
Relapsing immunoglobulin G4-related sclerosing cholangitis during maintenance treatment with low-dose steroids: a case report
Introduction
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is the biliary manifestation of immunoglobulin G4-related disease (IgG4-RD), which is an immune-mediated multi-system fibroinflammatory disease and related to an elevated serum IgG4 level and the infiltration of IgG4 positive plasma cells (1). Hepatopancreaticobiliary system is the most frequently involved organ of the IgG4-RD with a proportion of 77% (2). Steroids are the first-line option of IgG4-SC with an initial remission rate of approximately 90% (3,4). The Japanese clinical practice guidelines recommend that the dosage of initial remission-induction therapy should be 0.6–1.0 mg/kg/day of prednisolone for 2–4 weeks, the dosage of prednisolone should be reduced by 5 mg every 1–2 weeks to the maintenance dosage by 2–3 months after imaging and serological improvement, and the recommended dosage of prednisolone is 5 mg per day for 3 years (5). However, the relapse of IgG4-SC is still a clinical challenge. It is reported that the relapse rate ranges from 24% to 59% in IgG4-RD patients treated with steroids alone (2,3,6-14). The international consensus (15) and Japanese practice guideline (5) recommend re-administration or up-dose of steroids, immunosuppressors, and rituximab for disease relapse. However, the standard protocol of steroids, including optimal starting dosage, adequate maintaining dosage, and withdrawal time, remain controversial (16). Herein, we reported a case of relapsing IgG4-SC, which was successfully treated by re-administration of steroids, and discussed the predictors and treatment strategy of disease relapse by reviewing the current evidence. We present the following case in accordance with the CARE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-111/rc).
Case presentation
On November 21, 2020, a 57-year-old male was admitted to our department due to abdominal distension and worsening liver function for 10 days. He had no underlying comorbidities. Physical examination revealed icteric sclera and skin without any tenderness or rebound pain in the abdomen. Laboratory tests on admission showed elevated levels of total bilirubin (102.7 µmol/L, reference range, 5.1–22.2 µmol/L) (Figure 1), direct bilirubin (68.4 µmol/L, reference range, 0–8.6 µmol/L), alkaline phosphatase (953.92 U/L, reference range, 45–125 U/L), gamma-glutamyltransferase (1,407.5 U/L, reference range, 10–60 U/L), alanine aminotransferase (1,030.07 U/L, reference range, 9–50 U/L), aspartate aminotransferase (589.62 U/L, reference range, 15–40 U/L), serum amylase (192.00 U/L, reference range, 30–110 U/L), serum lipase (2,956.0 U/L, reference range, 23–300 U/L), and IgG4 (499 mg/dL, reference range, 1.2–201 mg/dL). He had negative immunoglobulin A, G, and M, autoantibodies (i.e., anti-nuclear antibody, anti-mitochondrial antibody, and anti-smooth muscle antibody), hepatitis virus A, B, C, and E, and tumor markers (i.e., alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen-199, 50, 24-2, 125, and 153). Contrast-enhanced magnetic resonance imaging (MRI) showed diffuse enlargement of pancreas with its delayed enhancement (Figure 2). Additionally, the inner and outer layers of the bile duct were smooth. Magnetic resonance cholangiopancreatography (MRCP) showed pancreatic duct stricture, intrahepatic bile duct and upper middle segment of common bile duct dilatation, and lower common bile duct stricture with slight wall thickness (Figure 3). A diagnosis of primary sclerosing cholangitis was excluded due to the presence of elevated serum IgG4 level and the absence of short strictures and beaded appearance of biliary trees on MRCP. A diagnosis of cholangiocarcinoma was also excluded due to the absence of a two-layer bile duct wall at the arterial phase of contrast-enhanced MRI scans. Finally, a diagnosis of type 1 autoimmune pancreatitis (AIP) combined with IgG4-SC was established. Since November 23, steroid therapy was started with methylprednisolone sodium succinate at 40 mg/day for 4 days, and then adjusted to oral methylprednisolone tablets at 36 mg/day. Serum IgG4 level decreased to 394 mg/dL on the 11th day of steroid treatment. MRI and MRCP also showed the improvement of pancreatic enlargement and biliary stricture on December 3, 2020 (Figures 2,3). Subsequently, methylprednisolone tablets were regularly reduced by 4 mg per week within 2 months and finally maintained at 2 mg/day. Serum IgG4 level and liver function markers gradually recovered during follow-up (Figure 1).
