Transanal drainage tube: alternative option to defunctioning stoma in rectal cancer surgery?
Introduction
Anastomotic leakage (AL) is a major complication after rectal cancer surgery with incidence rates of 3–23%, leading to increased postoperative morbidity and mortality rates, increased length of hospital-stay and increased hospital costs (1-3). Moreover, oncologic outcome may be significantly impaired by AL (4-6). Most reported risk factors include advanced age, male gender, malnutrition, obesity, preoperative radiochemotherapy, larger tumour size, low anastomosis, number of stapler firings used to transect the rectum and blood transfusions (1-3). Defunctioning stoma (DS) has been used in order to reduce the incidence and severity of AL after rectal cancer surgery. However, stoma construction carries clinical disadvantages such as patient discomfort, stoma-related complications, the need for reversal procedure and increased hospital costs (7-10). Transanal drainage tube (TDT) placement has been recently introduced with promising results, mostly coming from Japanese experiences (11-16). The aim of this study was to evaluate the role of TDT on AL incidence, severity and hospital costs as alternative to DS.
Methods
This study was a single-centre retrospective analysis of a prospectively maintained database of patients who underwent sphincter-preserving low anterior resection for rectal adenocarcinoma between January 2000 and December 2016 at our Department. For the purpose of this study, patients were divided into two groups: the TDT group (A) and the non-TDT group (B). Written informed consent from all individual participants included in the study were obtained. The medical records were reviewed for patients’ demographic (age and gender), distance from the anal verge, tumour stage and grading, surgical approach, postoperative mortality, morbidity and management. Exclusion criteria were abdominoperineal resection, Hartmann’s procedure, transanal local excision, proctocolectomy, bypass or palliative DS only creation. Patients with distant metastases were also excluded. Preoperative staging included endoscopy and whole body computed tomography (CT) scan. Endorectal ultrasound and/or pelvic magnetic resonance imaging (MRI) were used to determine the extent of local tumours. Rectal cancers were grouped according to their distance from the anal verge: lower (<5 cm), middle (5–10 cm) and upper (>10 cm). Surgical procedures were standardized and performed by the same team and laparoscopic approach was introduced since 1997. Preoperative standard radiochemotherapy was administered in most locally advanced mid to low tumours (cT3/4 or N+) and patients operated 8–10 weeks after the completing of treatment. No short-course radiation therapy was performed. All patients underwent mechanical bowel preparation and prophylactic antibiotic therapy in accordance with the hospital guidelines. Standard surgical technique included full mobilization of the splenic flexure and high ligation of the inferior mesenteric artery and vein. Middle and lower cancers underwent nerve-sparing Total Mesorectal Excision (TME) while upper rectal cancers received Partial Mesorectal Excision (PME). Methods of reconstruction were end-to-end anastomosis by double stapling technique or straight coloanal hand-sewn anastomosis in most cases. A closed system drainage was inserted around the anastomosis site in all patients and removed within 6 days if AL was not defined. Pathologic stages were based on the 7th American Joint Committee on Cancer TNM Staging System (17). Postoperative overall 30-day mortality included all deaths occurring during hospitalization. Postoperative overall 30-day morbidity included medical and surgical complications.
