Clinical outcomes: endoscopic resection of duodenal ampullary lesions
Highlight box
Key findings
• When considering lesions treated with APC, a significantly higher number displayed recurrence compared to residual lesion during the follow-up period.
• While the average follow-up time for pathology-proven adenomas was 14 months, time to recurrence was 31 months.
• Endoscopic success was observed in approximately 70% of patients.
What is known and what is new?
• Endoscopic papillectomy is a widely accepted technique for the management of duodenal adenomas.
• This manuscript calls special attention to surveillance periods, and how they may need adjustment depending on a patient’s risk for recurrence.
What is the implication, and what should change now?
• Future studies should investigate outcomes in patients treated with APC and determine if they require longer surveillance periods.
• Pathology-proven adenomas would likely benefit from a surveillance period of at least 31 months.
Introduction
Ampullary adenomas are lesions found at the major papilla of the duodenum. They are commonly associated with familial adenomatous polyposis (FAP) but also occur sporadically. Sporadic adenomas are often asymptomatic and diagnosed incidentally, most commonly among patients aged 60–80. When symptomatic, ampullary adenomas may cause obstructive jaundice, pancreatitis, and hemorrhage (1). Sporadic adenomas carry a 30–50% risk for malignant conversion, and patients with hereditary adenomas have a 3–5% lifetime risk for adenocarcinoma (1-3). Historically, ampullary adenomas were removed surgically by either transduodenal local resection or pancreaticoduodenectomy. Since surgical resection carries a risk for adverse events and morbidity, endoscopic resection has emerged as a preferred method of resection (4).
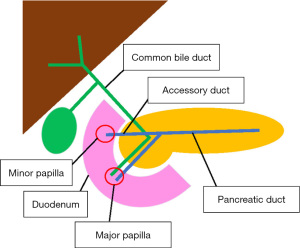
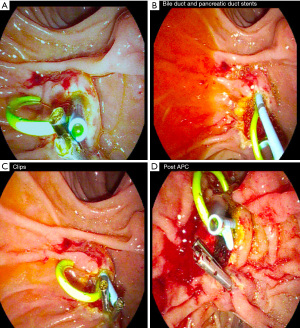
Endoscopic papillectomy, sometimes referred to as ampullectomy, was first described in 1983 by Suzuki et al. as an alternative to surgical resection, offering improved morbidity and mortality and success rates exceeding 80% in complete resection cases (4-6). Often solitary and sessile, sporadic ampullary lesions are removed endoscopically by snare excision, resecting the mucosa of the duodenal wall around the major papilla. This includes the tissue surrounding the orifices of the bile and pancreatic ducts (PD) (Figure 1) (6,7). Following endoscopic resection, close surveillance is important to monitor for recurrence (4). Though less invasive than surgical resection, endoscopic papillectomy confers potential complications, including hemorrhage, perforation, and pancreatitis, with an overall adverse event rate of 8% to 31% (1).
Most of the literature on management of duodenal adenomas are small single-center retrospective reviews. Clinical interest continues to grow, and multiple systematic reviews have been published in recent years to offer a more comprehensive understanding of duodenal adenomas (8,9). The first ampullary adenoma management guideline was published by American Society for Gastrointestinal Endoscopy (ASGE) in 2015, which was followed by one from the European Society of Gastrointestinal Endoscopy (ESGE) in 2021, highlighting continued interest in the topic (10,11). Significant updates to the recommendations proposed by ESGE include elimination of submucosal injection use and implementation of a post-papillectomy surveillance period for at least 5 years. However, it is important to recognize that all of the recommendations made by ASGE and ESGE are based on low to moderate quality evidence. Thus, further studies are required to investigate current recommendations.
The objective of this study is to describe endoscopic papillectomy outcomes to further refine management guidelines. We paid special attention to complications, recurrence, and surveillance periods in the setting of adjunctive therapies, such as the use of submucosal injection, argon plasma coagulation (APC), and prophylactic PD stents in management of both pathology-proven adenomas and all adenomatous lesions treated with papillectomy. We present the following article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-87/rc).