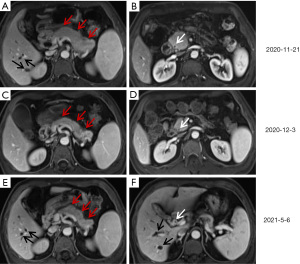
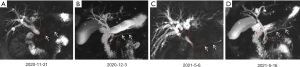
On May 6, 2021, he was re-admitted to our department for icteric sclera and skin after a 3-month maintenance treatment of methylprednisolone tablets at a dosage of 2 mg/day. Except for an elevated serum IgG4 level of 388 mg/dL, laboratory tests revealed significantly elevated levels of total bilirubin (161.8 µmol/L, reference range, 5.1–22.2 µmol/L), direct bilirubin (112.9 µmol/L, reference range, 0–8.6 µmol/L), alkaline phosphatase (566.75 U/L, reference range, 45–125 U/L), gamma-glutamyltransferase (1,510.32 U/L, reference range, 10–60 U/L), alanine aminotransferase (758.53 U/L, reference range, 9–50 U/L), aspartate aminotransferase (321.94 U/L, reference range, 15–40 U/L) (Figure 1). MRI showed a slightly swollen pancreas, which was not significantly deteriorated compared to the previous images (Figure 2). MRCP showed intrahepatic bile duct dilatation as well as hilar bile duct and lower common bile duct stricture with wall thickness (Figure 3). A relapse of IgG4-SC was considered. Methylprednisolone sodium succinate was given at 40 mg/day for 1 week. His liver dysfunction and jaundice gradually improved. Then, it was adjusted to oral methylprednisolone tablets at 36 mg/day for 1 week. On the 10th day of steroid therapy, MRCP showed a significant improvement of bile duct stricture (Figure 3). After induction of remission, methylprednisolone tablets were reduced by 4 mg per week to a maintenance dosage of 4 mg/day. IgG4 level and liver function normalized after a 1.5-month steroid therapy (Figure 1). At the last follow-up in December 2021, he was still stable with methylprednisolone tablets at 4 mg/day.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
IgG4-RD relapse may increase the risk of sclerosis and permanent damage of organs affected (17). It is very necessary to recognize the management and predictors of disease relapse. Most previous studies focused on the management of relapse of IgG4-RD or type 1 AIP, but few studies on IgG4-SC. Currently, a relapse of IgG4-SC is often defined as the recurrence of symptomatic, serologic, radiologic, and/or histological abnormality after complete or partial remission of this disease, but not all of these abnormalities may appear at the same time (18). After IgG4-SC remission, our patient developed recurrent obstructive jaundice, elevated serum IgG4 level, and more aggressive biliary stricture as compared to the imaging presentations at the first onset of his diseases. This suggests that clinical symptoms, serum IgG4 level, and imaging should be closely screened in such patients to catch relapsing flames in time.
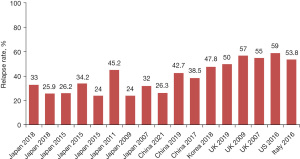
Among the Asian population with AIP or IgG4-RD, the relapse rate after steroid therapy alone ranges between 24% and 47.8% (3,6-12,19-23). By comparison, a few studies from Western countries show a higher relapse rate of 50–59% (2,13,14,24,25). Such a heterogeneity in relapse rate may be related to the difference in dosage and duration of steroids and follow-up duration. Maintenance treatment can reduce the relapse rate. A large retrospective study of 459 patients with type 1 AIP showed that the relapse rate was significantly lower in patients receiving the maintenance steroid therapy than those with drug withdrawal (23% vs. 34%, P=0.048) (3). In addition, it seems that a longer duration of steroid therapy leads to a lower relapse rate. A retrospective study by Kubota et al. (8) showed that the relapse rates were 80% (12/15) and 25.9% (7/27) in AIP patients who had received steroids for less and more than 12 months, respectively (P<0.01). Long-term follow-up is required to monitor the patients’ conditions and assess the disease relapse because there is an increasing trend of relapse rate over time (Figure 4) (3,10).
