Therapeutic endoscopic ultrasound
Introduction
Endoscopic ultrasound (EUS) was developed in 1980 after modifying a side viewing scope by adding an ultrasound probe at the tip of the scope (1). Since then, EUS has been continuously evolving and its use has become widely available. EUS was initially used for better characterization of lesions visible during endoscopy; over time, improvements in the device have allowed not only imaging but sampling, injection, and most recently drainage and anastomosis creation of structures within and adjacent to the gastrointestinal tract (2,3). These advancements have led to a new era of EUS known as therapeutic EUS (TEUS). TEUS procedures include drainage procedures (pancreatic fluid collections, gallbladder, pancreatic duct and biliary duct) as well as gastrointestinal anastomoses creation [gastroenterostomy (GE) and EUS-directed endoscopic retrograde cholangiopancreatography (ERCP)]. All generally require the following critical steps: access, fistula creation and stent deployment (4). The training for TEUS requires the acquisition of skills beyond those obtained during a traditional GI fellowship. There are no specific guidelines regarding the requirements to credential a trainee in TEUS. However, proficiency and high volume in ERCP and diagnostic EUS procedures seems to be the foundation (4-6).
EUS-guided gallbladder drainage
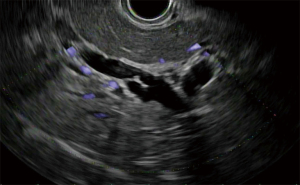
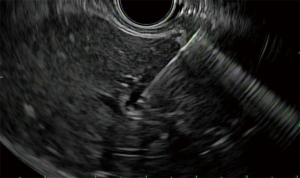
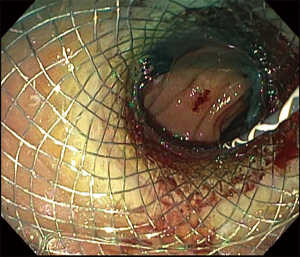
Acute cholecystitis is a highly prevalent pathology. The gold standard treatment is surgical cholecystectomy (7). For non-surgical candidates, treatment requires antibiotics and early gallbladder drainage, traditionally percutaneously (PC-GBD) and more recently via transpapillary ERCP (TP-GBD), and now EUS-guided drainage (EUS-GBD) (8). The first use of EUS in the treatment of acute cholecystitis was reported by Baron in 2007 performing a cholecystoduodenostomy with a double pigtail stent (DPS) (9). Since then, different techniques have been used, including placement of a nasocystic drainage catheter or metal biliary stent. With the advent of lumen-apposing metal stents (LAMSs), the technique has been standardized and currently LAMS are widely used in EUS-GBD (10,11). LAMS allow for gallbladder drainage in addition to interventions such as stone removal, lithotripsy, magnifying endoscopy or polyp removal (4,11). Both transgastric and transduodenal approaches have been used for EUS-GBD. Usually during the transgastric approach the gallbladder is accessed at its body from the gastric antrum (Figures 1-3). The transduodenal approach is from the duodenal bulb to the neck of the gallbladder. There are advantages and disadvantages with each method. The transduodenal approach is theoretically easier to perform since the duodenum is less mobile than the stomach. It also carries less risk of stent migration or food reflux into the gallbladder. On the other hand, the transgastric approach gives access to the gallbladder body, which constitutes a larger entry site to the gallbladder. Additionally, future cholecystectomies are easier to be performed with the anastomosis in the stomach, since gastric fistula closure is technically less complex than duodenal fistula closure. To date, there is no evidence of one approach being superior to the other. Another unresolved controversy is the placement of DPS through the LAMS. The advantage of placing DPS is to decrease the rate of clogging by food or tissue growth as well as the possibility of keeping the fistula open in case there is LAMS migration, but it’s uncertain if this practice improves outcomes (11).
Outcomes
EUS-GBD is an efficacious and safe procedure, with technical and clinical success rate of EUS-GBD ranges from 90–100% and 72–99% respectively, and adverse event (AE) rate ranging from 7–50% based on the largest series of EUS-GBD (Table 1). When EUS-GBD was compared to PC-GBD and TP-GBD, EUS-GBD had a technical success slightly lower than PC-GBD (94–95% vs. 98–99%) but better than TP-GBD (83–88%). The clinical success for EUS-GBD (90–96%) was similar to PC-GBD (89–97%), but better than TP-GBD (80–88%). In terms of AEs, there was no difference between all groups. However, there was a need for repeated procedures after PC-GBD in up to 25% due to tube dislodgement or obstruction (19,24-26).
Table 1
| Author | Patients | Technical success, % | Clinical success, % | AEs, % |
|---|---|---|---|---|
| Jang 2012 (12) | 30 | 97 | 97 | 7 |
| Choi 2014 (13) | 63 | 98 | 85 | 10 |
| Irani 2017 (14) | 45 | 97 | 95 | 17 |
| Kahaleh 2016 (15) | 35 | 91 | 89 | 25 |
| Walter 2016 (16) | 30 | 90 | 86 | 50 |
| Dollhopf 2017 (17) | 75 | 98 | 95 | 10 |
| Teoh 2017 (18) | 59 | 96 | 89 | 32 |
| Tyberg 2018 (19) | 42 | 95 | 95 | 21 |
| Oh 2019 (20) | 76 | 99 | 99 | 7 |
| Higa 2019 (21) | 40 | 97 | 95 | 17 |
| Tyberg 2020 (22) | 48 | 100 | 72 | 19 |
| Teoh 2020 (23) | 80 | 97 | 92 | 25 |
EUS, endoscopic ultrasound; AE, adverse event.