Methods
Subjects
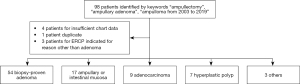
This was a retrospective study of 90 patients with suspected ampullary adenoma referred for endoscopic papillectomy to a single tertiary center from 2003 to 2019. Using our database from the endoscopy software platform EndoPro, 98 patients were initially identified using keywords “ampullectomy”, “ampullary adenoma”, and “ampulloma”. We included patients with pathology-proven adenoma, adenocarcinoma, and hyperplastic polyp. Patients were excluded from the study if no record was available in the electronic medical record or if they underwent endoscopic retrograde cholangiopancreatography (ERCP) for indication other than papillectomy (Figure 2).
The study (#2024) was approved by the MedStar Health Research Institute’s Institutional Review Board (IRB) (No. IRB00011931). All patient information was obtained from the electronic medical record. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). A waiver for informed consent was obtained from the IRB, as this was a retrospective study and obtaining consent from all subjects was deemed impractical given the number of subjects included and the time frame of their procedures.
Endoscopic technique and management
Papillectomy was performed via snare loop excision during ERCP (Figure 3). Hot snare polypectomy was employed with a blended cut using ERBE VIO 300D electrosurgical unit (EndoCut Q, Effect 2, Cut duration 1, cut interval 4). Adjunctive submucosal injection with saline or methylene blue was utilized for some lesions to facilitate en bloc resection in earlier years; however, it was used less frequently over the period of the study. APC after resection was employed if adenomatous appearing tissue remained following papillectomy. PD stenting was attempted in all patients to prevent pancreatitis, with stent removal scheduled at the next follow-up. En bloc resection was attempted in all patients. Piecemeal resection was done if en bloc resection was not possible. Endoscopic submucosal dissection is not conducted at this institution and was not used in the treatment of any of the patients in this study. Biliary sphincterotomy was attempted in all patients to allow easier access to the bile duct for interventions and allow for more complete excision in the case of intraductal involvement. Pancreatic sphincterotomy is not routinely practiced at this institution. All procedures were performed by two board-certified gastroenterologists with combined 34 years of post-fellowship experience. Endoscopic follow-up was recommended within one to three months of procedure.
All specimens were sent for pathologic evaluation. Follow-up endoscopic papillectomy was scheduled in the event of incomplete excision noted endoscopically, positive margins on pathology or tumor recurrence on first follow up. In some cases, aggressive or malignant characteristics on pathology warranted surgical referral.
Chart review methodology
Data from the remaining 90 patients were extracted by chart review. Pre-operative symptoms, management, operative notes, pathology results, and postoperative course were documented. Patient-specific data included age, gender, comorbidities, and history of FAP were collected. Indication, associated symptoms, and prior imaging such as computed tomography, magnetic resonance imaging, or endoscopic ultrasound were also assessed. From the procedure notes, endoscopic impression, lateral spread, evidence of malignancy, flat-appearing lesions, adenoma size, method of resection, use of submucosal injection, use of rectal indomethacin, and PD or bile duct stenting were obtained. If adenoma size was not explicitly mentioned in the procedure note, the size was estimated using the greatest dimension measured in the pathology report. Papillectomy pathology reports were compared with prior outside referral biopsy reports, making note if the prior report under- or overestimated the papillectomy specimen. The need for surgical intervention such as pancreaticoduodenectomy was also included. If the patient required surgery, the surgical pathology report was compared to the papillectomy pathology report. In the postoperative period, the number of follow-ups, instances of tumor recurrence, and post-operative complications including pancreatitis, bleeding, perforation, or post-papillectomy stenosis were noted.
Statistical analysis
Papillectomy data were stratified by sporadic adenomas, recurrence, residual tumor, post-operative pancreatitis, and presence of symptoms for all lesions and pathology-proven adenomas separately. Missing values were excluded from the analysis. Patients lost to follow-up were removed from analysis. Summary statistics included frequency with percentage for categorical variables, mean with standard deviation for normal continuous variables and median with second and third quartile [interquartile range (IQR)] for non-normal continuous variables. The Shapiro test was used to test normality. Chi-squared tests, Kruskal-Wallis rank-sum tests, and t-tests were performed to compare differences between groups.
