
Esophageal carcinoma cuniculatum: a narrative review to understand this rare and commonly misdiagnosed variant of well-differentiated esophageal squamous cell carcinoma
Introduction
Carcinoma cuniculatum (CC) is a rare variant of a well-differentiated squamous cell carcinoma (SCC). CC was first described in the plantar skin by Aird et al. in 1954 as an unusual variant of a SCC of the skin in a 64-year-old man who presented with a forefoot swelling (1,2). This disease was initially considered a variant of a verrucous carcinoma (VC); however, the World Health Organization (WHO) has come to recognize it as a unique variant of a well-differentiated SCC (3,4). CC has a distinctive histomorphology characterized by burrowing channels lined by well-differentiated squamous epithelium (5,6). This hallmark has been described in cutaneous and non-cutaneous sites, such as the oral mucosa and the pharynx (7). However, only several cases involving the esophagus have been reported in the English literature starting in 2005 (2,3,8-15). Esophageal CC is often diagnosed at a locally advanced stage contributing to the patients’ overall morbidity (10). Unfortunately, the rarity of the disease and difficulty in the preoperative diagnosis has led to a delayed or missed diagnosis. Here, we review the available literature on esophageal CC to shed light on the epidemiology, clinicopathological features, diagnostic challenges, treatment, and prognosis. We present this article according to standardized guidelines set forth by the Narrative Review reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-37/rc).
Methods
Search strategies were developed for PubMed, Embase, Scopus, and Google Scholar databases from inception till date (Table 1). The search was conducted in March 2022 using controlled vocabulary and keywords (Table S1). An updated search was carried out in December 2022. Two independent researchers reviewed each article to ensure they met inclusion criteria and excluded duplicates. Reference of articles included were examined to capture articles missed in previous searches.
Table 1
| Items | Specification |
|---|---|
| Date of search | Inception to 1/20/2022; updated search on 12/12/2022 |
| Databases and other sources searched | PubMed, Embase, Scopus, and Google Scholar |
| Search terms used | Carcinoma AND Cuniculatum AND Esophagus (see Table S1 for additional search term used for the individual databases) |
| Timeframe | Inception till 12/12/2022 |
| Inclusion and exclusion criteria | Inclusion criteria: articles published in the English literature and human subjects |
| Exclusion criteria: articles published in languages other than English, non-human subjects, and other close variants of SCC not CC | |
| Selection process | Two independent reviewers conducted the initial database search using the defined search terms. Consensus was achieved in cases of disagreement by a third independent reviewer |
SCC, squamous cell carcinoma; CC, carcinoma cuniculatum.
Inclusion criteria: articles published in the English literature and containing human subjects were included. Abstracts of interests on esophageal CC were identified and full articles were obtained.
Exclusion criteria: articles published in languages other than English, non-human subjects, and other close variants of SCC and not CC were excluded.
Potential risk factors, clinical presentations, diagnostic evaluations, histopathological findings, treatment, and disease prognosis were evaluated and reported.
Search results
A total of 11 full articles on esophageal CC were identified. These included case reports, case series, and retrospective studies on esophageal CC. A total of 28 patient cases were identified. Table 2 contains a list of all publication on esophageal CC cases, patient characteristics, symptoms at presentation, endoscopic findings and staging, pathological staging, and mortality. The article by Yin et al. [2022] was included because it contained important sociodemographic information and pathological staging of disease (16) despite missing information on clinical presentation, potential risk factors, endoscopic and biopsy findings, diagnostic modalities, and patient outcomes. A decision was made to exclude the article by Landau et al. [2012] because it contained duplicate cases reported by Chen et al. in 2013 (Table 2) (7,8).
