Modular step-up approach to robot-assisted transthoracic esophagectomy—experience of a German high volume center
Introduction
The use of robot-assisted technology in general surgery is dramatically increasing. This may be due to the increasing worldwide distribution of robotic systems and the associated increased experience of surgeons using them. On the other hand, the technical development of the systems with advanced instrumentation has favored their use in complex visceral and thoracic surgical procedures (1). Transthoracic esophageal surgery is among the most complex minimally invasive surgical procedures, and especially the reconstruction phase with gastric interposition and esophagogastric anastomosis is currently a highly debated part of the surgical procedure (2).
One possibility to diminish the complexity of a minimally invasive procedure is the use of hand-assisted or hybrid approaches (3,4). Another more recent approach is the division of complex procedures into different modules and to perform a step-by-step approach to a complex procedure (5). This has been first proposed for trainees in open pancreatic surgery, but this idea has also been used in laparoscopic and lately in the implementation process of robotic technology to complex procedures (6).
In the recent past, a considerable number of reports were published on anastomotic techniques and early postoperative results of robotic esophageal surgery (2). In particular, the techniques for the esophagogastrostomy are reported to involve a variety of non-standardized techniques. Some surgeons use a robot-assisted manual hand-sewn technique or a linear-stapled side to side esophagogastrostomy or interestingly more rarely the standardized circular stapled esophagogastrostomy, even if this technique is among the most commonly performed and most standardized technique in open esophageal surgery (2,4,7,8).
Our center was among the contributors to a recently published report on standardization of the robotic assisted Ivor-Lewis procedure including 7 large German academic centers (9). From the initial implementation of robotic technology in our department, we followed a strict modular approach to finally perform a fully robotic assisted transthoracic esophagectomy. The purpose of this paper is to present this modular approach to robotic assisted Ivor-Lewis esophagectomy with a standard circular-stapled anastomosis.
Methods
Patients who undergo esophagectomy for esophageal cancer in our high-volume center (n>300 upper gastrointestinal surgeries per year) are entered into our prospectively maintained database. This study includes data from March 2017 to December 2018. All patients who underwent robotic surgery on our modular pathway to robotic esophagectomy were included in this present study after approval from our institutional review board.
Demographic data, endoscopic findings, and tumor histology and stage were recorded. Complications were classified according to Dindo-Clavien.
We defined five different key steps (modules) of the complex operation. A step-up was only performed if the operating surgeon felt safe to do so and if operative time of the previously performed module was comparable to a standard open or laparoscopic/thoracoscopic case.
Treatment pathway of patients with resectable esophageal cancer
Treatment of patients with esophageal cancer is undertaken in accordance with national and international guidelines (10-13): patients with esophageal cancer in our national center of excellence undergo routine staging including but not limited to endoscopy, endoscopic ultrasound, CT-scans (thorax and abdomen) as well as pulmonary function testing with spirometry. Additionally, bronchoscopy is performed in squamous cell cancer patients to rule out tracheobronchial infiltration.
Early mucosal carcinomas may be resected endoscopically, advanced cT1b and cT2NxM0 cancers should be treated surgically. cT3 stage esophageal cancers or resectable higher stages, including node positive cT2 stage, are treated in a multimodal setting with neoadjuvant chemotherapy (FLOT) or chemoradiation (CROSS). Non resectable cases undergo palliative definitive chemoradiation. Restaging after neoadjuvant treatment includes gastroscopy and CT-scans. The surgical resection is performed 4 to 6 weeks after neoadjuvant treatment as a standardized Ivor-Lewis procedure with a high intrathoracic esophagogastrostomy after two-field lymphadenectomy (14). The standard surgical procedure in our institution is a hybrid Ivor-Lewis esophagectomy (abdominal part performed laparoscopically, thoracic part performed open). Our hybrid procedure has been described before (15). We perform an intense preoperative risk scoring and assessment before surgery (16,17). This preoperative risk management produces an ultra low in-hospital mortality rate after esophagectomy of around 1.5% also including high risk patients over the last years. If patients are classified as low risk patients in this risk assessment, we offer the patients a totally minimally invasive procedure. Since 02/2017 we have access to a da Vinci XI system that is used for this purpose.
Technique description—abdominal phase
Our robotic technique has been adapted and modified from colleagues from Mainz and Utrecht (7,18). The laparoscopic gastric mobilization (gastrolysis) is the most common laparoscopic procedure performed in our department, also performed by fellows and surgical trainees. Due to this standardization and high performance level, this phase of the operation is hard to improve from an oncological standpoint as well as operative speed. Therefore, we often maintain this technique even in the robotic era.
