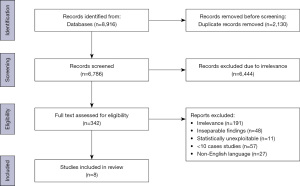
Our search initially yielded 8,916 studies, of which 2,130 duplicates were removed prior to abstract screening. A total of 6,444 studies were excluded following abstract and title screening due to irrelevance and a further 334 studies were excluded after full text screening, leaving 8 studies eligible for inclusion in our review (Figure 1).
Pre-operative features
Of the 8 studies identified (6-13), 7 used staging computed tomography (CT) (6,8-13) and 2 also used magnetic resonance imaging (MRI) (11,12). One study did not state the image modality used for staging (7). All studies included tumours of the pancreatic head and uncinate process, with the pancreatic head been the commonest reported location. Three studies reported the classification of tumours, with the most commonly reported tumor being borderline resectable (6,9,12). Where reported, National Comprehensive Cancer Network (NCCN) guidelines were used to classify each cancer (6,9). Arterial resection was carried out in a total of 170 patients [median 18, interquartile range (IQR), 15–21.5] (6-13) (Table 1).
Table 1
| Study | No. patients | Age (years) | Gender (male, %) | Neoadjuvant Chemo. (%) | Neoadjuvant Radio. (%) | Adjuvant Chemo. (%) ǂ | Adjuvant Radio. (%)ǂ | Adjuvant Chemrad. (%)ǂ |
|---|---|---|---|---|---|---|---|---|
| Marichez et al. (6) | 16 | NR | NR | 50 | 0 | 0 | 0 | 12.5 |
| Ramia et al. (7) | 17 | 59 | 64.7 | 47.1 | 50 | NR | NR | NR |
| Bachellier et al. (8)* | 51 | NR | NR | 74.5 | NR | NR | NR | NR |
| Yang et al. (9) | 14 | 67.5 | 57.1 | 7.1 | NR | 100 | NR | NR |
| Zhang et al. (10) | 10 | 58 | 40 | 0 | 0 | 0 | 0 | 0 |
| Miyazaki et al. (11) | 21 | 66 | 42.9 | 42.9 | NR | 90.4 | NR | NR |
| Amano et al. (12) | 22 | 64 | 45.5 | NR | NR | NR | NR | NR |
| Wang et al. (13)* | 19 | 46 | 78.9 | NR | NR | NR | NR | NR |
| Overall median (IQR) | 18 (15–21.5) | 61.5 [58–66] | 51.3 (42.9–64.7) | 45 (7.1–50) | 0 (0–50) | 45.2 (0–95.2) | 0 (0–0) | 6.3 (0–12.5) |
*, denotes where baseline characteristics were not available due to the data being extrapolated from a subgroup. ǂ, denotes where ranges were used as insufficient data was available to calculate IQR. Chemo., chemotherapy; Radio., radiotherapy; Chemrad., chemoradiotherapy; NR, not reported; IQR, interquartile range.
Baseline patient characteristics are presented in Table 1. The overall median age was 61.5 years (IQR, 58–66 years) (6-13). Neoadjuvant chemotherapy was utilised in a median of 45.0% of patients (IQR, 7.1–50%) (6-11). One study (7) reported neoadjuvant radiotherapy, however no information was available on the specifics of the treatment. Only one study reported American Society of Anaesthesiology (ASA) grades for their patients, with 76.9% ASA 2 and the remaining ASA 3 (7). Adjuvant chemotherapy was used in a median of 45.2% of patients (6,9-11), whilst adjuvant chemoradiotherapy was used only in one study (6). The only reported chemotherapy regimen was the neoadjuvant FOLFINIROX in two studies (6,8) and the length and type of chemotherapy or radiotherapy regimens used were otherwise unclear or not reported.
Operative results
PD was carried out in 90.5% of patients (IQR, 55.2–100%) and TP in 9.5% of patients (IQR, 0–44.8%) (6-13). The types of arterial resections performed can be found on Table 2. Of the 4 studies that reported aberrant right hepatic artery (aRHA) resection (6,8,9,12), only two clarified that this was a replaced rather than an accessory artery (6,12). The median overall operative time was 561 minutes (IQR, 426–669.8 minutes) (6-9,11,12), and the median blood loss per case was 1,221 mL (IQR, 509.8–2,577.5 mL) (6,7,9,11,12).