Patients with pathologies described as “tubulovillous adenoma with high grade dysplasia”, “adenoma with focal hyperplastic change”, “villous adenoma”, “tubular adenoma”, “tubulovillous adenoma”, “ampullary adenoma” were then analyzed separately. Data were further stratified by “submucosal injection”, “APC”, “Complete resection”, and “PD stent”. The groups were compared using t-tests and Kruskal-Wallis rank-sum tests.
Recurrence was analyzed via a survival analysis. The longest follow-up from papillectomy in months was used as the time to event for patients without recurrence. All analyses were conducted with R and SPSS software. Statistical significance was set at P values less than 0.05.
Definitions
“Complete resection” was defined as complete excision of the lesion without evidence of remaining lesion. “Endoscopic success” was defined as resection that did not yield residual lesion or recurrent lesion on follow-up endoscopy that required referral for surgical management. Lesions that were managed endoscopically, or without surgery, were considered successful. “Endoscopic failure” was defined as the presence of recurrent lesion or any lesion requiring referral for surgical management. “Residual” was defined as presence of lesion at the first follow-up endoscopy. “Recurrence” was defined as presence of lesion after initial negative results on a previous follow-up endoscopy.
Results
Patient demographics and tumor characteristics
A total of 98 patients were referred to MedStar Georgetown University Hospital for endoscopic papillectomy of suspected adenomas during the 16-year study period. Eight patients were excluded from the study, because four did not have data pertaining to their lesion available in the chart, three patients underwent ERCP for indication other than ampullary adenoma resection, and one patient was a duplicate. A total of 90 patients were included for analysis. After review of the pathology, 60% of the resected samples (54 of 90) were found to be adenoma, while 18.9% (17 of 90) were ampullary or intestinal mucosa, 10% were adenocarcinoma (9 of 90), 7.8% (7 of 90) were hyperplastic polyp, and 3.3% were other lesions (3 of 90). The median age of patients was 60 years (range, 25–93 years). 51.1% of patients were male (46 of 90). 32.2% of adenomas caused patients to be symptomatic (29 of 90), while the remainder were incidental. Among all lesions, 13.3% of patients had FAP (12 of 90). Of pathology-proven adenomas, 18.5% of patients had FAP (10 of 54). The average size of all resected masses was 13.5 mm. The average size of all pathology-proven adenomas was 15.3 mm. Sessile morphology was observed in 16.7% of all lesions (15 of 90) and 18.5% of adenomas (10 of 54).
For all lesions that had a prior biopsy (65 of 90), 24.6% overestimated the pathology obtained by papillectomy (16 of 65) and 21.5% underestimated the pathology obtained by papillectomy (14 of 65). For all pathology-proven adenomas that had a prior biopsy (36 of 54), 16.7% overestimated the pathology obtained by papillectomy (6 of 36) and 19.4% underestimated the pathology obtained by papillectomy (7 of 36) (Table 1).