Although many patients never proceed to cholecystectomy after gallbladder drainage, EUS-GBD can be performed as a bridge to a definitive surgery. A paper by Saumoy et al. compared cholecystectomy after EUS-GBD vs. PC-GBD and found no differences in terms of AEs or the rate of conversion to an open procedure. Cholecystectomy after EUS-GBD had a shorter operative time compared to the group that had PC-GBD prior to surgery (24).
The learning curve for EUS-GBD is unknown, though one study suggested a learning rate of 19 cases (22).
Conclusions and future directions
EUS-GBD is an alternative to PC-GBD with comparable or, in some series, improved safety and efficacy, with the associated benefit of less pain after the procedure and less morbidity from having an external drain (22,27). The most recent Tokyo consensus has made changes to its recommendations and has included EUS-GBD as one of the options for treatment of cholecystitis in high volume centers (27). Further randomized controlled trials comparing EUS-GBD and PC-GBD will likely illustrate the dominance of this procedure and ultimately lead to EUS-GBD as the main modality for non-surgical gallbladder drainage.
EUS-guided pancreatic duct drainage
The most common indications for pancreatic duct drainage include: chronic pancreatitis with stricture and/or pancreatolithiasis, post-Whipple procedure with suspected stenosis of the pancreatic anastomosis, prophylactic main pancreatic duct (MPD) stent prior to ampullectomy and disruption of the MPD (28). Endoscopic retrograde pancreatography (ERP) is the standard of care for the management of MPD pathology. Surgery could be used after an unsuccessful ERP or in patients with altered anatomy but it is associated with increased morbidity and mortality (29).
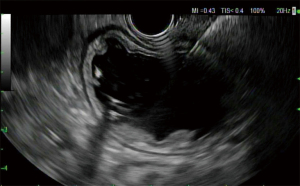
The first EUS pancreatography was described by Harada in 1994 (30). The first use of EUS-guided pancreatic drainage (EUS-PD) was described in 2002 by Francois, developing a pancreaticogastrostomy (31), and by Bataille, performing a pancreaticoduodenostomy with “pancreatic rendezvous” (32). The term “rendez-vous approach” was coined by Ghattas in 1992 and was described in patients with difficult cannulation of the MPD, by accessing the MPD via the minor papillae (33). In 1999 Dumonceau described the “transduodenal rendezvous” where the MPD was cannulated after duodenal puncture (34). EUS-PD can be performed in different ways, the MPD can be accessed from an area that starts at the gastric cardia and extends to the third portion of the duodenum, and the site is selected based on the shortest space between the MPD and the EUS probe, and the structures in the pathway of the needle. MPD stent placement can be performed via retrograde approach also known as “rendezvous procedure”, crossing the papilla or anastomosis and then inserting a duodenoscope for conventional ERP (Figures 4-6), or antegrade from the gastrointestinal lumen into the MPD with or without traversing the site of obstruction. Typically, plastic stents are used, but metal stents can be used as well when the MPD is significantly dilated (28,35).
Outcomes
EUS-PD is a technically challenging procedure with a variable technical success rate ranging from 69% to 100%, clinical success from 69% to 100%, and an AE rate of 6% to 35% in the largest reported series (Table 2). The most common AEs include: abdominal pain, pancreatitis, pancreatic fluid collection, abscess, bleeding and perforation (28,35).
Table 2
| Author | Procedure | Patients | Technical success, % | Clinical success, % | AEs, % |
|---|---|---|---|---|---|
| Tessier 2007 (36) | EUS-PD A | 36 | 91 | 69 | 13 |
| Ergun 2011 (37) | EUS-PD A, R | 20 | 90 | 72 | 10 |
| Shah 2012 (38) | EUS-PD A, R | 22 | 54 | NR | 18 |
| Fujii 2013 (35) | EUS-PD A, R | 43 | 74 | NR | 6 |
| Will 2015 (39) | EUS-PD A, R | 83 | 56 | 81* | 21 |
| Oh 2016 (40) | EUS-PD A | 25 | 100 | 100 | 20 |
| Tyberg 2017 (29) | EUS-PD A, R | 80 | 89 | 81 | 20 |
| Chen 2017 (41) | EUS-PD A, R | 40 | 92 | 87 | 35 |
| Matsunami 2018 (42) | EUS-PD A | 30 | 100 | 92 | 23 |
*, Reported improvement of symptoms even when stent placement was not possible. EUS-PD, endoscopic ultrasound-guided pancreatic drainage; AE, adverse event; A, antegrade; R, retrograde; NR, not reported.