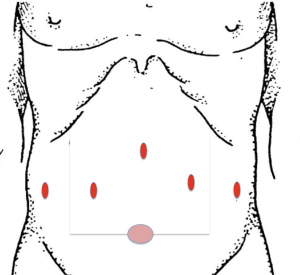
The following steps for the creation of the gastric conduit are performed in a standardized fasion: the surgery is performed with the patient in French and anti-Trendelenburg position. Our trocar placement is shown in Figure 1. The liver is elevated with a 5 mm cuschieri retractor through the right 5 mm trocar, which is fixed to the OR table. The scope is inserted through the upper subxiphoidal 5 mm trocar (45 degrees angled scope, 5 mm Stryker ICG) or 8 mm robotic trocar (45 degrees angled scope, 8 mm intuitive ICG). The hiatal dissection is performed without opening the right pleura whenever possible. We continue the procedure with the D2 lymphadenectomy along the hepatic ligament, the hepatic artery and the celiac trunk which will be extended from the lesser curvature along to the splenic artery. The greater curvature is dissected, starting from the corpus region beyond the epiploic vessels toward the left crus of the diaphragm; here we leave a part of the greater omentum at the region just below the spleen for a later omentum wrap covering the anastomosis. The stomach is dissected on the Crowfoot region, where the tri-stapler [Endo Gia (Covidien)—violet, 45 mm] is applied to do the first bite for the later gastric sleeve. The gastric conduit is completed with at least two additional Endo Gia 60 mm violet stapling magazines. Indocyanine green (ICG) can be used in combination with the da Vinci Xi and Stryker system to identify the gastroepiploic vessels via fluorescence.
Technique description—thoracic phase
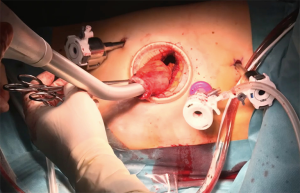
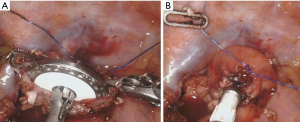
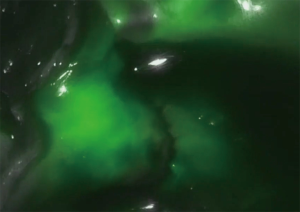
The thoracic phase is when the robotic potential becomes evident. The dissection of lymph nodes especially in the carina region can be performed extremely radical and controlled. Our trocar placement and anastomotic set up is shown in Figure 2. The patient is bedded in a left lateral semiprone position with the right lung not ventilated. The robot is docked from the patient right side resulting in a view from the left, comparable to the assistants view in an open Ivor Lewis case. Our standardized steps of the operation remain unchanged. For dissection, the monopolar cautery hook is used, starting the dissection from the pulmonary ligament upward along the pericardial layer toward the azygos vein, which is stapled with a tri-stapler [Endo Gia (Covidien)—gold, 45 mm]. The thoracic duct is always dissected and clipped with 2 polymer clips (Grena Click'aV®). The subcarinal esophagus is dissected along the pericardium as well as from the aortic side. The esophagus does not need to be looped in all cases, as the triangulating robotic instruments allow for enough traction and counter-traction. The dissection of the carinal, retrotracheal, and paratracheal tissue can be performed extremely radical and controlled leaving the vagal and recurrent nerves without damage. A monofilament purse string suture is then performed robotically, and the gastric conduit is brought into the right thorax after opening the hiatus into the abdominal cavity. A variation of this technique is an esophagogastric anastomosis using the orvil system (Covidien). One 12-mm assistant trocar is then removed, and this incision is extended to a mini thoracotomy with a standardized length of 7cm. An Alexis S Wound Protector/Retractor (Alexis Laparoscopic System, Applied Medical) is inserted. The stapler head is inserted and sutured into the esophageal stump using the prepared purse string suture using the robotic instruments (Figure 3A,B). The set-up can be seen in Figure 2. A second purse string suture may be placed. The da Vinci Xi system is then disconnected, and the camera is held by hand by the assistant similar to conventional thoracoscopy. The surgeon then luxates the transected esophagus out of the chest. The conduit is completed using an Endo GIA (Covidien) and a various amount of stapler loads. The specimen is removed via the minithoracotomy. The circular stapled esophagogastric anastomosis is then performed as depicted on Figure 2 using a circular stapler (25 or 28 mm) inserted through the minor curvature of the stomach. The omentum wrap may be used to cover the anastomosis and we always perform a quality control using ICG (Figure 4).