Table 1
| Characteristic | All lesions, N=90 (%) | Pathology-proven adenomas, N=54 (%) |
|---|---|---|
| Age (years, mean) | 58.9 (range, 25–93) | 58.8 (range, 25–89) |
| Gender | ||
| Male | 46 (51.1) | 27 (50.0) |
| Female | 44 (48.9) | 27 (50.0) |
| Race | ||
| White | 49 (54.4) | 11 (20.4) |
| Black | 20 (22.2) | 14 (25.9) |
| Other | 21 (23.3) | 29 (53.7) |
| Comorbidities | ||
| Cardiovascular disease | 41 (45.6) | 12 (22.2) |
| Diabetes | 15 (16.7) | 12 (22.2) |
| Malignancy | 11 (12.2) | 3 (5.6) |
| Pulmonary disease | 8 (8.9) | 6 (11.1) |
| Familial adenomatous polyposis | 12 (13.3) | 10 (18.5) |
| Symptoms | ||
| Abdominal pain | 19 (21.1) | 5 (9.3) |
| Pancreatitis | 4 (4.4) | 3 (5.6) |
| Weight loss | 4 (4.4) | 1 (1.9) |
| Nausea | 3 (3.3) | 2 (3.7) |
| Jaundice | 3 (3.3) | 1 (1.9) |
| Lesion size, largest dimension (mm, mean) | 13.5 (range, 4–46) | 15.3 (range, 5–46) |
| Pathology | ||
| Adenoma | 54 (60.0) | 54 (100.0) |
| Ampullary/intestinal mucosa | 17 (18.9) | 0 |
| Adenocarcinoma | 9 (10.0) | 0 |
| Hyperplastic polyp | 7 (7.8) | 0 |
| Other | 3 (3.3) | 0 |
| Sessile morphology | 15 (16.7) | 10 (18.5) |
| Biopsy concordance | n=65 | n=36 |
| Biopsy overestimated papillectomy | 16 (24.6) | 6 (16.7) |
| Biopsy underestimated papillectomy | 14 (21.5) | 7 (19.4) |
Endoscopic papillectomy
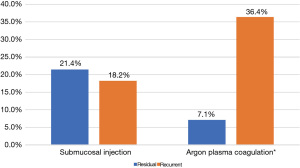
14.4% of all lesions (13 of 90) and 18.5% of adenomas (10 of 54) were treated with adjunctive APC. Among all patients who were treated with APC and had follow-up data available, 36.4% developed recurrence (4 of 11) and 7.1% developed residual lesions (1 of 14) (P=0.019) (Figure 4). Among adenomas, complete resection was achieved in 90% of patients treated with adjunctive APC (9 of 10) vs. 75% of those without APC (33 of 44) (P=0.426).
10% of all lesions (9 of 90) and 9.3% of adenomas (5 of 54) had submucosal injection prior to resection. Among all patients who developed recurrence, 18.2% were treated with submucosal injection (7 of 11) compared to the 21.4% of those who developed residual lesion (3 of 14) (P=0.795) (Figure 4). Among adenomas, complete resection was achieved in 60% of those treated with submucosal injection (3 of 5) and 79.6% of those without submucosal injection (39 of 49) (P=0.306).
Among the patients whose notes documented completeness of resection, 83.9% of all lesions (47 of 56) and 90.9% of adenomas (29 of 33) achieved complete resection. When comparing recurrent lesions with non-recurrent lesions, data on 61 patients were available, because 12 patients were lost to follow-up and 17 had residual lesions. 66.7% of recurrent lesions (10 of 15) were completely resected at index procedure vs. 82.6% of non-recurrent lesions (38 of 46) (P=0.344).
PD stents were deployed in 85.6% of all lesions (77 of 90) and 87% of adenomas (47 of 54) (Figure 3B).
Complications
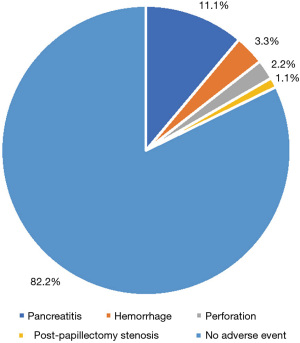
15.6% of patients with all lesions (14 of 90) reported complications, including 11.1% pancreatitis (10 of 90), 3.3% hemorrhage (3 of 90), 2.2% perforation (2 of 90), and 1.1% post-papillectomy stenosis (1 of 90). Among patients with adenomas, 18.5% reported adverse outcomes (10 of 54), including 5.6% pancreatitis (3 of 54), 3.7% hemorrhage (2 of 54), 1.9% perforation (1 of 54), and 1.9% post-papillectomy stenosis (1 of 54) (Figure 5). 83.8% of patients who had PD stents placed did not develop pancreatitis (67 of 80) vs. 100% of patients who had PD stents placed that did develop pancreatitis (10 of 10) (P=0.368).