Only one study has compared EUS-PD versus enteroscopy-assisted ERP in the setting of prior pancreaticoduodenectomy. In this study, technical success was considerably higher after EUS-PD (92%) than after enteroscopy-assisted ERP (20%). AEs were more common after EUS-PD (35%) than after ERP (2%), but were all mild or moderate. AEs were more common after EUS-PD since the PD was not manipulated in most of the ERP cases (41). Stent migration has been reported to be more common with straight plastic stents compared to DPS (28).
Conclusions and future directions
With the development of EUS-PD, an effective and minimally invasive option has become available for the management of pathology of the pancreatic region. The rendezvous approach should be used as the first modality since AEs are lower with this technique. Pancreaticoduodenostomy is recommended for stenosis of the MPD at the level of the head, since the scope is in a more stable position, whereas pancreaticogastrostomy can be used during surgically altered anatomy, although this method is technically more difficult and associated with more complications (43). Although AEs are common with EUS-PD, the safety of the procedure is acceptable and can be implemented in properly selected patients by experienced teams (41,43).
EUS-guided biliary drainage
ERCP is the first-line procedure to provide access to the bile ducts in the vast majority of patients requiring biliary drainage, with a success rate of 93% to 95% (44). However, other options are required in those patients not amenable to conventional drainage or in whom biliary cannulation is unable to be achieved. The most common alternative is percutaneous transhepatic biliary drainage (PTBD). However, PTBD is associated with significant AEs in up to 33% of the patients (44,45). The close proximity of the bile ducts to the stomach and small intestine allows the use of EUS to delineate and access the bile ducts. Wiersema in 1996 described the use of EUS to perform cholangiopancreatography in patients with a failed ERCP, and those patients with abnormalities found during the cholangiopancreatography had an ERCP performed subsequently (3). Later on, in 2001 and 2003, Giovannini reported a palliative choledochoduodenostomy in a patient with pancreatic cancer, and a hepaticogastrostomy to relieve cholangitis in a patient with a hilar obstruction (46,47).
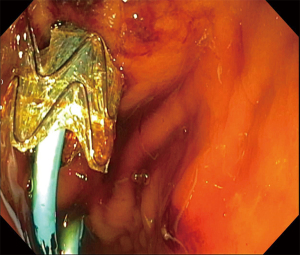
The concept of insertion of a guidewire in the bile ducts to assist ERCP was initially described in 1985 by Shorvon in patients with a failed initial ERCP, using a combination of percutaneous transhepatic cholangiography with guidewire placement into the duodenum to facilitate conventional ERCP (48). Since the first descriptions of its use (46,47,49), EUS-BD has become more widespread with numerous publications about its efficacy and safety (50). However, a major innovation was the adaptation and further development of the rendezvous approach to TEUS by Kahaleh in 2004 where EUS was used to access the bile ducts for guidewire placement to facilitate conventional ERCP, decreasing the morbidity associated with the percutaneous approach (51). EUS bile drainage (EUS-BD) can be performed via an intrahepatic (Figures 7-9) or extrahepatic route, with stent placement transluminally (hepaticogastrostomy or choledochoduodenostomy) or transpapillary (antegrade or using a rendezvous approach assisted by conventional ERCP) (51,52).
Outcomes
The reported technical and clinical success rate of EUS-BD ranges from 90–100% and 62–100% respectively across the largest published series; AE rate ranges from 8–21% (Table 3) (64). Hepaticogastrostomy has been compared to choledochoduodenostomy with no difference in technical success, clinical success or AEs (65).
Table 3
| Author | Procedure | Patients | Technical success, % | Clinical success, % | AEs, % |
|---|---|---|---|---|---|
| Park 2011 (53) | EUS-BD IH, EH | 57 | 96 | 85 | 19 |
| Poincloux 2015 (54) | EUS-BD IH, EH | 96 | 97 | 91 | 12 |
| Sharaiha 2016 (55) | EUS-BD IH, EH | 47 | 93 | 62 | 13 |
| Sportes 2017 (56) | EUS-BD IH | 31 | 100 | 86 | 16 |
| Khashab 2016 (57) | EUS-BD IH, EH | 121 | 92 | 83 | 16 |
| Kunda 2016 (58) | EUS-BD EH | 57 | 98 | 94 | 15 |
| Lee 2016 (59) | EUS-BD IH, EH | 34 | 94 | 87 | 8 |
| Nakai 2016 (60) | EUS-BD IH | 33 | 100 | 100 | 9 |
| Cho 2017 (61) | EUS-BD IH, EH | 54 | 100 | 94 | 16 |
| Minaga 2017 (62) | EUS-BD IH | 30 | 96 | 73 | 10 |
| Bang 2018 (63) | EUS-BD EH | 33 | 90 | 87 | 21 |
EUS-BD, endoscopic ultrasound guided biliary drainage; AE, adverse event; IH, intrahepatic; EH, extrahepatic.