Table 2
| Author | Number of cases | Cases | Age/sex | Clinical presentation | Associated factors | CT/PET scan | EGD findings | Location | Biopsies | Endoscopic stage | Pre-surgical diagnosis/diagnosis | Surgery | Chemo/radiation | Final stage | Follow-up | Mortality | Recurrence/progression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yin et al., 2022 (16) | 5 | 1 | 58/F | – | – | – | – | – | – | – | – | Yes | – | ypT3N0 | – | – | – |
| 2 | 37/F | – | – | – | – | – | – | – | – | Yes | – | pT3N0 | – | – | – | ||
| 3 | 67/F | – | – | – | – | – | N/A (EMR) | – | – | – | – | N/A | – | – | – | ||
| 4 | 73/F | – | – | – | – | – | Yes | – | – | – | – | N/A | – | – | – | ||
| 5 | 67/M | – | – | – | – | – | Yes | – | – | – | – | N/A | – | – | – | ||
| Liu et al., 2020 (2) | 2 | 1 | 67/M | Dysphagia (solids and liquids) + weight loss | – | Esophageal thickening/avid mass | Villous, nodular partially obstructive mass | GEJ | Yes | T3N2Mx | Yes (endoscopic biopsies)/esophageal CC | Yes | Neoadjuvant chemotherapy + radiation | Surgery/ypT3N0Mx | 1 month | No | No |
| 2 | 62/M | Chest pan + dysphagia + weight loss | – | Esophageal thickening/avid mass | 5 cm partially obstructing + friable mass lesion | Distal esophagus | Yes | T3N1Mx | Yes (endoscopic biopsies)/esophageal CC | Yes | Neoadjuvant chemotherapy + radiation | Surgery/ypT3N0Mx | 36 months | No | No | ||
| Fatima et al., 2020 (14) | 1 | 1 | 86/M | Dysphagia to solids+ weight loss | Smoking (previous history) | – | Keratinized esophageal mucosa + papillary + filiform protrusion | Distal esophagus | – | – | Yes (EMR)/well differentiated SCC/CC | No | Treated with cryotherapy | – | – | – | – |
| Lai et al., 2019 (3) | 2 | 1 | 74/F | Dysphagia + weight loss | Smoking | Esophageal thickening+ stenosis/no lymphadenopathy | Pseudomembranous lesions + lumen stenosis | Distal esophagus | No | – | No/esophagitis dissecans superficialis | Yes | – | Surgery/T3N0 | 6 months | No | No |
| 2 | 46/M | Dysphagia + melena + severe anemia | Smoking | – | Stenotic lesion | Distal esophagus | Yes | – | Yes (endoscopic biopsies)/well differentiated SCC | Yes | No | Surgery/T4N0 | 9 months | No | No | ||
| Dick et al., 2018 (9) | 1 | 1 | 52/M | Dysphagia + chest discomfort | Reflux esophagitis + smoking | Esophageal wall thickening at GEJ + lymphadenopathy/partially obstructing esophageal cancer + lymphadenopathy | Papillary and nodular esophageal mass | GEJ | Yes | T4N3M0 | No/nonspecific (bland squamous epithelium + mild cytoplasmic atypia + granulomatous changes+ lymphocytosis) | Yes | No | Surgery/pT2N0 | 15 months | No | No |
| Koch et al., 2018 (12) | 1 | 1 | 68/M | Dysphagia + weight loss | – | Avid mass with no evidence of spread | Firm mass | GEJ | Yes | T3N0M0 | No/reactive changes (atypical squamous proliferation) | Yes | No | Surgery/No wall invasion/N0 | – | No | – |
| Goh et al., 2014 (13) | 1 | 1 | 72/M | Dysphagia + weight loss | Smoking | Tumor in esophagus | Irregular tumorous lesion | Distal esophagus | No | – | No/hyperplastic changes | No | – | – | 14 months | No | No |
| Coman et al., 2014 (15) | 1 | 1 | 65/F | – | – | Esophageal mass, no lymphadenopathy | Large circumferential partially obstructing mass | 22–30 cm | Yes | – | Yes (subsequent ESD)/esophageal CC | Yes | No | Surgery/T1bN0 | – | – | – |
| Chen et al., 2013** (7) | 11 | 1 | 48/F | Dysphagia + weight loss | – | – | Nodular mass | 16–28 cm | – | Wall invasion. Thickening of all layers | Yes (EMR)/esophageal CC | – | – | – | 19 months | No | – |