Except for drug withdrawal, there are some other predictors of disease relapse. First, serum IgG4 level is a predictor of IgG4-SC relapse. Tsang et al. (26) found a significant correlation between a raised serum IgG4 level (greater than twice the normal upper limit) at the time of first diagnosis and disease relapse. Similarly, our patient’s serum IgG4 level at the time of initial onset was greater than two times the upper limit of reference range, which suggests a higher risk of IgG4-SC relapse. In addition, a more rapid rate of decrease in serum IgG4 level during steroid therapy correlated with a lower risk of relapse after steroid therapy (27). Re-elevation of serum IgG4 level during steroid therapy is also a useful predictor of disease relapse (6,19). Second, the degree of bile duct involvement during the initial attack is also related to IgG4-SC relapse. Multiple bile duct strictures and thicker bile duct walls can increase the risk of disease relapse (5,12,28). Third, a long duration from first diagnosis to initiation of treatment and history of allergy are risk factors for disease relapse (10). Fourth, serum tumor necrosis factor-alpha (TNF-alpha) ≥13 pg/mL and soluble interleukin-2 receptor (sIL-2R) ≥1,010 U/mL are the independent risk factors for IgG4-SC relapse (29).
There is no standard treatment regimen for IgG4-SC relapse. First, steroids are appropriate for the treatment of disease relapse. Accordingly, our patient’s condition improved significantly after re-administration of methylprednisolone tablets. However, during long-term follow-up, we cannot ignore the side effects of steroids, such as osteoporosis and diabetes (3). Second, immunosuppressors have also been used for relapsing IgG4-SC. It was reported that seven patients with relapsing IgG4-SC were treated with azathioprine at 2.0–2.5 mg/kg/day or mycophenolate mofetil at 750 mg twice daily at the Mayo Clinic. During a median follow-up period of 6 months (range, 2–19 months), they all achieved remission (30). In a retrospective single-center study in China, 17 patients with relapsing or refractory IgG4-RD were treated with iguratimod at 25 mg twice daily, of whom 16 patients responded well without any serious drug related side effect (31). Third, rituximab, an antiCD20 antibody, is an appropriate alternative when some relapsing cases are resistant or intolerant to immunosuppressors or steroids. Among 12 relapsing AIP cases who were intolerant or resistant to steroids or immunosuppressors at Mayo Clinic, the complete remission rate was 83% after rituximab treatment (375 mg/m2 intravenously per week for 4 weeks, and then repeat infusions every 2–3 months for 24 months), and side effects related to rituximab were uncommon (32).
In conclusion, based on the case report and current evidence reviewed, IgG4-SC is likely to relapse after steroid treatment, especially in patients who have high serum IgG4 level at initial onset and receive low-dose steroids as a maintenance treatment. In addition, steroids interruption, more severe bile duct stricture, long duration from diagnosis to treatment, history of allergy, and high serum TNF-alpha and sIL-2R levels are the predictors of disease relapse. Re-administration or up-dose of steroids and initiation of immunosuppressors and rituximab are useful for the treatment of relapsing disease. In the future, more large-scale cohort studies are needed to clarify the optimal treatment strategy and long-term outcomes of IgG4-RD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-111/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-21-111/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mahajan VS, Mattoo H, Deshpande V, et al. IgG4-related disease. Annu Rev Pathol 2014;9:315-47. [Crossref] [PubMed]
- Sekiguchi H, Horie R, Kanai M, et al. IgG4-Related Disease: Retrospective Analysis of One Hundred Sixty-Six Patients. Arthritis Rheumatol 2016;68:2290-9. [Crossref] [PubMed]
- Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009;58:1504-7. [Crossref] [PubMed]
- Wang S, Xu X, Bai Z, et al. IgG4-related disease with multiple organs involvement was effectively controlled by glucocorticoids: a case report. AME Case Rep 2020;4:22. [Crossref] [PubMed]
- Kamisawa T, Nakazawa T, Tazuma S, et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 2019;26:9-42. [Crossref] [PubMed]
- Sasaki T, Akiyama M, Kaneko Y, et al. Risk factors of relapse following glucocorticoid tapering in IgG4-related disease. Clin Exp Rheumatol 2018;36:186-9. [PubMed]
- Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680. [Crossref] [PubMed]