When comparing EUS-BD to PTBD, a meta-analysis found that EUS-BD has superior clinical success with lower rates of reintervention. Bile leak is the most common AE reported with both techniques and is more common after PTBD (7%) than after EUS-BD (3%). Bleeding and cholangitis are also more common after PTBD than after EUS-BD (4.3% vs. 2.7%; 5.1% vs. 0.3% respectively). The assessment of cost-effectiveness of the procedures also favors EUS-BD against PTBD (45).
A randomized trial compared EUS-BD to conventional ERCP as primary treatment in patients with biliary obstruction from pancreatic cancer, and there were no differences in terms of outcomes or related AEs between the two groups (63).
Conclusions and future directions
The available evidence favors EUS-BD over PTBD due to higher clinical success with less AEs (45). The type of EUS-BD approach, either intrahepatic or extrahepatic, remains up to the endoscopist discretion unless dictated by patient anatomy. Although currently EUS-BD is considered as an alternative only when ERCP fails, emerging studies suggest that EUS-BD could potentially be considered as the primary option for biliary drainage before conventional ERCP, given its great technical and clinical success and negated risk of pancreatitis. Currently further trials are needed.
EUS-GE
Gastric outlet obstruction (GOO) can be secondary to benign or malignant etiology and is treated with enteral stent (ES) placement or diversion of the gastric content to the jejunum by the creation of a GE, either using surgery or endoscopy. The endoscopic approach by means of ES or EUS-GE has been favored in patients who are poor surgical candidates or have very short life expectancy (66). The development of LAMS allowed lumen to lumen communication with anchorage between non-adherent structures, preventing migration of the stent as well as leakage of content outside of the communication. The covered ends of the stents also decreased tissue trauma caused by the end of regular tubular stents, resulting in the creation of durable anastomoses that previously were only able to be performed by conventional surgery (67). The first use of LAMS to perform a gastrojejunostomy was reported by Binmoeller in 2012 (68), and since then multiple techniques have been described for the performance of EUS-GE for malignant or benign GOO as well as superior mesenteric syndrome (66,69,70). The three main methods for EUS-GE follow the same principle of creating a fistula between the stomach and the small intestine upstream from the obstruction, but the difference lies in the method of localizing the small bowel before the site is accessed from the stomach. The direct EUS-GE method offers the advantage to be performed even with complete GOO. Assisted EUS-GE and EUS-guided balloon-occluded gastrojejunostomy bypass (EPASS) require the ability to access the upstream small bowel through the obstruction but offer the advantage of performing the procedure in a distended jejunum. Infusion of methylene blue is also widely used to confirm placement after stent deployment by visualization of methylene-blue tinted fluid (Figures 10-12). Usually a liquid diet can be started the next day after the procedure (66,71).
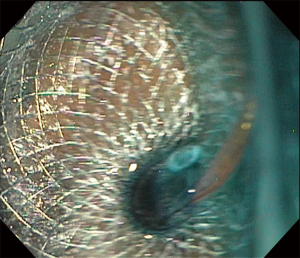
Outcomes
Only few comparative studies exist comparing EUS-GE against surgical GE or ES. A recent pooled analysis demonstrated that EUS-GE is slightly more difficult to perform with somewhat lower technical success (91%) than ES (97%) or surgical GE (100%). However, the clinical success of EUS-GE (88%) was similar to a surgical GE (87%) and better than the ES (73%) with less AEs (15%) than the surgical GE (30%) and ES (30%) (72). Stent occlusion requiring reintervention is less common after EUS-GE compared with ES (73). Table 4 includes the largest series of EUS-GE.
Table 4
| Author | Procedure | Patients | Technical success, % | Clinical success, % | AEs, % |
|---|---|---|---|---|---|
| Itoi 2016 (74) | EPASS | 20 | 90 | 90 | 10 |
| Tyberg 2016 (75) | EUS-GE A, D | 26 | 92 | 85 | 11 |
| Khashab 2017 (76) | EUS-GE A, D, EPASS | 30 | 86 | 86 | 16 |
| Perez-Miranda 2017 (77) | EUS-GE A, D | 25 | 92 | 82 | 12 |
| Kerdsirichairat 2018 (78) | EUS-GE A, D | 37 | 94 | 81 | 2 |
| Chen 2018 (79) | EUS-GE A, D | 74 | 93 | 91 | 6 |
| Ge 2019 (73) | EUS-GE A | 22 | 100 | 90 | 22 |
| Widmer 2019 (80) | EUS-GE A | 10 | 100 | 100 | 0 |
EUS-GE, endoscopic ultrasound-guided gastroenterostomy; AE, adverse event; EPASS, EUS-guided balloon-occluded gastrojejunostomy bypass; A, assisted technique; D, direct technique.
Conclusions and future directions
EUS-GE should be considered as an alternative to surgery for patients with GOO in centers with capability of performing this procedure (71,72). Other applications of EUS-GE are also emerging. Enteroentero and enterocolonic anastomosis using EUS and LAMS have also been reported recently for the treatment of strictures or malignancy, as well as to access the small bowel limb hosting the pancreaticobiliary anastomoses in post-surgical patients requiring pancreaticobiliary intervention (81-85). The possibility of bypassing the duodenum with an EUS-GE coupled with an endoscopic gastroplasty and/or pyloric exclusion is being explored in bariatric endoscopy and in duodenal injuries in which diversion of gastric contents is required for healing (86,87).