| 2 | 67/F | Dysphagia | – | – | Obstructing mass | 12–30 cm | – | N/A | Yes (EMR)/esophageal CC | – | – | – | 8 months | Yes (tracheal recurrence) | – | ||
| 3 | 63/M | Dysphagia + weight loss | Smoking (72 pack years) | – | Warty stenosis | Distal | Yes | N/A | Yes (endoscopic biopsies)/invasive SCC | Yes | – | –/Adventitia and lungs invasion/N0 | 0 | Yes (V fib) | – | ||
| 4 | 73/M | Change in bowel habits | – | – | Mass | Distal/GEJ | Yes | N/A | No/esophagitis with candida | Yes | – | –/adventitia/N0 | 214 months | Yes (–) | – | ||
| 5 | 40/M | Dysphagia + weight loss | Smoking (8.5 pack years) | – | Fond like mass | Distal | Yes | T1N0M0 | Yes (endoscopic biopsies)/invasive well differentiated carcinoma | Yes | – | Surgery/adventitia/N0 | 12 days | Yes (ARDS) | – | ||
| 6 | 46/M | Dysphagia + weight loss + chest pain | Smoking (50 pack years) | – | Fungating + ulcerating mass | Distal | Yes | T2N0M0 | No/ulcer with acute inflammatory changes and atypical squamous epithelium | Yes | – | Surgery/muscularis propria/N0 | 157 months | No | – | ||
| 7 | 62/M | Epigastric pain + regurgitation | Smoking (45 pack years) | – | Ulcerated mass | Distal EGJ | Yes | T3N1M0 | No/active esophagitis | Yes | – | Surgery/muscularis propria/N0 | 49 months | Yes (empyema + respiratory failure) | – | ||
| 8 | 44/M | Dysphagia + weight loss + GERD | Smoking (30 years) | – | Fungating and friable mass | Distal | Yes | T3N1 | No/fungal esophagitis/parakeratosis and atypical maturation changes | Yes | – | Surgery/adventitia/N0 | 87 months | No | – | ||
| 9 | 63/M | Dysphagia | Smoking (40 pack years) | – | Polypoid obstructing | Distal | Yes | T3N1M0 | No/fungal esophagitis | Yes | – | Surgery/muscularis propria/N0 | 68 months | Yes (metastatic lung cancer) | – | ||
| 10 | 48/F | Dysphagia + weight loss | Smoking (3 pack years) | – | Polypoid, ulcerating | 25 cm | Yes | N/A | Yes (endoscopic biopsies)/SCC | Yes | – | Surgery/muscularis propria/N0 | 84 months | No | – | ||
| 11 | 72/F | Dysphagia + odynophagia | – | – | Wary lesion | 25–27 cm | Yes | T1N0M0 | No/esophagitis + fungal organism present | Yes | – | Surgery/3 tumors mucosal ×2 and submucosal ×1/N0 | 48 months | No | – | ||
| De Petris et al., 2005 (11) | 2 | 1 | 73/M | Dysphagia + weight loss | Smoking (60 packs) + alcohol | Stricture, no adenopathy | Verrucous lesion + stricture + ulceration | Distal | Yes | Distorted architecture + diffuse hypoechoic thickening | No/– | Yes | – | Surgery/muscularis propria/N0 | 12 months | No | – |
| 2 | 58/M | Dysphagia + weight loss | Smoking | Mass at GEJ no adenopathy | Mass | GEJ | Yes | – | No/– | Yes | – | – | 7 months | No | – | ||
| Long et al., 2020 (10) | 1 | 1 | 63/F | Dysphagia + weight loss | GERD + smoking | Mass in distal esophagus and proximal stomach/no lymph node | Inflammatory and, fungating lesion | Lower third of esophagus + extending into cardia | Yes | T3N1M0 | Yes (EMR)/esophageal CC on multiple biopsies | No (declined/poor surgical candidate) | Chemotherapy + radiation | – | 17 months | Yes (progressive disease) | Yes |
**, article from Chen et al. [2013] contained 11 cases which included 9 cases from previously reported case-series by Landau et al. [2012] (7,8). CT, computed tomography; PET, positron emission tomography; EGD, esophagogastroduodenoscopy; F, female; M, male; N/A, not available; EMR, endoscopic mucosa resection; GEJ, gastroesophageal junction; CC, carcinoma cuniculatum; ESD, endoscopic submucosal dissection; SCC, squamous cell cancer; ARDS, acute respiratory distress syndrome; GERD, gastroesophageal reflux disease; N0, no lymph node involvement.