Follow up
Patients who underwent surgery for esophageal cancer are subsequently followed with routine check-ups performed in our outpatient clinic. Even if not required by national and international guidelines for esophageal cancer, we routinely follow a specific scheme for this follow-up program, including physical examination, blood samples and tumor markers as well as endoscopic evaluations and CT-scans at specific time points. During a 5-year follow-up period, typically 7 endoscopies and 7 CT scans are performed.
Data collection and statistical analysis
Data were collected prospectively, including but not limited to, patient demographics, operative and oncologic parameters, conversions, intraoperative complications, postoperative complications, readmissions, length of hospital stay, mortality, and reoperations. Our main outcome of measure was the definition of modules for a safe introduction of a new technology to a complex procedure. The incidence of in hospital mortality following esophagectomy was also recorded and compared to our benchmark population that was not operated using the surgical robot. Continuous variables are presented as means and range. Categorical data are presented as numbers and percentages. The Student t test (for continuous variables) and Chi Square test (for nominal or categorical variables) were used for all bivariate analyses. All tests were 2-sided, with statistical significance set at P≤0.05. Data were analyzed by Stata 11.0 (StataCorp., College Station, TX).
Results
As derived from previous reports in other surgical fields, the following modules were defined before surgical implementation of the new technology. Two prep modules were established in our case, as the da Vinci Xi robot was at that time a new device in our hospital. The six esophageal modules divide the approach to robotic assisted esophagectomy into straightforward and manageable steps that should not take more than 1–2 hours, depending on proficiency level.
Definition of modules and modular approach:
- Prep module: simulation, inanimate and animate training;
- Prep module: simple training procedures with increasing difficulty (robotic cholecystectomy, fundoplication);
- Esophageal module: abdominal phase (gastric mobilization);
- Esophageal module: thoracic docking, thoracoscopy, first steps of dissection and lymphadenectomy;
- Esophageal module: esophageal dissection, division of azygos vein;
- Esophageal module: paracarinal lymphadenectomy;
- Esophageal module: high intrathoracic lymphadenectomy and high intrathoracic esophageal transection;
- Esophageal module: esophagogastric anastomosis.
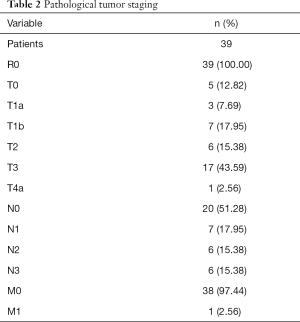
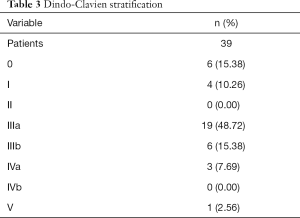
A total of 70 patients (9 females) fulfilled inclusion criteria to our study. On our modular pathway, we completed a total of 30 cases in prep module 2, mainly cholecystectomies (n=6) and benign esophageal cases (n=19). This resulted in a good proficiency level for two esophageal surgeons to feel comfortable with the robotic technology and to safely perform benign esophageal cases with an acceptable OR time. A total of 41 patients underwent upper gastrointestinal cancer surgery. Two patients underwent (transhiatal extended) gastrectomy, 39 patients underwent Ivor Lewis esophagectomy, using the robot for the abdominal and/or the thoracic phase. Of these patients, 38 underwent neoadjuvant treatment (92.7%) due to clinically advanced cT3 cancer. Esophageal adenocarcinoma was present in 34 cases. Mean length of stay was 16 days (range, 12–52 days). All patients underwent an R0 resection. One patient died due to pulmonary dysfunction and severe ARDS on the ICU (in-hospital mortality 1/41=2.4%). This patient underwent surgery in the first set of 10 patients and therefore had an open reconstruction phase (esophageal modules 1–3 were performed in this patient). Mean OR time of the first 10 cases was 7:25 hours (range, 6:21–10:40 hours), mean OR time of the last 10 cases was 6:43 hours (range, 5:16–8:06 hours). This difference was not statistically significant. The detailed demographic and oncologic information of all patients with esophageal cancer can be found in Tables 1-3. There were no intraoperative complications and no unplanned conversions to open surgery. Two surgeons were able to sequentially train and perform a completely robotic transthoracic esophagectomy using this modular approach. A total of 10 cases per surgeon were necessary to complete all modules in one case.