Follow-up and recurrence
Residual lesions were observed in 18.9% for all lesions (17 of 90) and 24.1% for adenomas (13 of 54). Recurrent lesions were observed in 16.7% for all lesions (15 of 90) and 20.4% for adenomas (11 of 54).
The median follow-up time was 8 months for all lesions (range, 1–177 months) and 14 months for adenomas (range, 1–177 months). Median time to recurrence was 30 for all lesions and 31 for adenomas (range, 3–137 months). Median follow up time was 58 months for FAP lesions (range, 1–177 months) with median time to recurrence 25 months (range, 3–69 months).
Twelve patients were lost to follow-up, 5 of which had adenomas; 10 lesions required surgery and 15 developed recurrences. Regarding adenomas specifically, 4 required surgery and 11 developed recurrences. Overall, there were 24 endoscopic failures, as one patient with recurrence also required surgery. After removing patients lost to follow-up, endoscopic success was achieved in 69.2% of all lesions (54 of 78) and 71.4% of adenomas (35 of 49).
Discussion
We chose to include all 90 masses referred for papillectomy, including pathology-proven adenomas, because pathology is only confirmed after the procedure, and all these masses are affected by initial endoscopic intervention. Prior studies have examined adenomas as well as ampullary lesions, which include other pathologies (12-15). FAP was observed in 13.3% of all lesions and 18.5% of adenomas. This is consistent with prior studies, observing FAP in 13.8% to 30% of duodenal adenoma cohorts (16-18).
Among our patients with pathology-confirmed adenoma, the average age was 59 years (range, 25–89 years), which is consistent with the literature (10,11). We observed a smaller average polyp size, at 15.3 mm (range, 5–46 mm) compared to the 20 mm average size previously reported (16). This may reflect increased awareness and early detection by the referring providers.
Collecting biopsies prior to papillectomy is a valuable method of determining appropriate management for patients. The ASGE recommends obtaining biopsy specimens from all ampullary lesions suspicious for neoplasia prior to proceeding with endoscopic resection (10). We found that 53.9% of the prior biopsies correlated with final pathology from papillectomy. This is consistent with previously reported biopsy concordance rates of approximately 40–60% (3,12,19). Moreover, in our study, 21.5% of prior biopsies underestimated the papillectomy specimen. Similarly, the literature reports missed adenocarcinoma diagnoses in 30% of forceps biopsies (20). The unpredictable nature of biopsy results emphasizes the importance of thorough examination and the importance of complete removal for accurate histopathologic examination, which is supported by expert opinion (21).
The technique of endoscopic resection varies and provides an opportunity for clinicians to maximize benefit and minimize harm to patients. En bloc excision with adequate identification of tumor margins is preferable to piecemeal resection. In larger lesions, some providers may prefer submucosal injection with epinephrine or methylene blue to reduce bleeding and enhance tumor margin visualization, though this technique has largely fallen out of favor (11). In our group with 54 adenomas, 9.2% underwent submucosal injection prior to papillectomy. We observed complete resection in 60% of submucosal injection papillectomies vs. 79.6% of simple snare papillectomies (P=0.306). In a prospective multi-center study of 50 patients with pathology-proven adenomas, 50% of submucosal injection papillectomies resulted in complete resection compared to 80.8% of simple snare papillectomies but showed no difference in tumor persistence at one month and recurrence at 12 months (22). There was also no difference in adverse effects. Our findings in conjunction with the literature indicate that there is no benefit in utilizing submucosal injection, supporting the trend among clinicians to move away from this technique.