Table 2
| Study | PD, n (%) | TP, n (%) | CA, n (%) | HA, n (%) | SMA, n (%) | aRHA, n (%) | Time (min) | Blood loss (mL) |
|---|---|---|---|---|---|---|---|---|
| Marichez et al. (6) | 16 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (100.0) | 377 | 466 |
| Ramia et al. (7) | 4 (23.5) | 13 (76.5) | 17 (100.0) | 0 (0) | 0 (0) | 0 (0) | 600 | 1,221 |
| Bachellier et al. (8) | 51 (100.0) | 0 (0) | 1 (2) | 17 (33.3) | 31 (60.8) | 4 (7.8) | 669.8 | NR |
| Yang et al. (9) | 11 (78.6) | 3 (21.4) | 0 (0) | 6 (42.9) | 0 (0) | 5 (35.7) | 426 | 553.6 |
| Zhang et al. (10) | 10 (100.0) | 0 (0) | 0 (0) | 9 [90] | 1 [10] | 0 (0) | NR | NR |
| Miyazaki et al. (11) | 17 [81] | 4 [19] | 0 (0) | 21 (100.0) | 0 (0) | NR | 522 | 2,290 |
| Amano et al. (12) | 7 (31.8) | 15 (68.2) | 0 (0) | 8 (36.4) | 12 (54.5) | 5 (22.7) | 703 | 2,865 |
| Wang et al. (13) | 19 (100.0) | 0 (0) | NR | NR | NR | NR | NR | NR |
| Overall median (IQR) | 13.5 (8.5–18)/ 90.5 (55.2–100) |
1.5 (0–8.5)/ 9.5 (0–44.8) |
0 (0–1)/ 0 (0–2) |
8 (0–17)/ 36.4 (0–90) |
0 (0–12)/ 0 (0–54.5) |
4.5 (0–5)/ 15.3 (0–35.7) |
561 (426–669.8) |
1,221 (509.8–2,577.5) |
PD, pancreaticoduodenectomy; TP, total pancreatectomy; CA, coeliac axis; HA, hepatic artery; SMA, superior mesenteric artery; aRHA, aberrant right hepatic artery; NR, not reported; IQR, interquartile range.
Studies reporting rates of vascular reconstruction are detailed in Table 3. The most frequently reported reconstruction was a primary end to end anastomosis (9-12) if adequate vessel length was available (10). Use of autologous interposition graft with great saphenous vein (8,10), internal iliac or splenic artery (12) or synthetic graft (8) were also reported. The rational for use of each type of graft was not mentioned. Venous resection was performed in a median of 60% (IQR, 42.9–86.4%) (6,8-13).
Table 3
| Study | Recon. (%) | Primary anastomosis (%) | Venous autologous graft (%) | Autologous arterial graft (%) | Synthetic graft (%) | Venous resection (%) |
|---|---|---|---|---|---|---|
| Marichez et al. (6) | NR | NR | NR | NR | NR | 50 |
| Ramia et al. (7) | NR | NR | NR | NR | NR | NR |
| Bachellier et al. (8) | 92.2 | NR | NR | NR | 2 | 98 |
| Yang et al. (9) | 21.4 | 100 | 0 | 0 | 0 | 42.9 |
| Zhang et al. (10) | 100 | 90 | 10 | 0 | 0 | 60 |
| Miyazaki et al. (11) | 4.8 | 100 | 0 | 0 | 0 | 71.4 |
| Amano et al. (12) | 100 | 90.9 | 0 | 13.6 | 0 | 86.4 |
| Wang et al. (13) | NR | NR | NR | NR | NR | 0 |
| Overall median (IQR) | 99.2 (13.1–100) | 95.5 (90.5–100) | 0 (0–5) | 0 (0–6.8) | 0 (0–1) | 60 (42.9–86.4) |
NR, not reported; IQR, interquartile range.