Full table
Full table
Full table
Discussion
The modular step up approach allowed us to introduce a complex new device to clinical practice without quality compromises in 41 patients with esophageal cancer. Using this approach and having two robotic daVinci Xi consoles in our OR setup allowed for a significant reduction of the learning curve that we faced with new devices or techniques in the past. Even if there was a tendency of a reduction of 43 min of OR time to the end of this series, there was no significant difference when comparing the first set of robotic assisted surgeries to the last set in our cohort. This shows how effective the modular approach is and how minimal the effect of the learning curve is for the patient. One Dindo-Clavien V complication in the postoperative course was likely unrelated to the robotic approach as the entire reconstruction phase was performed open in this early patient set (first 10 cases). Nevertheless, a critical discussion of reasons for every severe complication is the routine in our M&M conference. It is too early to statistically compare the complication rate in this early case series with our standardized hybrid approach (laparoscopic gastrolysis—open transthoracic esophagectomy), but for the moment the mortality in this present series does not seem to differ significantly from mortality in our benchmark population (1.5%).
Some points of criticism regarding this used new technology need to be addressed in this manuscript. When introducing a new technology as few compromises as possible should be present. The robotic technology allows for excellent triangulation and allows for probably even more precise surgery than in an open or laparoscopic/thoracoscopic case, especially in narrow anatomic regions. Nevertheless, the robotic instrumentarium is currently very limited, especially regarding sealing devices for fast and accurate dissection. Hopefully more precise devices will be developed, maybe the upcoming new robotic platforms will also provide better instruments. This limitation leads to a compromise: we and most other robotic esophageal surgeons use monopolar energy for the most part in the thoracic phase, whereas we do not do this in an open/thoracoscopic case (4,7,18).
Grimminger et al. reported recently results after their first 25 robotic assisted esophageal resections after implementing the robotic system to their hospital (19). In concordance with our report they conclude that the introduction of the robot can be done without quality compromises when compared to hybrid or laparoscopic/thoracoscopic cases. No modular approach is reported in this paper, but a learning curve cutoff of 16 cases is reported for one surgeon. It is interesting to compare this when looking at our data, as no clear learning curve exists when looking at OR time using our modular approach. As mentioned in our results section, 20 out of our 41 robotic cases were performed just in stepwise modules by two surgeons, with increasing complexity but without a dramatically increased OR time when compared to our standard approach. So when looking at cases needed to gain proficiency in totally minimally invasive esophagectomy, our approach seems to be comparable or even more effective (n=10) than in the previously reported series, where learning curve cutoffs up to 40 cases in terms of OR time are reported (20,21). Overall, the OR time in our collective is comparable to other reports using the same approach. When compared to our standard technique in our benchmark population, we face an increase of around 60 min at this time.
When looking at in-hospital mortality we are luckily facing an extremely low rate in our cohort. As reported earlier, the mortality in the present series was 1/41=2.4% and 1.5% in the benchmark population at our institution. Mortality in smaller centers has been reported to be still higher than 10% in recent series (22,23). We recently performed an analysis of the nationwide inpatient sample in the United States and found an overall mortality rate of 7.7% for the period of 1998–2011 (16). Birkmeyer even reported mortality rates exceeding 20% for smaller centers in 2002 (24). On the other hand, high volume centers such as ours are able to drop this rate even below 1%, also using minimally invasive techniques (25). We reported this very strong effect of hospital volume on in-hospital mortality analyzing more than 24,000 cases too (23).
The use of the presented technique reveals the robotic advantages. The precise dissection is one fact, in addition the reconstruction is not changed compared to open and compared to most robotic approaches by using a “robotic hand-sewed” method. This allows for a maximum of standardization in minimally invasive esophagectomy which has already been stated by our coworkers (18). A modular approach has been described for other specialties but also for open surgery and during the transition to laparoscopic and thoracoscopic general surgery (5,26-29). Especially complex open general surgery procedures in hepatobiliary and pancreatic surgery belong to the first reports (30). Giulianotti performed the first robotic Whipple procedure in 2001 and recently the University of Chicago reported their step-by-step approach (5). Similar reports exist for colorectal and thoracic surgery, and Urology. We believe that our first description of such an approach to upper gastrointestinal surgery—definitely belonging to the most complex surgeries performed—will help to implement robotic technology to this field and guide future coworkers.
