
Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis
Introduction
Both alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) are leading indications for liver transplantation in the United States and important causes of hepatocellular carcinoma (HCC) and cirrhotic complications. NAFLD affects at least a quarter of the global adult population (1). Its active spectrum, non-alcoholic steatohepatitis (NASH), is associated with accelerated fibrosis progression and increased risk of liver-related morbidity and mortality (2,3). While lifestyle intervention can reverse NASH and fibrosis, only a minority of patients can achieve sufficient weight reduction and histological improvement (4,5). At present, five drugs have entered phase 3 development for the treatment of NASH, and the first drug may be registered for this indication in 2019.
The epidemiology of ALD is highly variable across countries (6). Due to cultural and policy differences, there is substantial variation in per capita alcohol consumption (7). Nonetheless, in the era of direct-acting antivirals, ALD has surpassed chronic hepatitis C as the leading indication for liver transplantation in the United States since 2016 (8). Alcohol abstinence is the most effective treatment for ALD but is difficult to achieve. Systemic corticosteroids remain the most established treatment for severe alcoholic hepatitis (AH), but do not improve long-term survival and are associated with infective complications (9).
It is thus clear that NASH and ALD represent two common conditions with limited treatment options. In this article, we review emerging therapies that may come to the clinic and transform clinical management in the future.
NAFLD
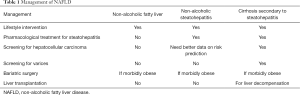
NAFLD is strongly associated with obesity and metabolic syndrome (10). Like other metabolic diseases, lifestyle management in terms of dietary restriction and regular physical exercise is the key management (Table 1). There is a dose-response relationship between weight reduction and improvement in NAFLD/NASH (4,5). In particular, a ≥10% weight reduction can lead to resolution of NASH in the majority and improvement in liver fibrosis in almost half of the patients. However, few patients can achieve this degree of weight reduction, and the presence of musculoskeletal problems in obese patients often limits the degree of physical activity. Therefore, some patients would still require pharmacological treatment. Nonetheless, because only a minority of patients with NASH will eventually die from liver disease, pharmacological treatment should be reserved for patients at risk of liver-related complications. Based on the current knowledge on the natural history of NAFLD, this would mean selecting NASH patients with at least significant fibrosis (F2 disease) (3,11). Among currently available drugs (none has specific indication for NASH), vitamin E and pioglitazone have demonstrated beneficial effects on histological NASH (12). A single-center study even suggests that vitamin E may prevent liver decompensation and improve survival in patients with advanced fibrosis (13). Current regional guidelines have endorsed the use of these two agents in selected patients with NASH (14-16). In addition, glucagon-like peptide 1 agonists such as liraglutide are important treatments for diabetes and obesity and may also improve NASH (17). These drugs have been discussed in previous reviews and will not be the focus of this article (18).
Full table
Furthermore, patients with NASH-related cirrhosis should receive regular surveillance for HCC and varices. While bariatric surgery can reverse most histological features of NASH (19), until further evidence the procedure should only be performed in patients with morbid obesity.
Currently, clinical trials in NASH are often based on histological response. This is because histological NASH and fibrosis are associated with liver-related outcomes in longitudinal studies (20,21). The US Food and Drug Administration (FDA) and European Medicines Agency also allow conditional approval of a drug for NASH if it demonstrates resolution of NASH without worsening of fibrosis and/or an improvement in fibrosis without worsening of NASH (18). Since the correlation between histological improvements and clinical outcomes remains to be established, current phase 3 trials are required to continue for 5 years or more to confirm the impact on clinical outcomes. On the other hand, studies using histological or clinical endpoints are expensive and take a long time to conduct. Therefore, early phase studies may rely on non-invasive surrogate endpoints such as changes in magnetic resonance imaging-based proton-density fat fraction (MRI-PDFF) (22).