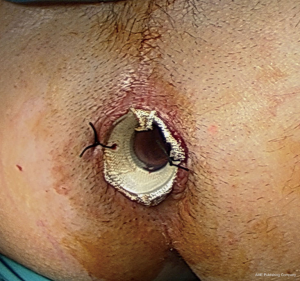
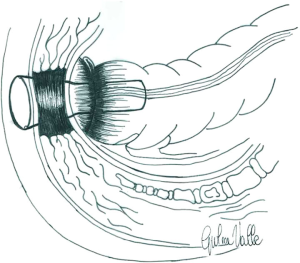
Since January 2007, a 6–8 cm long, 2 mm thick radiopaque soft silicon tube with a 2 cm calibre, named No Coil® (18) was placed in all patients after rectal surgery. At the end of the operation, it is secured to the perineal skin by two stitches (Figure 1). The device decreases intraluminal pressure by draining the watery stool and gas flow and keeping the anal sphincter open. In extremely low anastomosis, it was used with the intent to cover the newly formed anastomosis preventing faecal content from contaminating the anastomotic site (Figure 2). Generally, the tube was removed on the 5th postoperative day. The removal was made for two reasons. Since all patients had passing feces and gas after five days surgery, and to stop analgesic intake. AL was defined according to the International Study Group of Rectal Cancer (ISGRG) as “any defect of the intestinal wall integrity at the colorectal or coloanal anastomotic site (including suture or staple line of neorectal reservoirs) leading to a communication between the intra- and extraluminal compartments” and severity was classified into 3 grades (19). Grade A requiring no therapeutic intervention. Grade B requiring active intervention without relaparotomy, including medical (fasting, antibiotics, total parenteral nutrition, somatostatin analogues), radiological (percutaneous drainage) or surgical (transanal drainage or repair) treatment. In Grade C patients exhibited signs of peritonitis, severe sepsis or septic shock requiring emergency surgical management and Intensive Care Unit support. Diagnosis was suspected on the basis of digital rectal palpation, clinical symptoms and signs (pain, fever, ileus and abnormal drain discharge) and/or biological parameters (leukocytosis and recently elevated biomarkers level) and confirmed by enteral water-soluble contrast CT scan exam or endoscopy. Water-soluble contrast enema was performed only at the beginning of our experience.
Patients in group 1 were matched at a ratio of 1:1 with patients who had undergone resection in group 2. The matching was achieved on the basis of propensity scores (PSs), including the following covariables: Age, Gender, BMI and preoperative radiochemotherapy.
In addition to the clinical results, costs analysis was another purpose of our study. For an approximation of values, each different procedure in the patient population was assigned a total mean cost in Euros (€), according to the “Regione Lazio” refunded cost per each surgery and economic benefit estimation was calculated.
PS matching
PS matching was performed on the cohort to adjust any difference in average outcomes for patients’ selection bias. PS matching was performed by considering all significant variables between the two groups in the preliminary analysis. PSs were generated by logistic regression and relied on the following covariables: age, gender, BMI, and preoperative radiochemotherapy. After estimation of PSs, a regular 1:1 nearest-neighbor matching process was performed. A small caliper (0.1) was specified to improve balance.
Statistical analysis
The collected patient data were reviewed. Continuous patient and tumour data are presented as median (range). Categorical variables are presented as number and percentage. All variables were compared using the χ2 or Fisher’s exact test for categorical data, the Mann–Whitney U-test for non-normally distributed continuous data, and the Student’s t-test for normally distributed continuous variables. All data are expressed as mean ± standard deviation or median and range. A P value of <0.05 was considered as statistically significant. The statistical program SPSS version 22.0® (Chicago, Illinois, USA) was used for analysis.
Results
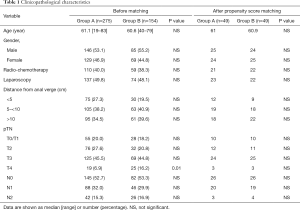
A total of 429 operated patients at our Department with at least 1 month of postoperative follow-up were identified. The TDT was placed in 275 (Group A) and not placed in 154 cases. Among the latter, in a subgroup of 54 cases (35%) a DS was created. Table 1 outlines clinicopathological characteristics of patients. Group A included significantly less T4 tumors (P=0.01).