Morbidity and mortality
The median 90-day mortality was 4.5% (IQR, 0–14.3%) (6-12) (Table 4). The median 90-day morbidity was 43.5% (IQR, 33.3–81%) (6-11), with 15.8% (IQR, 6.3–41.2%) of patients experiencing a complication of Clavien-Dindo grade 3 or higher (6-11). A median of 19.7% (range, 14.3–25%) of patients were readmitted within 90 days (6,9) and 7.3% (range, 2.4–12.8%) underwent a re-operation or re-intervention (8,9,11,13). The median length of stay was 17 days (IQR, 12.3–18.6 days) (6,7,9,10,13).
Table 4
| Study | 90-day mortality (%) | 90-day morbidity (%) | Clavien-Dindo ≥3 (%) | 90-day re-admission (%)ǂ | Re-operation/intervention (%) | LOS (days) |
|---|---|---|---|---|---|---|
| Marichez et al. (6) | 13 | 44 | 6.3 | 25 | NR | 12 |
| Ramia et al. (7) | 23.5 | 88.2 | 41.2 | NR | NR | 19.2 |
| Bachellier et al. (8) | 3.9 | 33.3 | 21.6 | NR | 9.8 | NR |
| Yang et al. (9) | 0 | 42.9 | 0 | 14.3 | 0 | 12.6 |
| Zhang et al. (10) | 10 | 10 | 10 | NR | NR | 17 |
| Miyazaki et al. (11) | 0 | 81 | 52 | NR | 4.8 | NR |
| Amano et al. (12) | 4.5 | NR | NR | NR | NR | NR |
| Wang et al. (13) | NR | NR | NR | NR | 15.8 | 18 |
| Overall median (IQR) | 4.5 (0–14.3) | 43.5 (33.3–81) | 15.8 (6.3–41.2) | 19.7 (14.3–25) | 7.3 (2.4–12.8) | 17 (12.3–18.6) |
ǂ, denotes where ranges were used as insufficient data was available to calculate IQR. LOS, length of stay; NR, not reported; IQR, interquartile range.
Details on the specific post-operative complications can be found in Table 5. Interestingly, among the patients who had post-operative haemorrhage, where reported, none of these were related to the arterial resection or reconstruction site (6,10,12,13).
Table 5
| Study | Haemorrhage (%) | SSI (%) | POPF (%) | DGE (%) | Abdominal abscess/collection (%) | DVT/PE (%) | Hepatic infarct (%) | Pulmonary (%) |
|---|---|---|---|---|---|---|---|---|
| Marichez et al. (6) | 0 | 0 | 0 | 25 | 13 | 0 | 6 | NR |
| Ramia et al. (7) | NR | NR | NR | NR | NR | NR | NR | NR |
| Bachellier et al. (8) | NR | NR | 0 | NR | NR | NR | NR | NR |
| Yang et al. (9) | NR | 0 | 7.1 | 0 | 0 | NR | 0 | 7.1 |
| Zhang et al. (10) | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miyazaki et al. (11) | NR | 4.8 | 4.8 | 9.5 | 14.3 | 9.5 | 33 | 14.2 |
| Amano et al. (12) | 0 | 31.8 | 13.6 | 0 | 0 | 0 | 4.5 | 27.2 |
| Wang et al. (13) | 15.8 | 0 | 21.1 | 5.3 | 0 | 5.3 | NR | NR |
| Overall median (IQR) | 5 (0–12.9) | 0 (0–4.8) | 4.8 (0–13.6) | 2.7 (0–9.5) | 0 (0–13) | 0 (0–7.4) | 4.5 (0–19.5) | 10.7 (3.6–20.7) |
SSI, surgical site infection; POPF, post-operative pancreatic fistula; DGE, delayed gastric emptying; DVT/PE, deep vein thrombosis/pulmonary embolism; NR, not reported; IQR, interquartile range.
Survival
Survival outcomes are summarised in Table 6. Median OS was 12.7 months (range, 10.5–22.2 months), with 6.6% (0–42.4%) survival rate at 3 years and 3.3% at 5 years (range, 0–6.6 years). Only Ramia et al. reported DFS, which was 10.7 months (7).