Epidemiology of esophageal CC
The most frequent CC site is the skin, particularly the soles of the feet. Occurrence of CC has also been reported in non-cutaneous sites such as the oral cavity (gingiva, tongue, buccal mucosa) (6,13,17-23), esophagus (8,11), larynx (24), and nails (25). CC involving the oral cavity has been reported in a broad age range (9–87 years). Esophageal involvement was uncommon: fewer than 30 cases have been reported in the English literature. Because of this disease’s rarity and the difficulty in the preoperative diagnosis, its exact incidence and risk factors remain relatively unknown.
Esophageal CC tends to be found predominantly in males with a male to female ratio of 3:1 (22). Chen et al. have reported that 63% (7 of 11) of the cases were in males (7). Similarly, a 2019 review by Lai et al. has indicated that 64% (11 of 17) of the cases were in males (3).
Esophageal CC has been reported over a wide age range. In an extensive review study, Lai et al. reported an age range of 40–77 years with a median age of 63 years at diagnosis (3). The oldest patient with esophageal CC reported in the English-language literature was in an 82-year-old man who presented with a 6-year history of dysphagia (14).
Pathogenesis of esophageal CC
Rare examples of cutaneous CC have been described earlier in longstanding neuropathic ulcers secondary to leprosy (26), plantar keratoderma (27) and necrobiosis lipoidica (28). However, the exact etiopathogenesis of esophageal CC remains unclear. Potential risk factors of esophageal CC are chronic reflux esophagitis, smoking, alcohol consumption, immunosuppression, and achalasia (8).
A history of reflux or previous use of reflux medications has been reported in approximately 53–73% of diagnosed cases (3,7). Chen et al. reported active esophagitis on endoscopic biopsies in 72.7% (8 of 11) of the examined cases. However, most of these cases were diagnosed as fungal esophagitis or associated changes. Despite a high prevalence of gastroesophageal reflux symptoms in patients with esophageal CC, the exact role of gastroesophageal reflux disease (GERD) in the etiopathogenesis of CC is unclear, as biopsies do not consistently show evidence of chronic esophageal injury (11).
Smoking appears to be a consistent risk factor in most cases (2,3); studies have reported the prevalence of smoking to be 64–67% among patients with esophageal CC (3,7). This may indicate that patients with esophageal CC with a substantial smoking history warrants a high vigilance regarding synchronous or metachronous SCC in the aerodigestive and respiratory tracts. Alcohol consumption has a relatively weaker association and has been reported at much lower rates in patients with esophageal CC (3,7).
A linkage between CC and human papillomavirus (HPV) type-11 has been suggested previously; this is believed to be site specific and has been reported only in cutaneous sites (29). Goh et al. have reported of no significant expression of p16 according to immunohistochemistry, thus arguing against high-risk HPV as an etiological factor (13). This finding is consistent with other reports of CC in extracutaneous sites that have not demonstrated any association with HPV (8,11). Landau et al. and De Petris et al. have analyzed numerous diagnosed cases through chromogenic in situ hybridization for HPV and immunostaining. These cases were negative for both low- and high-risk HPV (8,11), thus further refuting the involvement of HPV in the pathogenesis of esophageal CC.