Full table
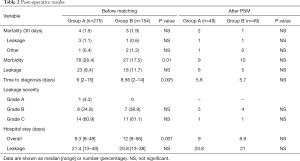
Postoperative results are shown in Table 2. No complications related to the tube placement occurred. Overall mortality rate was 1.5% in Group A and 3% in Group B. Overall morbidity was higher in first group, rate was 28.4% A and 17% respectively. The incidence of AL was significantly higher in Group B (18% vs. 8.4%; P=0.01), accounting for two thirds of all surgical complications and most of those requiring relaparotomy in both groups. According to the severity grading, there was no difference of AL Grade between the two groups. Median time to diagnosis was significantly higher in Group B (8.56 vs. 6 days; P=0.005) and median hospital stay was higher in patients with AL in both groups (21.4 vs. 20.8 days). In regards to treatment, 5 Grade B complications in Group A recovered with conservative medical and/or radiological treatment. The remaining 3 underwent transanal repair and TDT repositioning. Grade C patients underwent emergency surgery with Hartmann’s procedures (3), coloanal anastomosis with DS (6) and DS creation only (7). Postoperative mortality rate was 21.42% in this group (3 cases).
Full table
After propensity score match, there was no more difference of T4 rate in the two groups (Table 1). Postoperative courses were comparable with no difference between the two groups. Notable, time to diagnosis leakage and length of stay was similar (Table 2).
Discussion
AL remains the most dreaded complication after rectal cancer surgery with a significantly high incidence rate. Several risk factors have been identified but since there is no consensus on the definition, available data are difficult to interpret and compare. According to Caulfield et al., it is a spectrum of clinical entities with different treatment and outcomes (20). In the current study we used the definition proposed by ISGRG since it is a valid classification system enabling a reliable correlation between AL and the severity of its impact in the clinical course of the patient. Grade C leaks comprised most of our leakage cases, as it occurred in other recent publications (7,21,22). Patients required prompt operative re-intervention showing higher mortality rate and longer hospital stay. About one third of leakages were classified as Grade B and patients were successfully managed without re-laparotomy. The unique Grade A case observed in this study may be explained in two ways. First, patients are usually asymptomatic and diagnosis is made during routine postoperative control or later during routine workup before stoma closure. Second, the presence of a DS may hide some clinically silent cases. It is reasonable to assume that the estimated number of undetected cases actually is higher.
Although there is currently no consensus on which is the best imaging modality to detect AL, CT scan with enteral contrast is becoming the gold standard. The relatively low sensitivity should be taken into account when relying on CT imaging to prevent delay in diagnosis (23,24). Besides the potential to visualize AL, CT scan has the advantage to detect alternative adverse events. According to the definition of ISGRG, an abscess in the vicinity of the anastomotic site without an obvious faecal fistula should also be classified as AL (19). Three (12%) patients in Group A (2 Grade B and 1 Grade C) had such an abscess in our series.
Since AL after cancer rectal surgery may cause life threatening complications with increased risk of postoperative mortality, stoma creation has been advocated in order to decrease its incidence or the severity of septic complications and need for reoperation. Despite diversion, a considerable number of patients will still develop clinical signs of AL. In a large series, DS leads to a lower AL rate, though it was associated with higher rates of complications, prolonged hospital stay and mortality (25). In diverted anastomosis, leakage could become clinically evident beyond 30 days, especially after preoperative chemoradiotherapy (26,27). Stomal problems should also not be ignored (i.e., discomfort, dehydration, stoma-related complications) and reversal procedure is associated with morbidity and increased hospital costs (4,7-10). Patients with anastomosis-related complications have been also reported to be at high risk of permanent stoma (2,28,29) and impaired functional results and quality of life (30,31). As a consequence, more selective use has been increasingly recommended only in high risk anastomosis in the last years (7,10,32,33). Our policy is to routinely divert patients with severe co-morbidity, intraoperative complications and extremely low anastomosis after preoperative chemoradiation.