Conclusions
To our knowledge, no modular approach for complex robotic assisted esophageal cancer surgery has been previously reported. Our main goal was to minimize the learning curve and to present an innovative way to successfully do so. The effects of minimally invasive esophageal resection for patients have already been clearly described before and were again confirmed by our study.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci 2018;1434:149-55. [Crossref] [PubMed]
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Wahba R, Kleinert R, Hellmich M, et al. Optimizing a living kidney donation program: transition to hand-assisted retroperitoneoscopic living donor nephrectomy and introduction of a passive polarizing three-dimensional display system. Surg Endosc 2017;31:2577-85. [Crossref] [PubMed]
- Grimminger PP, Fuchs HF. Minimally invasive and robotic-assisted surgical management of upper gastrointestinal cancer. Chirurg 2017;88:1017-23. [Crossref] [PubMed]
- Giulianotti PC, Mangano A, Bustos RE, et al. Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique: Lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc 2018;32:4329-36. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc 2010;24:1533-41. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- van der Sluis PC, van Hillegersberg R. Robot assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer. Best Pract Res Clin Gastroenterol 2018;36-37:81-3. [Crossref] [PubMed]
- Egberts JH, Biebl M, Perez DR, et al. Robot-Assisted Oesophagectomy: Recommendations Towards a Standardised Ivor Lewis Procedure. J Gastrointest Surg 2019;23:1485-492. [Crossref] [PubMed]
- Fuchs HF, Hölscher AH. Therapeutic decisions in patients with operable, non-metastatic oesophageal cancer. Zentralbl Chir 2014;139:32-6. [PubMed]
- Moehler M, Al-Batran SE, Andus T, et al. German S3-guideline "Diagnosis and treatment of esophagogastric cancer". Z Gastroenterol 2011;49:461-531. [Crossref] [PubMed]
- Moehler M, Baltin CT, Ebert M, et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer 2015;18:550-63. [Crossref] [PubMed]
- Hölscher AH, Stahl M, Messmann H, et al. New S3 guideline for esophageal cancer: Important surgical aspects. Chirurg 2016;87:865-72. [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Hölscher AH, Schneider PM, Gutschow C, et al. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 2007;245:241-6. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Simple preoperative risk scale accurately predicts perioperative mortality following esophagectomy for malignancy. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Schröder W, Bollschweiler E, Kossow C, et al. Preoperative risk analysis--a reliable predictor of postoperative outcome after transthoracic esophagectomy? Langenbecks Arch Surg 2006;391:455-60. [Crossref] [PubMed]
- Grimminger PP, Hadzijusufovic E, Lang H. Robotic-Assisted Ivor Lewis Esophagectomy (RAMIE) with a Standardized Intrathoracic Circular End-to-side Stapled Anastomosis and a Team of Two (Surgeon and Assistant Only). Thorac Cardiovasc Surg 2018;66:404-6. [Crossref] [PubMed]
- Grimminger PP, Tagkalos E, Hadzijusufovic E, et al. Change from Hybrid to Fully Minimally Invasive and Robotic Esophagectomy is Possible without Compromises. Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Guo W, Zou YB, Ma Z, et al. One surgeon's learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc 2013;27:1346-52. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Lapar DJ, Stukenborg GJ, Lau CL, et al. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg 2012;144:1152-7. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31:2491-7. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Veronesi G, Dorn P, Dunning J, et al. Outcomes from the Delphi process of the Thoracic Robotic Curriculum Development Committee. Eur J Cardiothorac Surg 2018;53:1173-9. [Crossref] [PubMed]
- Jutric Z, Warner S, Fong Y. A practical guide to development of a successful robotic abdominal surgery program: The path to implementation. Rozhl Chir 2017;96:49-53. [PubMed]
- Schiavina R, Borghesi M, Dababneh H, et al. The impact of a structured intensive modular training in the learning curve of robot assisted radical prostatectomy. Arch Ital Urol Androl 2018;90:1-7. [Crossref] [PubMed]
- Marino M, Gulotta G, Komorowski AL. Robotic Pancreaticoduodenectomy: Technical Considerations. Indian J Surg 2018;80:118-22. [Crossref] [PubMed]
- Ciancio G, Gonzalez J, Shirodkar SP, et al. Liver transplantation techniques for the surgical management of renal cell carcinoma with tumor thrombus in the inferior vena cava: step-by-step description. Eur Urol 2011;59:401-6. [Crossref] [PubMed]
Cite this article as: Fuchs HF, Müller DT, Leers JM, Schröder W, Bruns CJ. Modular step-up approach to robot-assisted transthoracic esophagectomy—experience of a German high volume center. Transl Gastroenterol Hepatol 2019;4:62.