Clinical and endoscopic findings in patients with esophageal CC
Dysphagia (either long-standing or progressive) appears to be the most common presentation of patients with esophageal CC (3,7,8). In a 2019 review of 15 cases by Lai et al., dysphagia was found in approximately 80% (12 of 15) of cases (3), similarly to previously reported rates (8).
GERD, particularly at the time of diagnosis, has been reported in 53–73% of cases (3,7,8). Weight loss has been reported in 66% (10 of 15) of diagnosed cases (3).
The preliminary modality for evaluating patients with an esophageal pathology is an esophagogastroduodenoscopy (EGD). Most cases of esophageal CC presented with a mass on EGD (7,12). In a review of 15 patients, Lai et al. have reported endoscopic growth patterns: stenosis or obstruction in approximately 40%, ulcerative patterns in approximately 33%, and warty patterns in 20% of diagnosed cases (3). The tumor appears to primarily affect the distal part of the esophagus (3,7). Lai et al. have reported that the distal esophagus was affected in 75% (10 of 15) of cases (3), and the gastroesophageal junction (GEJ) was affected in the remaining one-third (5 of 15). Chen et al. have reported similar frequencies in the distal esophagus and GEJ. However, they have also reported involvement of the cervical and/or mid-esophagus in approximately one-third (36%, 4 of 11) of examined cases (7).
Histological findings in esophageal CC
Histologically, CC is characterized by a diverse pattern of hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic micro-abscesses, focal cytologic atypia, koilocyte-like cells, and keratin-filled cysts/burrows (Figures 1,2) (3,7,8,11,13,15). Lai et al. have observed the presence of hyperkeratosis, dyskeratosis, acanthosis, cysts or burrows, and deep keratinization in 100% of the reviewed cases; furthermore, authors observed intraepithelial neutrophilic micro-abscesses in 93.3% of cases (3). In a similar review conducted by Landau et al., the presence of hyperkeratosis, acanthosis, dyskeratosis, abnormal keratinization, and intraepithelial neutrophils was noted in 100% of examined cases, while intraepithelial neutrophilic micro-abscesses occurred in 91% (10 of 11) of cases (8).

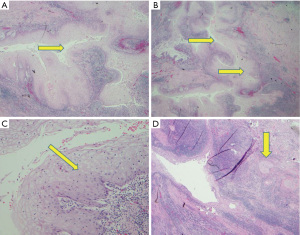
Preoperative diagnosis on the basis of mucosal biopsies obtained through EGD is highly challenging because of the bland cytology and the presence of inflammation (2,7-9). Presurgical diagnosis is difficult but can be suspected in endoscopic specimens if the biopsy obtained is sufficiently deep and adequately oriented (3), with a squamous proliferation devoid of cytological atypia and a non-aggressive pattern of invasion (3). Difficulties in presurgical diagnosis have often led to a misdiagnosis of esophageal CC as an active esophagitis, candida esophagitis, papilloma, esophageal Crohn’s disease, or an inconclusive diagnosis (7,8,13,15).
Prior studies have proposed a histologic scoring system to aid in the diagnosis with mucosal biopsies (2,7,8). Thirty-five esophageal mucosal biopsies from 25 upper endoscopies in 11 patients with a resection-validated diagnosis of esophageal CC have been evaluated and compared with 92 esophageal biopsies from 69 patients with a benign diagnosis (7). All biopsies were assessed for the presence of hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic micro-abscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cysts/burrows, which are the typical findings in patients with esophageal CC. Each feature, if present, was assigned 1 point, and final histologic score was calculated for each biopsy by adding the points. A cut off score of 7 for endoscopic mucosal biopsies from an esophageal mass greatly improved the diagnostic sensitivity to 57% and specificity to 100%. Early recognition of the histopathologic features can improve the pre-surgical diagnosis of esophageal CC (10). The use of this scoring system needs to be validated in other prospective studies.