APC is another adjunctive technique used to treat the remaining tissue after resection, with goal of reducing risk for residual and recurrent adenomas. In addition, APC is also used to treat bleeding following resection. When examining the use of APC, we found that 36.4% had recurrence while 7.1% had residual lesion (P=0.019). This finding supports the use of APC in the prevention of residual adenoma. Similarly, Catalano et al. showed that the use of APC significantly reduced the risk for persistent tumor but did not find differences in either success rates or recurrence in patients treated with APC (17). It is not immediately clear what these results represent. One possible explanation is that proper visualization of residual tumor on initial follow-up may be difficult to achieve in a patient who were treated with APC at the time of endoscopy. In other words, coagulation of the superficial mucosa may obscure residual adenoma lying deep to the area of coagulation. The observed difference between the two groups may also be a result of confounding, where patients who are predisposed to recurrent lesions are more likely to be treated with APC due to a large lesion size or lesions that necessitate piecemeal resection. Nonetheless, we believe that patients treated with APC warrant careful consideration when clinicians are determining surveillance plans, as recurrence may occur later than expected, especially in patients without evidence of residual tumor during follow-up examinations.
Determining the appropriate surveillance period is crucial for monitoring patients post-procedure for residual tumor and recurrence. We observed a median follow-up time of 8 months among all lesions and 14 months among pathology-proven adenomas, with the median time to recurrence being 30 months in all lesions and 31 months in pathology-proven adenomas. This raises the question of whether patients without recurrence are truly without recurrence of simply have not been monitored for long enough. Contrary to our findings, Kang et al. found that after monitoring patients for a median follow-up time of 44.2 months, recurrence is most likely to occur within the first six months of the procedure (12). Several other similar studies demonstrate average follow-up time of 9 to 48 months and time to recurrence of approximately 9 to 36 months (13,16,18). One study even identified recurrence up to 65 months from papillectomy, proposing indefinite surveillance in select patients (23). Our findings suggest that longer follow-up would be beneficial.
Anticipating adverse outcomes can guide clinicians when making management decisions. Of our cohort, post-papillectomy adverse effects were seen in 18.5% of pathology-proven adenomas compared to 15.6% of all lesions. While adverse outcome rates vary across institutions, our findings are comparable to that of other studies that report a range from 9.7% to 24.9% (9,17). In a retrospective single center study of 87 patients with adenoma, complications were observed in 25.3%, predominantly hemorrhage, followed by perforation, and papillary stenosis (18). In our cohort, pancreatitis was the most common complication, followed by hemorrhage, perforation, and stenosis. According to other studies, pancreatitis rates range from 3.4% to 20%, which is consistent with our findings of 11.1% in all lesions and 5.6% in adenomas (18,22). Papillary stenosis has been observed in 2.4%, which is also consistent with our findings of 1.1% in all lesions and 1.9% in adenomas (9).
Prophylactic PD stent placement is accepted as standard of care and is attempted in all patients at our institution. The practice was found to be protective in a small single-center randomized controlled trial (RCT) of 19 patients, with 33% of the stented group and zero of the unstented group developing pancreatitis (24). Similarly, in a meta-analysis of RCTs, Wang et al. suggested that prophylactic PD stent placement reduced the odds of developing pancreatitis (25). By contrast, all 10 patients in our cohort who developed pancreatitis also had PD stents placed. While it is most likely that our findings are coincidental, we acknowledge the results from a single-center retrospective study that suggested pancreatitis may occur in patients who are treated with a PD stent due to foreign body reaction or inflammation following papillectomy (26). Other factors may also affect the outcome, including use of lactated ringers and rectal indomethacin in the prevention of post-operative pancreatitis. Furthermore, use of pure coagulation is said to increase the risk for pancreatitis, due to resulting edema.