Table 6
| Study | Median survival (months) | 1-year survival (%)ǂ | 2-year survival (%)ǂ | 3-year survival (%)ǂ | 5-year survival (%)ǂ |
|---|---|---|---|---|---|
| Marichez et al. (6) | NR | NR | NR | NR | NR |
| Ramia et al. (7) | NR | NR | NR | NR | NR |
| Bachellier et al. (8) | 14.3 | NR | NR | NR | NR |
| Yang et al. (9) | 30 | 81.8 | 63.6 | 42.4 | NR |
| Zhang et al. (10) | NR | NR | NR | NR | NR |
| Miyazaki et al. (11) | 11 | 47.6 | NR | 6.6 | 6.6 |
| Amano et al. (12) | 10 | NR | NR | NR | NR |
| Wang et al. (13) | NR | 16 | 0 | 0 | 0 |
| Overall median (IQR) | 12.7 (10.5–22.2) | 47.6 (16–81.8) | 31.8 (0–63.6) | 6.6 (0–42.4) | 3.3 (0–6.6) |
ǂ, denotes where ranges were used as insufficient data was available to calculate IQR. NR, not reported; IQR, interquartile range.
Resection margins and histopathology
The resection margins were clear (R0) in a median of 75% of patients (IQR, 50–92.9%) (6-12). Only one study (12) reported macroscopically positive (R2) resection margins at a notable rate of 18.2% (12). Arterial involvement histologically was observed in a median of 57.9% of patients (IQR, 42.1–61.6%) (8,9,11,12). Histopathological tumour characteristics can be found in Table 7.
Table 7
| Study | R0 (%) | R1 (%) | R2 (%) | Tumour size (cm) | PAC (%) | Other (%) | Perineural invasion (%)ǂ | Venous invasion (%)ǂ |
|---|---|---|---|---|---|---|---|---|
| Marichez et al. (6) | 75 | 25 | 0 | 2.9 | 100 | 0 | NR | NR |
| Ramia et al. (7) | 58.8 | 41.2 | 0 | 5.1 | 88.2 | 11.8 | NR | NR |
| Bachellier et al. (8) | 50 | NR | NR | NR | 100 | 0 | NR | 74.5 |
| Yang et al. (9) | 92.9 | NR | NR | 3.5 | 100 | 0 | 64.3 | 28.6 |
| Zhang et al. (10) | 100 | 0 | 0 | NR | 90 | 10 | NR | NR |
| Miyazaki et al. (11) | 42.9 | 57.1 | 0 | 3 | NR | NR | 95.2 | NR |
| Amano et al. (12) | 77.3 | 4.5 | 18.2 | NR | NR | NR | 59.1 | 100 |
| Wang et al. (13) | NR | NR | NR | NR | 100 | 0 | NR | NR |
| Overall median (IQR) | 75 (50–92.9) | 25 (2.3–49.2) | 0 (0–9.1) | 3.3 (3–4.3) | 100 [90–100] | 0 (0–0) | 64.3 (59.1–95.2) | 74.5 (28.6–100) |
ǂ, denotes where ranges were used as insufficient data was available to calculate IQR. PAC, pancreatic adenocarcinoma; NR, not reported; IQR, interquartile range.
Discussion
Arterial involvement remains a challenging clinical presentation in pancreatic cancer. Neoadjuvant treatment with systemic chemotherapy with or without chemoradiation is the first indicated step in management as suggested by the international guidelines (2,4). Following neoadjuvant treatment, surgical resection has been shown to achieve oncological outcomes comparable to earlier stages of the disease and should be considered in selected cases (3,4). Nonetheless, due to the technical complexity and increased peri-operative risks, such cases are still not commonly performed even in tertiary pancreatic centres.