When diversion is not absolutely indicated, TDT may be a suitable alternative. It is supposed to be beneficial for reducing intraluminal pressure, allowing adequate gas and watery stool drainage, resulting in a protective effect on anastomotic healing. In presence of a possible early postoperative spasm of the anal sphincter, it may provide partial incontinence that ensures the patency of anastomosis. Previous studies showed that measured rectal resting pressure was lower in patients with transanal tube (13,18). It also appears to function as a target drainage allowing easy drainage of bowel contents, reducing the degree of extraluminal sepsis in case of AL (11,15). Promising results have been reported in studies limited by small sample size, varied indications and use of nonrandomized trials (11-15). Differences in each study such as tube material, shape and diameter of the device, length of insertion and duration of its placement should be also considered when results are evaluated. Nevertheless, effectiveness of prophylactic TDT placement has been confirmed in two recently published systematic review and meta-analysis (34,35). Retrospective comparative studies showed that TDT supports anastomotic site protection avoiding stoma-related complications (1,14-16). The optimal time of tube placement has not been determined and mean reported duration is between 4 and 6 days. Excluding late leakages related to vascular or septic complications, the patency of anastomosis is usually consolidated after 5 days in most cases and this period was considered enough for removal of TDT.
In our experience, it was used mainly to lower intraluminal pressure in 176 cases (64%) and provide direct protection of the anastomosis in 99 cases (36%). Our 8.4% AL incidence rate was consistent with those reported in the literature. Even though more risk factors were present in this group of patients, tube placement was associated with a reduction of AL incidence and Grade C cases compared to the control group. All the laparoscopic procedures were performed by senior surgeons, but the higher AL incidence rate in the first period of the study may be partially explained considering the learning period of the technique.
One of the main end points of the study was to determine the patient’s quality of life without DS. The TDT is well tolerated since it requires only a 5-day placement versus an average of 3 months for the stoma. Significant stoma-related disadvantages are also avoided, keeping in mind that it does not safely prevent AL occurrence. Tube placement has been reported to increase patient discomfort and inconvenience in few cases, mostly including perineal pain (11,13-15). At the beginning of our experience, 3 cases of urinary retention with pain were observed, so continuous administration of analgesics until TDT removal was introduced with beneficial effects.
Another purpose of our study was to estimate the hospital costs. Leakage occurrence has been clearly associated with increased health-care costs in recent publications (36). A randomized multicentric trial showed that DS creation was more expensive, despite the cost-savings associated with a reduced incidence of AL (37). In our experience, an estimated economic benefit of €4,000 for each patient with TDT compared with those requiring DS and reversal procedure was obtained regardless of AL occurrence (Table 3), allowing a significant decrease in overall economic burden.
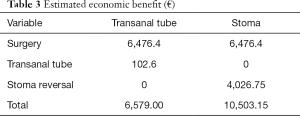
Full table
Despite advances in surgical technology, studies on patients and methods of prevention, AL continues to occur at a high rate and the fundamental pathogenesis remains unknown. According to Shogan et al., the fundamental pathogenesis of leakage remains unknown and often anastomosis healing occurs without any incidents in high-risk patients while many patients without a single risk factor will develop leakage (38). It is of critical importance to identify predictors of AL to enable early diagnosis and appropriate treatment. The interval between surgery and clinical onset suggests a preclinical phase in which reliable biomarkers could be used to predict AL (24). Treatment should be based on leakage location and severity and patient’s clinical condition but non operative management may be safely and successfully adopted in many cases (39-41).
The study presents evident limitations. A relatively small sample size covering a long period was enrolled. The analysis was nonrandomized and retrospective and no strict criteria were established for the selection of patients undergoing tube insertion, clearly creating a selection bias. Nevertheless, it includes 429 homogeneously operated patients in a Western single-centre. Estimated economic cost-saving was also calculated for the first time.
In conclusion, leakage of the anastomosis remains the Achilles heel of rectal cancer surgery and even a DS does not safely prevent its occurrence. In our opinion, TDT may be considered a suitable option in many cases. Although the AL incidence was similar in our experience, the tube allows to avoid a stoma-related consequence and the need for reversal procedure. Overall estimated economic burden was also reduced. Considering the promising results, large prospective randomized clinical trials are needed to confirm the benefits.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the IFO - Istituto Regina Elena Institutional board review (IRB #2016-73) and written informed consent was waived.