EUS-directed transgastric ERCP
Due to the obesity epidemic, bariatric procedures have become widespread and Roux-en-Y gastric bypass (RYGB) is one of the most common bariatric procedures. Due to rapid weight loss, these patients are at increased risk of developing cholelithiasis and choledocholithiasis. However, the surgically altered anatomy represents a challenge when attempting to perform ERCP. To access the ampullary region, balloon enteroscopy ERCP (BE-ERCP) or laparoscopy-assisted ERCP (LA-ERCP) was traditionally required (88). A new procedure called EUS-directed transgastric ERCP (EDGE) was described by Kedia et al. in which EUS was used to create a gastrogastric fistula between the gastric pouch and the gastric remnant, providing access to the native ampulla through the LAMS (Figures 13-15) to facilitate conventional ERCP (89).
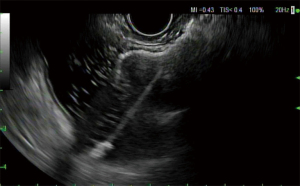
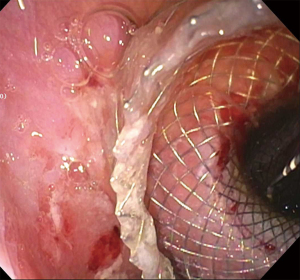
Outcomes
The reported technical and clinical success rates and AE rates of EDGE ranges from 92–100% and 0–21% across the largest published series (Table 5). The learning curve for the EDGE procedure is unknown, though one study showed a learning rate of nine cases and an estimated requirement of 25–35 procedures to achieve mastery (6). Compared to the conventional LA-ERCP or BE-ERCP, EDGE has a technical success similar to LA-ERCP (95.5% vs. 95.3%) and better than BE-ERCP (71.4%). The calculated clinical success for EDGE (95.9%) is better than LA-ERCP (92.9%) and BE-ERCP (58.7%) although the difference between EDGE and LA-ERCP was not significant. Regarding AEs, the rate of perforation is similar between the three groups but bleeding is lower in BE-ERCP when compared to LA-ERCP and EDGE. Some of the AEs are unique to the EDGE procedure including stent migration (13.3%), although the risk can be decreased by allowing maturation of the fistula and performing the ERCP during a second procedure. Another concern is the persistence of the gastrogastric fistula which can lead to weight gain, although most studies report persistent weight loss after EDGE (88). Cost effectiveness has also been evaluated between EDGE, BE-ERCP and LA-ERCP, and after taking into consideration the cost of hospitalization, AEs and subsequent procedures, EDGE represents the most cost-effective strategy in patients with surgically altered anatomy after RYGB (94).
Table 5
| Author | Patients | Technical success, % | Clinical success, % | AEs, % |
|---|---|---|---|---|
| Bukhari 2018 (90) | 30 | 100 | 100 | 6 |
| Chiang 2018 (91) | 66 | 92 | NR | 19 |
| James 2018 (92) | 19 | 100 | 100 | 0 |
| Wang 2019 (93) | 10 | 100 | 100 | 20 |
| Tyberg 2020 (6) | 19 | 100 | 94 | 21 |
EUS, endoscopic ultrasound; AE, adverse event; ERCP, endoscopic retrograde cholangiopancreatography; NR, not reported.
Conclusions and future directions
Although a relatively new procedure, the EDGE procedure has been widely used and adopted at many centers. However, the vast majority of the procedures that have been published have been performed by an experienced endoscopist and the results may not be generalizable (88,94). One of the limitations of the procedure is that it is safest when done in two steps to allow for a mature fistula to decrease stent migration (95). With the availability of LAMS of larger diameter, it could be expected that dislodgement during the initial procedure will decrease and the procedure can be performed as a single step in all cases (58).