Diagnosis of esophageal CC from endoscopic biopsies is difficult but possible (14). Several cases have been reported to be diagnosed in specimens through an endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), the latter of which has enabled successful tumor staging (2,7,10,15). However, the use of these techniques to either diagnose or stage disease has not been validated, and specimens obtained from EMR can be inconclusive and non-diagnostic (10). Long et al. have reported a case in which EMR was performed on an area of congested and inflamed mucosa with a fungating mass in the distal esophagus: initial histologic evaluation of a simple biopsy specimen revealed six of the characteristic features of esophageal CC, but findings were not sufficient for the diagnosis. A subsequent EMR, on the other hand, met the cutoff of 7, and the most distinguished characteristic feature was burrowing channels lined by well-differentiated squamous epithelium (10).
The common differential diagnoses for esophageal CC are VC and benign papilloma (11). Differentiating VC from CC has been suggested to be essential because of the local invasion and potentially faster growth of VC (11). VC usually presents as a T4 disease (7,8,30) in comparison to CC and is associated with a poor prognosis and a higher mortality (31,32). Squamous papilloma are rare benign lesions with endophytic patterns that are difficult to distinguish from the extremely well-differentiated squamous carcinomas (11). Another uncommon differential diagnosis suggested for esophageal CC with an endophytic growth pattern is esophageal intramural pseudo diverticulosis (8).
Treatments of esophageal CC
The preferred treatment for esophageal CC is surgical excision. Radical surgery is typically curative because of the aggressive nature of the tumor. Advanced local aggressiveness does not correspond to contextual systemic involvement (3). The role of perioperative endoscopic ultrasound (EUS) in staging remains unclear and may be limited. Landau et al. have reported inaccurate assessment of lymph node involvement and tumor depth in 50% (3 of 6) of cases. They suggested that stromal inflammation and mural fibrosis are likely to be responsible for over-staging of the disease (8). Endoscopic submucosal dissection has been examined as a therapeutic alternative to surgery but has shown limited success, owing to the severe submucosal fibrosis from tumor invasion encountered during the procedure (15). The roles of other ancillary treatment modalities such as radiotherapy and chemotherapy are not well defined, but these modalities have been used as primary treatments. Long et al. have reported an initial response to chemoradiation treatment in a poor surgical candidate with uT3N1M0 disease (10). However, disease progression occurred within 3 months, and death occurred at 15 months (10). Surgical excision of the tumor by esophagectomy provides long-term survival even in T3 tumors and continues to be the best therapeutic modality available for the treatment of esophageal CC.
Evolution and prognosis of esophageal CC
Patients with isolated CC usually have a favorable prognosis. Post-surgical resection outcomes in patients with esophageal CC appear to be generally better than those in patients with other types of esophageal cancer (3). Although the diagnostic route of CC can be arduous and exacerbated by surgical complications from deeply invasive tumors, the tentative prognosis appears to be quite favorable, on the basis of the 9 patient cases published by Landau et al. (8). Seven patients who received esophagectomy were followed for a median duration of 84 months and showed no disease recurrence, despite evidence of invasion of the muscularis propria. However, in all these cases, there were no lymph node involvement, suggesting that these tumors are indolent and do not metastasize, although can be locally aggressive (10). Two other case reports of patients with esophageal CC have shown no recurrence or metastasis after a 14 months follow-up (11,13). The role of neoadjuvant chemoradiation therapy prior to surgery remains unclear (2,10). Prognosis with treatment primarily involving chemoradiation, rather than surgical resection, is less certain (10).