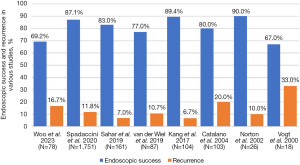
Endoscopic success is an essential measurement of the overall utility of the procedure. Among all lesions, our overall endoscopic success rate was 69.2% for all lesions and 71.4% among adenomas, which is slightly lower than prior studies that largely report a range from 75% to 90%, though Vogt et al. described an endoscopic success rate of 67% (Figure 6) (9,12,14,18,27). Recurrence has been reported in 7% to 39% of adenomas post-papillectomy (16,27). We observed recurrence in 16.7% of all lesions and 20.4% of adenomas, with significantly higher rates of recurrence among FAP-associated lesions than sporadic (P=0.031). We identified a longer time to recurrence, 31 months for pathology-proven adenomas, than most previously noted in the literature, ranging from 6 to 13 months, which suggests that surveillance times employed by other studies may be insufficient, thus contributing to the discrepancy in recurrence and endoscopic success rates (11,12). While FAP-associated lesions tended to have shorter times to recurrence than sporadic, the difference was not significant. Finally, we recognize these discrepancies may also be affected by variability in definitions used by authors. For example, some authors view ‘residual’ and ‘recurrence’ to be the same, which may increase recurrence rate from what we would expect using our definitions (28). There is also inconsistency across publications regarding types of lesions investigated, ranging from only pathology-proven adenomas to all ampullary lesions. For this reason, for future studies we encourage standardized use, or at least the inclusion of clear definitions, of terminology.
Interestingly, 26.7% of our cohort’s specimen was neither adenoma nor adenocarcinoma according to pathology, which is higher than most other studies, but very similar to the 26.1% reported by Kang et al. (12) We believe papillectomy may have been deemed clinically indicated due to the presence of the mass and in some cases symptoms. We acknowledge the potential for suboptimal detection by the operator, though this is unlikely given their extensive experience.
Conclusions
Endoscopic papillectomy is an effective method for managing duodenal adenomas, with an endoscopic success rate of approximately 70% and an adverse effect rate of less than 20%. We observed lower success rates of endoscopic resection of ampullary adenomas compared to rates reported in the literature. A multitude of different factors may have contributed to this finding, including differences in adenoma resection methods and surveillance times.
While our data suggest a trend between APC use and minimization of residual adenoma, the same cannot be said for recurrent tumor. As such, patients treated with APC may require closer follow up for longer periods of time, given the risk for recurrence. Our more cautious approach agrees with the ESGE recommendation to reserve APC for use on an individualized basis, due to inconclusive data. Finally, for all pathology-proven adenomas, we advocate for a surveillance period of at least 31 months, which exceeds the two-year surveillance period referenced by the ASGE and favors the 5-year recommendation made by the ESGE.
Though our study is limited by the small sample size and the single-center, retrospective design, our findings offer another perspective on an emerging topic. Future studies should focus on the success rates of different endoscopy resection methods, surveillance periods, and the use of risk-benefit analysis when employing adjunctive therapies.
Acknowledgments
We thank Dr. David B. Doman for his guidance in the development of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-87/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-87/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-87/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study (#2024) was approved by the MedStar Health Research Institute’s Institutional Review Board (IRB) (No. IRB00011931). All patient information was obtained from the electronic medical record. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). A waiver for informed consent was obtained from the IRB, as this was a retrospective study and obtaining consent from all subjects was deemed impractical given the number of subjects included and the time frame of their procedures.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol 2016;22:600-17. [Crossref] [PubMed]
- Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol 1992;87:37-42. [PubMed]
- Laleman W, Verreth A, Topal B, et al. Endoscopic resection of ampullary lesions: a single-center 8-year retrospective cohort study of 91 patients with long-term follow-up. Surg Endosc 2013;27:3865-76. [Crossref] [PubMed]
- Onkendi EO, Naik ND, Rosedahl JK, et al. Adenomas of the ampulla of Vater: a comparison of outcomes of operative and endoscopic resections. J Gastrointest Surg 2014;18:1588-96. [Crossref] [PubMed]
- Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endoscopic excision (in Japanese with English abstract). Prog Dig Endosc 1983;23:236-9.