Final conclusion
TEUS has provided groundbreaking procedures permitting safer, faster and more efficient treatment for diseases previously managed via percutaneous and/or surgical techniques. In a healthcare system constantly looking for noninvasive and cost saving interventions, TEUS has become a major player and is reshaping the way patients are being managed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Amy Tyberg) for the series “Innovation in Endoscopy” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-2020-12/coif). The series “Innovation in Endoscopy” was commissioned by the editorial office without any funding or sponsorship. MK reports Research Grant support from Boston Scientific, Apollo Endosurgery, Cook Endoscopy, NinePoint Medical, Merit Medical, Olympus, and Interscope Med. Consultant for Boston Scientific, Concordia Laboratories Inc, ABBvie and ERBE. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet 1980;1:629-31. [Crossref] [PubMed]
- Kimmey MB, Martin RW, Silverstein FE. Endoscopic ultrasound probes. Gastrointest Endosc 1990;36:S40-6. [Crossref] [PubMed]
- Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43:102-6. [Crossref] [PubMed]
- Shah SL, Perez-Miranda M, Kahaleh M, et al. Updates in therapeutic endoscopic ultrasonography. J Clin Gastroenterol 2018;52:765-72. [Crossref] [PubMed]
- Saumoy M, Kahaleh M. Progress in endoscopic ultrasonography: training in therapeutic or interventional endoscopic ultrasonography. Gastrointest Endosc Clin N Am 2017;27:749-58. [Crossref] [PubMed]
- Tyberg A, Kedia P, Tawadros A, et al. EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE): the first learning curve. J Clin Gastroenterol 2020;54:569-72. [Crossref] [PubMed]
- Glenn F. Cholecystostomy in the high risk patient with biliary tract disease. Ann Surg 1977;185:185-91. [Crossref] [PubMed]
- Mayumi T, Okamoto K, Takada T, et al. Tokyo Guidelines 2018: management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 2018;25:96-100. [Crossref] [PubMed]
- Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc 2007;65:735-7. [Crossref] [PubMed]
- Baron TH, Grimm IS, Swanstrom LL. Interventional approaches to gallbladder disease. N Engl J Med 2015;373:357-65. [Crossref] [PubMed]
- Perez-Miranda M. Technical considerations in EUS-guided gallbladder drainage. Endosc Ultrasound 2018;7:79-82. [PubMed]
- Jang JW, Lee SS, Song TJ, et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology 2012;142:805-11. [Crossref] [PubMed]
- Choi JH, Lee SS, Choi JH, et al. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy 2014;46:656-61. [Crossref] [PubMed]
- Irani S, Ngamruengphong S, Teoh A, et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol 2017;15:738-45. [Crossref] [PubMed]
- Kahaleh M, Perez-Miranda M, Artifon EL, et al. International collaborative study on EUS-guided gallbladder drainage: are we ready for prime time? Dig Liver Dis 2016;48:1054-7. [Crossref] [PubMed]
- Walter D, Teoh AY, Itoi T, et al. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut 2016;65:6-8. [Crossref] [PubMed]
- Dollhopf M, Larghi A, Will U, et al. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest Endosc 2017;86:636-43. [Crossref] [PubMed]
- Teoh AYB, Serna C, Penas I, et al. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy 2017;49:130-8. [PubMed]
- Tyberg A, Saumoy M, Sequeiros EV, et al. EUS-guided versus percutaneous gallbladder drainage: isn't it time to convert? J Clin Gastroenterol 2018;52:79-84. [Crossref] [PubMed]
- Oh D, Song TJ, Cho DH, et al. EUS-guided cholecystostomy versus endoscopic transpapillary cholecystostomy for acute cholecystitis in high-risk surgical patients. Gastrointest Endosc 2019;89:289-98. [Crossref] [PubMed]
- Higa JT, Sahar N, Kozarek RA, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent versus endoscopic transpapillary gallbladder drainage for the treatment of acute cholecystitis (with videos). Gastrointest Endosc 2019;90:483-92. [Crossref] [PubMed]
- Tyberg A, Jha K, Shah S, et al. EUS-guided gallbladder drainage: a learning curve modified by technical progress. Endosc Int Open 2020;8:E92-6. [Crossref] [PubMed]
- Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020;69:1085-91. [Crossref] [PubMed]
- Saumoy M, Tyberg A, Brown E, et al. Successful cholecystectomy after endoscopic ultrasound gallbladder drainage compared with percutaneous cholecystostomy, can it be done? J Clin Gastroenterol 2019;53:231-5. [Crossref] [PubMed]
- Siddiqui A, Kunda R, Tyberg A, et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an International, Multicenter Study. Surg Endosc 2019;33:1260-70. [Crossref] [PubMed]
- Mohan BP, Khan SR, Trakroo S, et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy 2020;52:96-106. [Crossref] [PubMed]
- Mori Y, Itoi T, Baron TH, et al. Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci 2018;25:87-95. [Crossref] [PubMed]
- Fujii-Lau LL, Levy MJ. Endoscopic ultrasound-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci 2015;22:51-7. [Crossref] [PubMed]
- Tyberg A, Sharaiha RZ, Kedia P, et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc 2017;85:164-9. [Crossref] [PubMed]