The technical approach to arterial involvement mainly includes two techniques: peri-adventitial dissection (also called arterial divestment) and arterial resection. The first describes the complete skeletonisation of the artery by dissection and eventually resection of the lymphoneural sheath that covers the adventitia. The technique is supported as a standard in pancreatic surgery, even in earlier stages of the disease by the American College of Surgeons (14) and can prevent the need for arterial resection in up to 49% of cases with arterial involvement on imaging (15,16). If peri-adventitial dissection is not possible then an arterial resection may be considered. However, this carries a substantial increase in peri-operative risk. Our review identified a 4.5% mortality (6-11) and 43.5% morbidity risk (6-12). This is comparable to the previously reported 90-day mortality rate of 5% and an overall morbidity of 52% (2). Furthermore, increasing centre and surgeon experience has shown to improve outcomes with 90-day mortality dropping from 8.8% to 4.8% (14). The 90-day mortality and 90-day morbidity rates identified in our review are comparable to head of pancreas tumour resections regardless of vascular involvement. Commonly quoted figures for 90-day mortality range from 4–7.4% in the literature (2,3,16-18). The vast majority of arterial reconstruction was via primary end to end anastomosis, which likely demonstrates that for most patients, malignancies with limited arterial involvement were considered for surgery. Whilst data on complications related to reconstruction was limited, the only patient that had a synthetic graft, died soon after from an arterial graft thrombosis (8).
With regards to the histopathological outcomes, R0 rate has been reported as low as 23% in one of the largest series recently published (15). In our review R0 rate was 75% which is comparable with the R0 rate of 66% reported by previous systematic review (2). This probably reflects the stricter patient and disease selection criteria used in the studies included, in contrast with the published experience from Heidelberg University Hospital (15), which included cases of advanced biological stage and aggressive behaviour, such as resections in the context of metastatic disease. Another possible explanation may be the lesser extent of local disease in studies of this review which can be inferred from the fact that adequate length of artery was present after the resection for a primary reconstruction in the majority of the cases. Equally importantly, there is controversy with regards to whether tumour involvement of the arteries corresponds to actual histopathological invasion of the arterial adventitia or the involvement is restricted in the peri-adventitial sheath. The latter is supported by clinical and macroscopic intra-operative observations, since dissection in the peri-adventitial plane can be successful in a substantial number of cases. However, even in these cases, the possibility of microscopic tumour cell invasion of the adventitia is still a possibility. Particularly, given that it has been histopathologically proved that pancreatic ductal adenocarcinoma (PDAC) cells grow in singletons and clusters in a distance from each other and notably in the periphery of the tumour (19). In our review, arterial invasion was only observed histologically in a median of 55.6% of patients (IQR, 39.8–61.6%) (7,8,10,11). However, there was little information about the definition of arterial invasion and whether cancer cells were histopathologically identified invading the layers of the arterial wall or just the peri-adventitial sheath. Nonetheless, similar arterial invasion findings were noted in the 2020 review by Haines et al. (2).
Surprisingly only about half of the patients (45%) received neoadjuvant treatment. This may indicate that the pre-operative staging did not accurately identify the arterial involvement or that the neoadjuvant approach was not in practice or favoured for arterial involvement in some of the reporting centres. One of the reasons for the latter may be the concern about the effects of chemotherapy in the liver and the potential risk for peri-operative liver ischaemia as reported in one included study (9). Liver ischaemia was reported from 0% (10) to 33% (11). Even though this is most likely related to the arterial resection and reconstruction, it is unclear from the current studies whether there is also a correlation with the use of neoadjuvant chemotherapy. Whilst most studies did not report their rationale for patients not receiving neoadjuvant chemotherapy, three of them suggested it was down to unit policy (7,9,10). The lack of adequate disease biology selection may explain the recorded median OS of 12.8 months, which is less that the expected OS with current palliative chemotherapy regimens, such as FOLFIRINOX and Gemcitabine/Abraxane (20,21). Interestingly, Miyazaki et al. found no statistically significant difference in survival between 9 patients who received neoadjuvant chemotherapy and 12 patients who didn’t (11). The rates for overall 3- and 5-year survival in this review remain low at 6.6% (range, 0–42.4%) and 3.3% (range, 0–6.6%) respectively (9,11,13), while in one study statistical difference in 5 year-survival (P=0.008) was identified between the arterial resection and the arterial palliation groups (13). Only one study reported DFS at 10.7 months (7).
Our study is limited by the heterogeneity and low numbers of patients across the studies included. The largest arterial resection cohorts and case series had to be excluded due to the pooling of the results across different tumour locations and stages. Whilst TPs were included in this study, if sufficient data was available, it would have been preferable to consider these cases separately due to the different peri-operative but also long-term risk profile, for example given no risk for post-pancreatectomy pancreatic leak/fistula but higher risk for long term complications from diabetes. Finally, as cohorts with fewer than 10 resections were excluded, low volume centers may be underrepresented in this study which may affect the generalisability of our findings and conclusions. Nonetheless, with a cohort of 170 patients, this is the largest systematic review of such cases published in the literature.