References
- Bennis M, Parc Y, Lefevre JH, et al. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg 2012;255:504-10. [Crossref] [PubMed]
- Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 2013;148:65-71. [Crossref] [PubMed]
- Wang S, Liu J, Wang S, et al. Adverse effects of anastomotic leakage on local recurrences and survival after curative resection for rectal cancer: a systematic review and meta-analysis. World J Surg 2017;41:277-84. [Crossref] [PubMed]
- Kulu Y, Tarantio I, Warschkow R, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol 2015;22:2059-67. [Crossref] [PubMed]
- Hain E, Maggiori L, Manceau G, et al. Oncological impact of anastomotic leakage after laparoscopic mesorectal excision. Br J Surg. 2017;104:288-95. [Crossref] [PubMed]
- Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 2015;29:3608-17. [Crossref] [PubMed]
- Hanna MH, Vinci A, Pigazzi A. Diverting ileostomy in colorectal surgery: when is it necessary? Langenbecks Arch Surg 2015;400:145-52. [Crossref] [PubMed]
- Messaris E, Connelly TM, Kulaylat AN, et al. Is a diverting ostomy needed in mid-high rectal cancer patients undergoing a low anterior resection after neoadjuvant chemoradiation? An NSQIP analysis. Surgery 2015;158:686-91. [Crossref] [PubMed]
- Anderin K, Gustafsson UO, Thorell A, et al. The effect of diverting stoma on postoperative morbidity after low anterior resection for rectal cancer in patients treated within an ERAS program. Eur J Surg Oncol 2015;41:724-30. [Crossref] [PubMed]
- Ihnát P, Gunkova P, Peteja M, et al. Diverting ileostomy in laparoscopic rectal surgery: high price of protection. Surg Endosc 2016;30:4809-16. [Crossref] [PubMed]
- Brandl A, Czipin S, Mittermair R, et al. Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: A case controlled study. Ann Med Surg (Lond) 2016;6:12-6. [Crossref] [PubMed]
- Hidaka E, Ishida F, Mukai S, et al. Efficacy of transanal tube for prevention of anastomotic leakage following laparoscopic low anterior resection for rectal cancers: a retrospective cohort study in a single institution. Surg Endosc 2015;29:863-7. [Crossref] [PubMed]
- Matsuda M, Tsuruta M, Hasegawa H, et al. Transanal drainage tube placement to prevent anastomotic leakage following colorectal cancer surgery with double stapling reconstruction. Surg Today 2016;46:613-20. [Crossref] [PubMed]
- Yang CS, Choi GS, Park JS, et al. Rectal tube drainage reduces major anastomotic leakage after minimally invasive rectal cancer surgery. Colorectal Dis 2016;18:O445-52. [Crossref] [PubMed]
- Kim MK, Won DY, Lee JK, et al. Comparative study between transanal tube and loop ileostomy in low anterior resection for mid rectal cancer: a retrospective single center trial. Ann Surg Treat Res 2015;88:260-8. [Crossref] [PubMed]
- Lee SY, Kim CH, Kim YJ, et al. Impact of anal decompression on anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis. Langenbecks Arch Surg 2015;400:791-6. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Montemurro S, De Luca R, Caliandro C, et al. Transanal tube NO COIL® after rectal cancer proctectomy. The "G.Paolo II" Cancer Centre experience. Tumori 2012;98:607-14. [Crossref] [PubMed]
- Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339-51. [Crossref] [PubMed]
- Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg 2013;148:177-82. [Crossref] [PubMed]
- Kulu Y, Ulrich A, Bruckner T, et al. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 2013;153:753-61. [Crossref] [PubMed]
- Matsuda K, Hotta T, Takifuji K, et al. Clinical characteristics of anastomotic leakage after an anterior resection for rectal cancer by assessing of the international classification on anastomotic leakage. Langenbecks Arch Surg 2015;400:207-12. [Crossref] [PubMed]