- Harada N, Kouzu T, Arima M, et al. Endoscopic ultrasound-guided pancreatography: a case report. Endoscopy 1995;27:612-5. [Crossref] [PubMed]
- François E, Kahaleh M, Giovannini M, et al. EUS-guided pancreaticogastrostomy. Gastrointest Endosc 2002;56:128-33. [Crossref] [PubMed]
- Bataille L, Deprez P. A new application for therapeutic EUS: main pancreatic duct drainage with a "pancreatic rendezvous technique". Gastrointest Endosc 2002;55:740-3. [Crossref] [PubMed]
- Ghattas G, Deviere J, Blancas JM, et al. Pancreatic rendez-vous. Gastrointest Endosc 1992;38:590-4. [Crossref] [PubMed]
- Dumonceau JM, Cremer M, Baize M, et al. The transduodenal rendezvous: a new approach to deeply cannulate the main pancreatic duct. Gastrointest Endosc 1999;50:274-6. [Crossref] [PubMed]
- Fujii LL, Topazian MD, Abu Dayyeh BK, et al. EUS-guided pancreatic duct intervention: outcomes of a single tertiary-care referral center experience. Gastrointest Endosc 2013;78:854-64.e1. [Crossref] [PubMed]
- Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc 2007;65:233-41. [Crossref] [PubMed]
- Ergun M, Aouattah T, Gillain C, et al. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy 2011;43:518-25. [Crossref] [PubMed]
- Shah JN, Marson F, Weilert F, et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc 2012;75:56-64. [Crossref] [PubMed]
- Will U, Reichel A, Fueldner F, et al. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol 2015;21:13140-51. [Crossref] [PubMed]
- Oh D, Park DH, Cho MK, et al. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: early and midterm outcomes (with video). Gastrointest Endosc 2016;83:366-73.e2. [Crossref] [PubMed]
- Chen YI, Levy MJ, Moreels TG, et al. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest Endosc 2017;85:170-7. [Crossref] [PubMed]
- Matsunami Y, Itoi T, Sofuni A, et al. Evaluation of a new stent for EUS-guided pancreatic duct drainage: long-term follow-up outcome. Endosc Int Open 2018;6:E505-12. [Crossref] [PubMed]
- Giovannini M. Endoscopic ultrasound-guided pancreatic duct drainage: ready for the prime time? Endosc Ultrasound 2017;6:281-4. [Crossref] [PubMed]
- Kedia P, Gaidhane M, Kahaleh M. Endoscopic guided biliary drainage: how can we achieve efficient biliary drainage? Clin Endosc 2013;46:543-51. [Crossref] [PubMed]
- Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:904-14. [Crossref] [PubMed]
- Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898-900. [Crossref] [PubMed]
- Giovannini M, Dotti M, Bories E, et al. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy 2003;35:1076-8. [Crossref] [PubMed]
- Shorvon PJ, Cotton PB, Mason RR, et al. Percutaneous transhepatic assistance for duodenoscopic sphincterotomy. Gut 1985;26:1373-6. [Crossref] [PubMed]
- Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc 2003;57:246-51. [Crossref] [PubMed]
- Hindryckx P, Degroote H, Tate DJ, et al. Endoscopic ultrasound-guided drainage of the biliary system: Techniques, indications and future perspectives. World J Gastrointest Endosc 2019;11:103-14. [Crossref] [PubMed]
- Kahaleh M, Yoshida C, Kane L, et al. Interventional EUS cholangiography: a report of five cases. Gastrointest Endosc 2004;60:138-42. [Crossref] [PubMed]
- Kahaleh M, Wang P, Shami VM, et al. EUS-guided transhepatic cholangiography: report of 6 cases. Gastrointest Endosc 2005;61:307-13. [Crossref] [PubMed]
- Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc 2011;74:1276-84. [Crossref] [PubMed]
- Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy 2015;47:794-801. [Crossref] [PubMed]
- Sharaiha RZ, Kumta NA, Desai AP, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc 2016;30:5500-5. [Crossref] [PubMed]
- Sportes A, Camus M, Greget M, et al. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: a retrospective expertise-based study from two centers. Therap Adv Gastroenterol 2017;10:483-93. [Crossref] [PubMed]
- Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open 2016;4:E175-81. [Crossref] [PubMed]
- Kunda R, Perez-Miranda M, Will U, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc 2016;30:5002-8. [Crossref] [PubMed]
- Lee TH, Choi JH. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol 2016;14:1011-9.e3. [Crossref] [PubMed]
- Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016;48:1125-8. [Crossref] [PubMed]
- Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc 2017;85:1067-75. [Crossref] [PubMed]
- Minaga K, Takenaka M, Kitano M, et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc 2017;31:4764-72. [Crossref] [PubMed]
- Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018;88:9-17. [Crossref] [PubMed]
- Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: a systematic review and meta-analysis. Dig Dis Sci 2016;61:684-703. [Crossref] [PubMed]
- Uemura RS, Khan MA, Otoch JP, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: a systematic review and meta-analysis. J Clin Gastroenterol 2018;52:123-30. [Crossref] [PubMed]
- Itoi T, Baron TH, Khashab MA, et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc 2017;29:495-502. [Crossref] [PubMed]
- Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy 2011;43:337-42. [Crossref] [PubMed]
- Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy 2012;44:499-503. [Crossref] [PubMed]