Conclusions
Arterial involvement is no longer considered a contraindication to surgical treatment for locally advanced pancreatic cancer of the head and uncinate process. Arterial resection can be performed with an acceptable peri-operative morbidity and 90-day mortality. However, oncological outcomes (OS and DFS) are still not convincing and future efforts should concentrate on patient and disease biology selection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-33/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-23-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882-93. [Crossref] [PubMed]
- Haines M, Chua TC, Jamieson NB, et al. Pancreatoduodenectomy With Arterial Resection for Locally Advanced Pancreatic Cancer of the Head: A Systematic Review. Pancreas 2020;49:621-8. [Crossref] [PubMed]
- Attard JA, Farrugia A, Pathanki A, et al. Treatment Strategies for the Optimal Management of Locally Advanced Pancreatic Adenocarcinoma With Curative Intent: A Systematic Review. Pancreas 2020;49:1264-75. [Crossref] [PubMed]
- Pancreatic Adenocarcinoma Guidelines [Internet]. NCCN. [cited 2023 January 23]. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Marichez A, Turrini O, Fernandez B, et al. Does pre-operative embolization of a replaced right hepatic artery before pancreaticoduodenectomy for pancreatic adenocarcinoma affect postoperative morbidity and R0 resection? A bi-centric French cohort study. HPB (Oxford) 2021;23:1683-91. [Crossref] [PubMed]
- Ramia JM, de Vicente E, Pardo F, et al. Spanish multicenter study of surgical resection of pancreatic tumors infiltrating the celiac axis: does the type of pancreatectomy affect results? Clin Transl Oncol 2021;23:318-24. [Crossref] [PubMed]
- Bachellier P, Addeo P, Faitot F, et al. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes?: A Single Institution’s Experience With 118 Patients. Ann Surg 2020;271:932-40. [Crossref] [PubMed]
- Yang F, Wang X, Jin C, et al. Pancreatectomy with Hepatic Artery Resection for Pancreatic Head Cancer. World J Surg 2019;43:2909-19. [Crossref] [PubMed]
- Zhang Q, Wu J, Tian Y, et al. Arterial resection and reconstruction in pancreatectomy: surgical technique and outcomes. BMC Surg 2019;19:141. [Crossref] [PubMed]
- Miyazaki M, Yoshitomi H, Takano S, et al. Combined hepatic arterial resection in pancreatic resections for locally advanced pancreatic cancer. Langenbecks Arch Surg 2017;402:447-56. [Crossref] [PubMed]
- Amano H, Miura F, Toyota N, et al. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg 2009;16:850-7. [Crossref] [PubMed]
- Wang C, Wu H, Xiong J, et al. Pancreaticoduodenectomy with vascular resection for local advanced pancreatic head cancer: a single center retrospective study. J Gastrointest Surg 2008;12:2183-90. [Crossref] [PubMed]
- American College of Surgeons. Operative standards for cancer surgery. Philadelphia: Lippincott Williams & Wilkins; 2023.
- Loos M, Kester T, Klaiber U, et al. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann Surg 2022;275:759-68. [Crossref] [PubMed]
- Butler JR, Ahmad SA, Katz MH, et al. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB (Oxford) 2016;18:305-11. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Strobel O, Hartwig W, Hackert T, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013;20:964-72. [Crossref] [PubMed]
- Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology 2011;59:1111-21. [Crossref] [PubMed]
- Ghaneh P, Palmer D, Cicconi S, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:157-68. [Crossref] [PubMed]
- Williet N, Saint A, Pointet AL, et al. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study. Therap Adv Gastroenterol 2019;12:1756284819878660. [Crossref] [PubMed]
Cite this article as: Dermanis AA, Halle-Smith J, Powell-Brett S, Roberts JK, Sutcliffe RP, Chatzizacharias N. Arterial resection during operative management of pancreatic head and uncinate process adenocarcinoma: a systematic review. Transl Gastroenterol Hepatol 2023;8:41.