- Huiberts AA, Dijksman LM, Boer SA, et al. Contrast medium at the site of the anastomosis is crucial in detecting anastomotic leakage with CT imaging after colorectal surgery. Int J Colorectal Dis 2015;30:843-8. [Crossref] [PubMed]
- Hirst NA, Tiernan JP, Millner PA, et al. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 2014;16:95-109. [Crossref] [PubMed]
- Bakker IS, Snijders HS, Wouters MW, et al. High complication rate after low anterior resection for mid and high rectal cancer; results of a population-based study. Eur J Surg Oncol 2014;40:692-8. [Crossref] [PubMed]
- Leahy J, Schoetz D, Marcello P, et al. What is the risk of clinical anastomotic leak in the diverted colorectal anastomosis? J Gastrointest Surg 2014;18:1812-6. [Crossref] [PubMed]
- Lim SB, Yu CS, Kim CW, et al. Late anastomotic leakage after low anterior resection in rectal cancer patients: clinical characteristics and predisposing factors. Colorectal Dis 2016;18:O135-40. [Crossref] [PubMed]
- Kim MJ, Kim YS, Park SC, et al. Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery 2016;159:721-7. [Crossref] [PubMed]
- Seo SI, Yu CS, Kim GS, et al. Characteristics and risk factors associated with permanent stomas after sphincter-saving resection for rectal cancer. World J Surg 2013;37:2490-6. [Crossref] [PubMed]
- Mongin C, Maggiori L, Agostini J, et al. Does anastomotic leakage impair functional results and quality of life after laparoscopic sphincter-saving total mesorectal excision for rectal cancer? A case-matched study. Int. J Colorectal Dis 2014;29:459-67. [Crossref] [PubMed]
- Schiergens TS, Hoffmann V, Schobel TN, et al. Long-term quality of life of patients with permanent end ileostomy: results of a nationwide cross-sectional survey. Dis Colon Rectum 2017;60:51-60. [Crossref] [PubMed]
- Shiomi A, Ito M, Maeda K, et al. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 2015;220:186-94. [Crossref] [PubMed]
- Mrak K, Uranitsch S, Pedross F, et al. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery 2016;159:1129-39. [Crossref] [PubMed]
- Yang Y, Shu Y, Su F, et al. Prophylactic transanal decompression tube versus non-prophylactic transanal decompression tube for anastomotic leakage prevention in low anterior resection for rectal cancer: a meta-analysis. Surg Endosc 2017;31:1513-23. [Crossref] [PubMed]
- Zhao WT, Li NN, Feng JY. Transanal tube for the prevention of anastomotic leakage after rectal cancer surgery: a systematic review and meta-analysis. World J Surg 2017;41:267-76. [Crossref] [PubMed]
- Hammond J, Lim S, Wan Y, et al. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg 2014;18:1176-85. [Crossref] [PubMed]
- Floodeen H, Hallbook O, Hagberg LA, et al. Costs and resource following defunctioning stoma in low anterior resection for cancer. A long-term analysis of a randomized multicenter trial. Eur J Surg Oncol 2017;43:330-6. [Crossref] [PubMed]
- Shogan BD, Carlisle EM, Alverdy JC, et al. Do we really know why colorectal anastomoses leak? J Gastrointest Surg 2013;17:1698-707. [Crossref] [PubMed]
- Blumetti J, Chaudhry V, Cintron JR, et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg 2014;38:985-91. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Alizadeh RF, et al. Contemporary management of anastomotic leak after colon surgery: assessing the need for reoperation. Am J Surg 2016;211:1005-13. [Crossref] [PubMed]
- Son GM, Kim JG, Lee JC, et al. Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A 2010;20:609-17. [Crossref] [PubMed]
Cite this article as: Carboni F, Valle M, Levi Sandri GB, Giofrè M, Federici O, Zazza S, Garofalo A. Transanal drainage tube: alternative option to defunctioning stoma in rectal cancer surgery? Transl Gastroenterol Hepatol 2020;5:6.