- Binmoeller KF, Boaventura S, Ramsperger K, et al. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc 1993;39:127-31. [Crossref] [PubMed]
- De Palma GD, Luglio G, Maione F, et al. Endoscopic snare papillectomy: a single institutional experience of a standardized technique. A retrospective cohort study. Int J Surg 2015;13:180-3. [Crossref] [PubMed]
- Heise C, Abou Ali E, Hasenclever D, et al. Systematic Review with Meta-Analysis: Endoscopic and Surgical Resection for Ampullary Lesions. J Clin Med 2020;9:3622. [Crossref] [PubMed]
- Spadaccini M, Fugazza A, Frazzoni L, et al. Endoscopic papillectomy for neoplastic ampullary lesions: A systematic review with pooled analysis. United European Gastroenterol J 2020;8:44-51. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 2015;82:773-81. [Crossref] [PubMed]
- Vanbiervliet G, Strijker M, Arvanitakis M, et al. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021;53:429-48. [Crossref] [PubMed]
- Kang SH, Kim KH, Kim TN, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol 2017;17:69. [Crossref] [PubMed]
- Attila T, Parlak E, Alper E, et al. Endoscopic papillectomy of benign ampullary lesions: Outcomes from a multicenter study. Turk J Gastroenterol 2018;29:325-34. [Crossref] [PubMed]
- Norton ID, Gostout CJ, Baron TH, et al. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc 2002;56:239-43. [Crossref] [PubMed]
- Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc 2004;60:757-64. [Crossref] [PubMed]
- Sahar N, Krishnamoorthi R, Kozarek RA, et al. Long-Term Outcomes of Endoscopic Papillectomy for Ampullary Adenomas. Dig Dis Sci 2020;65:260-8. [Crossref] [PubMed]
- Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc 2004;59:225-32. [Crossref] [PubMed]
- van der Wiel SE, Poley JW, Koch AD, et al. Endoscopic resection of advanced ampullary adenomas: a single-center 14-year retrospective cohort study. Surg Endosc 2019;33:1180-8. [Crossref] [PubMed]
- Choi SJ, Lee HS, Kim J, et al. Clinical outcomes of endoscopic papillectomy of ampullary adenoma: A multi-center study. World J Gastroenterol 2022;28:1845-59. [Crossref] [PubMed]
- Espinel J, Pinedo E, Ojeda V, et al. Endoscopic ampullectomy: a technical review. Rev Esp Enferm Dig 2016;108:271-8. [Crossref] [PubMed]
- Fritzsche JA, Klein A, Beekman MJ, et al. Endoscopic papillectomy; a retrospective international multicenter cohort study with long-term follow-up. Surg Endosc 2021;35:6259-67. [Crossref] [PubMed]
- Hyun JJ, Lee TH, Park JS, et al. A prospective multicenter study of submucosal injection to improve endoscopic snare papillectomy for ampullary adenoma. Gastrointest Endosc 2017;85:746-55. [Crossref] [PubMed]
- Ridtitid W, Schmidt SE, Al-Haddad MA, et al. Performance characteristics of EUS for locoregional evaluation of ampullary lesions. Gastrointest Endosc 2015;81:380-8. [Crossref] [PubMed]
- Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc 2005;62:367-70. [Crossref] [PubMed]
- Wang Y, Qi M, Hao Y, et al. The efficacy of prophylactic pancreatic stents against complications of post-endoscopic papillectomy or endoscopic ampullectomy: a systematic review and meta-analysis. Therap Adv Gastroenterol 2019;12:1756284819855342. [Crossref] [PubMed]
- Chang WI, Min YW, Yun HS, et al. Prophylactic pancreatic stent placement for endoscopic duodenal ampullectomy: a single-center retrospective study. Gut Liver 2014;8:306-12. [Crossref] [PubMed]
- Vogt M, Jakobs R, Benz C, et al. Endoscopic therapy of adenomas of the papilla of Vater. A retrospective analysis with long-term follow-up. Dig Liver Dis 2000;32:339-45. [Crossref] [PubMed]
- Alali A, Espino A, Moris M, et al. Endoscopic Resection of Ampullary Tumours: Long-term Outcomes and Adverse Events. J Can Assoc Gastroenterol 2020;3:17-25. [Crossref] [PubMed]
Cite this article as: Woo SM, Real MJ, Will BM, Kim EJ, Chou J, Alsaiari AA, Nakshabandi A, Chalhoub WM, Haddad NG. Clinical outcomes: endoscopic resection of duodenal ampullary lesions. Transl Gastroenterol Hepatol 2023;8:15.