Conclusions
Esophageal CC is a rare variant of a well-differentiated SCC associated with dysphagia and weight loss. It is more common in men than women and is associated with smoking. This condition is difficult to diagnose from endoscopic biopsies due to the nonspecific inflammatory and hyperkeratotic changes, which unfortunately leads to a delay or missed diagnosis. However, a semiquantitative method has been proposed based on specific histopathologic findings to enable a presurgical diagnosis from endoscopic biopsies. A high index of clinical suspicion for the disease is necessary along with close endoscopic follow-up with repeated biopsies for an early diagnosis and treatment. Histologic findings seen in esophageal CC include hyperkeratosis, acanthosis, dyskeratosis, deep keratinization, intraepithelial neutrophils, neutrophilic micro-abscess, focal cytologic atypia, koilocyte-like cells, and keratin-filled cyst/burrows. Surgery remains the gold standard for treatment and is associated with a favorable prognosis. The need for neoadjuvant chemoradiation is unclear and needs to be further studied.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-37/rc
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-37/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg 1954;42:245-50. [Crossref] [PubMed]
- Liu X, Yang D, Zhang X, et al. Esophageal Carcinoma Cuniculatum Diagnosed on Mucosal Biopsies Using a Semiquantitative Histologic Schema: Report of Two Esophagectomy-Confirmed Cases. Gastroenterology Res 2020;13:44-51. [Crossref] [PubMed]
- Lai Q, de Giacomo T, Consolo A, et al. Esophageal carcinoma cuniculatum: systematic review of the literature and report of two cases. Pathol Res Pract 2019;215:152602. [Crossref] [PubMed]
- Johnson NW, Franceschi S, Ferlay J, et al. Tumours of the Oral Cavity and Oropharynx. In: Barnes L, Eveson JW, Sidransky D, et al. editors. Pathology and genetics of head and neck tumours. Lyon: IARC Press, 2005:163-75.
- Barreto JE, Velazquez EF, Ayala E, et al. Carcinoma cuniculatum: a distinctive variant of penile squamous cell carcinoma: report of 7 cases. Am J Surg Pathol 2007;31:71-5. [Crossref] [PubMed]
- Yadav S, Bal M, Rane S, et al. Carcinoma Cuniculatum of the Oral Cavity: A Series of 6 Cases and Review of Literature. Head Neck Pathol 2022;16:213-23. [Crossref] [PubMed]
- Chen D, Goldblum JR, Landau M, et al. Semiquantitative histologic evaluation improves diagnosis of esophageal carcinoma cuniculatum on biopsy. Mod Pathol 2013;26:806-15. [Crossref] [PubMed]
- Landau M, Goldblum JR, DeRoche T, et al. Esophageal carcinoma cuniculatum: report of 9 cases. Am J Surg Pathol 2012;36:8-17. [Crossref] [PubMed]
- Dick TM, El Hag M, Mallery JS, et al. Esophageal Carcinoma Cuniculatum Associated with Non-Necrotizing Granulomatous Inflammation and Lymphadenopathy: Clinicopathologic Features and Diagnostic Challenges. Am J Case Rep 2018;19:790-5. [Crossref] [PubMed]
- Long DE, Al-Hader A, Emerson R, et al. A Case Report of an Early Response to Definitive Chemoradiation for Esophageal Carcinoma Cuniculatum. Case Rep Oncol Med 2020;2020:4674871. [Crossref] [PubMed]
- De Petris G, Lewin M, Shoji T. Carcinoma cuniculatum of the esophagus. Ann Diagn Pathol 2005;9:134-8. [Crossref] [PubMed]
- Koch LK, Templeton A, Westerhoff M. Carcinoma Cuniculatum: A Rare Cause of a Gastroesophageal Junction Mass. Clin Gastroenterol Hepatol 2018;16:A37-8. [Crossref] [PubMed]
- Goh GH, Venkateswaran K, Leow PC, et al. Carcinoma cuniculatum of the esophagus and tongue: report of two cases, including TP53 mutational analysis. Head Neck Pathol 2014;8:261-8. [Crossref] [PubMed]
- Fatima H, Wajid M, Cummings OW. A Rare Cause of Dysphagia. Gastroenterology 2020;159:e6-7. [Crossref] [PubMed]
- Coman RM, Collinsworth A, Draganov PV. Endoscopic submucosal dissection in a rare case of carcinoma cuniculatum of the esophagus initially misdiagnosed as benign squamous papilloma. Endoscopy 2014;46 Suppl 1 UCTN:E531-2.