- Kubota K, Watanabe S, Uchiyama T, et al. Factors predictive of relapse and spontaneous remission of autoimmune pancreatitis patients treated/not treated with corticosteroids. J Gastroenterol 2011;46:834-42. [Crossref] [PubMed]
- Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007;56:1719-24. [Crossref] [PubMed]
- Liu Y, Zeng Q, Zhu L, et al. Relapse predictors and serologically unstable condition of IgG4-related disease: a large Chinese cohort. Rheumatology (Oxford) 2020;59:2115-23. [Crossref] [PubMed]
- Zhu L, Xue HD, Zhang W, et al. Pancreaticobiliary involvement in treated type 1 autoimmune pancreatitis: Imaging pattern and risk factors for disease relapse. Eur J Radiol 2019;120:108673. [Crossref] [PubMed]
- Lee HW, Moon SH, Kim MH, et al. Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: long-term follow-up results after steroid therapy with short-duration maintenance treatment. J Gastroenterol 2018;53:967-77. [Crossref] [PubMed]
- Sandanayake NS, Church NI, Chapman MH, et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol 2009;7:1089-96. [Crossref] [PubMed]
- Church NI, Pereira SP, Deheragoda MG, et al. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007;102:2417-25. [Crossref] [PubMed]
- Okazaki K, Chari ST, Frulloni L, et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2017;17:1-6. [Crossref] [PubMed]
- Kamisawa T, Zen Y, Nakazawa T, et al. Advances in IgG4-related pancreatobiliary diseases. Lancet Gastroenterol Hepatol 2018;3:575-85. [Crossref] [PubMed]
- Khosroshahi A, Stone JH. Treatment approaches to IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:67-71. [Crossref] [PubMed]
- Kim HM, Chung MJ, Chung JB. Remission and relapse of autoimmune pancreatitis: focusing on corticosteroid treatment. Pancreas 2010;39:555-60. [Crossref] [PubMed]
- Suzuki D, Shimizu K, Tokushige K. Relative Rise of Serum IgG4 Levels After Steroid Therapy for Autoimmune Pancreatitis Predicts the Likelihood of Relapse. Pancreas 2018;47:412-7. [Crossref] [PubMed]
- Shimizu S, Naitoh I, Nakazawa T, et al. Correlation between long-term outcome and steroid therapy in type 1 autoimmune pancreatitis: relapse, malignancy and side effect of steroid. Scand J Gastroenterol 2015;50:1411-8. [Crossref] [PubMed]
- Yamamoto M, Nojima M, Takahashi H, et al. Identification of relapse predictors in IgG4-related disease using multivariate analysis of clinical data at the first visit and initial treatment. Rheumatology (Oxford) 2015;54:45-9. [Crossref] [PubMed]
- Peng L, Lu H, Zhou J, et al. Clinical characteristics and outcome of IgG4-related disease with hypocomplementemia: a prospective cohort study. Arthritis Res Ther 2021;23:102. [Crossref] [PubMed]
- Yunyun F, Yu C, Panpan Z, et al. Efficacy of Cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids. Sci Rep 2017;7:6195. [Crossref] [PubMed]
- Poo SX, Tham CSW, Smith C, et al. IgG4-related disease in a multi-ethnic community: clinical characteristics and association with malignancy. QJM 2019;112:763-9. [Crossref] [PubMed]
- Campochiaro C, Ramirez GA, Bozzolo EP, et al. IgG4-related disease in Italy: clinical features and outcomes of a large cohort of patients. Scand J Rheumatol 2016;45:135-45. [Crossref] [PubMed]
- Tsang KFP, Oppong WK, Leeds SJ, et al. Does IgG4 level at the time of diagnosis correlate with disease outcome in IgG4-Related disease? Pancreatology 2019;19:177-81. [Crossref] [PubMed]
- Shimizu K, Tahara J, Takayama Y, et al. Assessment of the Rate of Decrease in Serum IgG4 Level of Autoimmune Pancreatitis Patients in Response to Initial Steroid Therapy as a Predictor of Subsequent Relapse. Pancreas 2016;45:1341-6. [Crossref] [PubMed]
- You MW, Kim JH, Byun JH, et al. Relapse of IgG4-related sclerosing cholangitis after steroid therapy: image findings and risk factors. Eur Radiol 2014;24:1039-48. [Crossref] [PubMed]
- Zongfei J, Lingying M, Lijuan Z, et al. Prognostic factors in IgG4-related disease: a long-term monocentric Chinese cohort study. Clin Rheumatol 2021;40:2293-300. [Crossref] [PubMed]
- Uchida K, Okazaki K, Asada M, et al. Case of chronic pancreatitis involving an autoimmune mechanism that extended to retroperitoneal fibrosis. Pancreas 2003;26:92-4. [Crossref] [PubMed]
- Liu Y, Zhang Y, Bian W, et al. Efficacy and safety of iguratimod on patients with relapsed or refractory IgG4-related disease. Clin Rheumatol 2020;39:491-7. [Crossref] [PubMed]
- Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 2013;62:1607-15. [Crossref] [PubMed]
Cite this article as: Zhu M, Li H, Zhou W, Wang W, Yin Y, Xu S, Yu K, Qi X. Relapsing immunoglobulin G4-related sclerosing cholangitis during maintenance treatment with low-dose steroids: a case report. Transl Gastroenterol Hepatol 2023;8:22.