- Xu MM, Dawod E, Gaidhane M, et al. Reverse endoscopic ultrasound-guided gastrojejunostomy for the treatment of superior mesenteric artery syndrome: a new concept. Clin Endosc 2020;53:94-6. [Crossref] [PubMed]
- Itoi T, Itokawa F, Uraoka T, et al. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos). Gastrointest Endosc 2013;78:934-9. [Crossref] [PubMed]
- Tringali A, Giannetti A, Adler DG. Endoscopic management of gastric outlet obstruction disease. Ann Gastroenterol 2019;32:330-7. [Crossref] [PubMed]
- Duarte-Chavez R, Kim M, Marino D, et al. Endoscopic ultrasound-guided gastroenterostomy versus enteral stent versus surgical gastrojejunostomy: systematic review and pooled analysis. Gastrointest Endosc 2020;91:AB308-9. [Crossref]
- Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc 2019;33:3404-11. [Crossref] [PubMed]
- Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut 2016;65:193-5. [Crossref] [PubMed]
- Tyberg A, Perez-Miranda M, Sanchez-Ocana R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open 2016;4:E276-81. [Crossref] [PubMed]
- Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open 2017;5:E275-81. [Crossref] [PubMed]
- Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an international collaborative study. J Clin Gastroenterol 2017;51:896-9. [Crossref] [PubMed]
- Kerdsirichairat T, Yang J, Brewer Gutierrez OI, et al. Su1362 Long-term outcomes of endoscopic ultrasound-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction: a 4-year cohort. Gastrointest Endosc 2018;87:AB320-1. [Crossref]
- Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc 2018;87:1215-21. [Crossref] [PubMed]
- Widmer JL, Winner M, Allendorf J, et al. Su1154 Single center comparative study of endoscopic gastrojejunostomy versus surgical gastrojejunostomy for malignant gastric outlet obstruction. Gastrointest Endosc 2019;89:AB291. [Crossref]
- Amateau SK, Lim CH, McDonald NM, et al. EUS-guided endoscopic gastrointestinal anastomosis with lumen-apposing metal stent: feasibility, safety, and efficacy. Obes Surg 2018;28:1445-51. [Crossref] [PubMed]
- Sooklal S, Kumar A. EUS-guided enterocolostomy for palliation of malignant distal small-bowel obstruction. VideoGIE 2019;4:530-1. [Crossref] [PubMed]
- Lajin M, Catalano MF, Orr CE, et al. EUS-guided duodenojejunostomy by use of a 2-cm lumen-apposing metal stent to treat proximal jejunal stricture in a patient with chronic pancreatitis. VideoGIE 2019;5:75-6. [Crossref] [PubMed]
- Mai HD, Dubin E, Mavanur AA, et al. EUS-guided colo-enterostomy as a salvage drainage procedure in a high surgical risk patient with small bowel obstruction due to severe ileocolonic anastomotic stricture: a new application of lumen-apposing metal stent (LAMS). Clin J Gastroenterol 2018;11:282-5. [Crossref] [PubMed]
- Ichkhanian Y, Yang J, James TW, et al. EUS-directed transenteric ERCP in non-Roux-en-Y gastric bypass surgical anatomy patients (with video). Gastrointest Endosc 2020;91:1188-94.e2. [Crossref] [PubMed]
- Cirocchi R, Kelly MD, Griffiths EA, et al. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon 2017;15:379-87. [Crossref] [PubMed]
- Seamon MJ, Pieri PG, Fisher CA, et al. A ten-year retrospective review: does pyloric exclusion improve clinical outcome after penetrating duodenal and combined pancreaticoduodenal injuries? J Trauma 2007;62:829-33. [Crossref] [PubMed]
- Dhindsa BS, Dhaliwal A, Mohan BP, et al. EDGE in Roux-en-Y gastric bypass: How does it compare to laparoscopy-assisted and balloon enteroscopy ERCP: a systematic review and meta-analysis. Endosc Int Open 2020;8:E163-71. [Crossref] [PubMed]
- Kedia P, Sharaiha RZ, Kumta NA, et al. Internal EUS-directed transgastric ERCP (EDGE): game over. Gastroenterology 2014;147:566-8. [Crossref] [PubMed]
- Bukhari M, Kowalski T, Nieto J, et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc 2018;88:486-94. [Crossref] [PubMed]
- Chiang AL, Gaidhane M, Loren DE, et al. 338 Impact of EUS-directed transgastric ERCP (EDGE procedure) access route on technical success and adverse events: a multi-center experience. Gastrointest Endosc 2018;87:AB70-1. [Crossref]
- James TW, Baron TH. Endoscopic ultrasound-directed transgastric ERCP (EDGE): a single-center us experience with follow-up data on fistula closure. Obes Surg 2019;29:451-6. [Crossref] [PubMed]
- Wang TJ, Thompson CC, Ryou M. Gastric access temporary for endoscopy (GATE): a proposed algorithm for EUS-directed transgastric ERCP in gastric bypass patients. Surg Endosc 2019;33:2024-33. [Crossref] [PubMed]
- James HJ, James TW, Wheeler SB, et al. Cost-effectiveness of endoscopic ultrasound-directed transgastric ERCP compared with device-assisted and laparoscopic-assisted ERCP in patients with Roux-en-Y anatomy. Endoscopy 2019;51:1051-8. [Crossref] [PubMed]
- Tyberg A, Nieto J, Salgado S, et al. Endoscopic ultrasound (EUS)-directed transgastric endoscopic retrograde cholangiopancreatography or EUS: mid-term analysis of an emerging procedure. Clin Endosc 2017;50:185-90. [Crossref] [PubMed]
Cite this article as: Duarte-Chavez R, Kahaleh M. Therapeutic endoscopic ultrasound. Transl Gastroenterol Hepatol 2022;7:20.