- Yin F, Wang K, Hu M, et al. Deleterious mutations in esophageal carcinoma cuniculatum detected by next generation sequencing. Int J Clin Exp Pathol 2022;15:38-45. [PubMed]
- Sun Y, Kuyama K, Burkhardt A, et al. Clinicopathological evaluation of carcinoma cuniculatum: a variant of oral squamous cell carcinoma. J Oral Pathol Med 2012;41:303-8. [Crossref] [PubMed]
- Kahn JL, Blez P, Gasser B, et al. Carcinoma cuniculatum. Apropos of 4 cases with orofacial involvement. Rev Stomatol Chir Maxillofac 1991;92:27-33. [PubMed]
- Datar UV, Kale A, Mane D. Oral Carcinoma Cuniculatum: A New Entity in the Clinicopathological Spectrum of Oral Squamous Cell Carcinoma. J Clin Diagn Res 2017;11:ZD37-9. [Crossref] [PubMed]
- Baz S, Amer HW, Wahed AA. Oral carcinoma cuniculatum: an unacquainted entity with diagnostic challenges-a case report. J Egypt Natl Canc Inst 2022;34:3. [Crossref] [PubMed]
- Suzuki J, Hashimoto S, Watanabe K, et al. Carcinoma cuniculatum mimicking leukoplakia of the mandibular gingiva. Auris Nasus Larynx 2012;39:321-5. [Crossref] [PubMed]
- Thavaraj S, Cobb A, Kalavrezos N, et al. Carcinoma cuniculatum arising in the tongue. Head Neck Pathol 2012;6:130-4. [Crossref] [PubMed]
- Shakil M, Mohtesham I, Jose M, et al. Case Report Carcinoma Cuniculatum of the Oral Cavity–A Rare Entity. J Adv Med Dent Scie 2014;2:124-6.
- Puxeddu R, Cocco D, Parodo G, et al. Carcinoma cuniculatum of the larynx: a rare clinicopathological entity. J Laryngol Otol 2008;122:1118-23. [Crossref] [PubMed]
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology 1993;186:217-21. [Crossref] [PubMed]
- Ramadan WM, el-Aiat A, Hassan MH. Epithelioma cuniculatum in leprotic foot. Int J Lepr Other Mycobact Dis 1986;54:127-9. [PubMed]
- Affleck AG, Leach IH, Littlewood SM. Carcinoma cuniculatum arising in focal plantar keratoderma. Dermatol Surg 2007;33:745-8. [PubMed]
- Porneuf M, Monpoint S, Barnéon G, et al. Carcinoma cuniculatum arising in necrobiosis lipoidica. Ann Dermatol Venereol 1991;118:461-4. [PubMed]
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol 1989;29:33-7. [Crossref] [PubMed]
- Osborn NK, Keate RF, Trastek VF, et al. Verrucous carcinoma of the esophagus: clinicopathophysiologic features and treatment of a rare entity. Dig Dis Sci 2003;48:465-74. [Crossref] [PubMed]
- Di L, Wu X, Chen Z, et al. Early verrucous cell carcinoma of the esophagus: a case report and endoscopic and histologic features. BMC Gastroenterol 2021;21:466. [Crossref] [PubMed]
- Ramani C, Shah N, Nathan RS. Verrucous carcinoma of the esophagus: A case report and literature review. World J Clin Cases 2014;2:284-8. [Crossref] [PubMed]
Cite this article as: Enofe I, Venkataraj H, Hong P, Ding X, Haseeb A. Esophageal carcinoma cuniculatum: a narrative review to understand this rare and commonly misdiagnosed variant of well-differentiated esophageal squamous cell carcinoma. Transl Gastroenterol Hepatol 2023;8:20.