Obeticholic acid
Bile acids are steroid molecules produced by the liver to facilitate digestion and absorption of lipids from the gut. Activation of the bile acid receptors such as the farnesoid X receptor (FXR) in the gut and liver reduces further production of bile acids to achieve homeostasis (23). However, it was soon apparent that bile acid receptors do more than controlling the bile acid pool; they also have multiple actions on glucose and lipid metabolism. In particular, activation of FXR in the gut results in the secretion of fibroblast growth factor 19 (FGF19, or FGF15 in mice), which in turn activates FGF receptor 4 (FGFR4) in the liver to increase glycogen synthesis and fatty acid oxidation. In animal studies, activation of hepatic FXR also reduces gluconeogenesis (24).
Obeticholic acid is a potent synthetic FXR agonist and is the first agent entering phase 3 development for NASH. In the earlier phase 2 FLINT study, obeticholic acid 25 mg daily met the primary outcome of a ≥2-point improvement in the histological NAFLD activity score without worsening of fibrosis at 72 weeks (45% vs. 21%) (25). Importantly, 35% of patients taking obeticholic acid had improvement in liver fibrosis, compared with 19% in the placebo group.
The interim analysis of the phase 3 REGENERATE Study was completed in February 2019. The study (n=931) met the primary endpoint of fibrosis improvement by ≥1 stage without worsening of NASH in the intention-to-treat population (23% in the obeticholic acid 25 mg daily group, 18% in the obeticholic acid 10 mg daily group, and 12% in the placebo group) (26). In contrast, there was no significant difference in the proportion of patients achieving NASH resolution without worsening of fibrosis (12% in the 25 mg group, 11% in the 10 mg group, and 8% in the placebo group). Fifty-one percent of patients receiving obeticholic acid 25 mg daily experienced pruritus (compared with 19% in the placebo group), and 13% discontinued treatment because of treatment-emergent adverse events. Seventeen percent also had increased low-density lipoprotein (LDL)-cholesterol. Although obeticholic acid mainly increases the less atherogenic large LDL particles (27), the long-term cardiovascular safety and tolerability of obeticholic acid should be carefully monitored. Based on the positive interim results, obeticholic acid will likely be the first drug conditionally approved under the FDA’s subpart H pathway in the United States.
Apart from obeticholic acid, several other FXR agonists are in phase 1–2 development. The non-steroidal FXR agonists may cause less pruritus and atherogenic dyslipidemia, but this notion has to be proven in clinical studies.
Elafibranor
Peroxisome proliferator-activated receptors (PPAR) are nuclear receptors with numerous metabolic actions (Table 2). Elafibranor is a dual PPAR-α/δ agonist with beneficial effects on hepatic and peripheral insulin sensitivity (28). It also reduces hepatic steatosis, inflammation and fibrosis in small animals (29). In the phase 2 GOLDEN-505 study, resolution of NASH without worsening of fibrosis occurred after 1 year in 19% of patients taking elafibranor 120 mg, 13% of those taking elafibranor 80 mg, and 12% of patients in the placebo group (30). Patients on elafibranor had mild increase in serum creatinine (4 µmol/L over placebo) that was reversible after treatment cessation. Although the histological improvement was modest, elafibranor improves insulin sensitivity and the lipid profile. In the ongoing phase 3 RESOLVE-IT Study, if elafibranor continues to show a positive effect on NASH and also demonstrates an improvement in cardiovascular outcomes, it may still be a useful drug in this patient population.

Full table
A number of other PPAR agonists are already in clinical use. PPAR-α is the target of fibrates, which are used for the treatment of hyperglyceridemia and are probably useful in primary biliary cholangitis. PPAR-γ agonists (thiazolidinedione) are already used in patients with type 2 diabetes. Other than that, PPAR-δ agonists have diverse effects on adipose tissues and muscles. For example, seladelpar, a potent and selective PPAR-δ agonist, has been granted Breakthrough Therapy designation by the FDA for achieving substantial effect on serum alkaline phosphatase lowering in a phase 2 study for primary biliary cholangitis. It also demonstrates effects on NASH and fibrosis in mouse models (31).
Cenicriviroc
Cenicriviroc is a dual C-C chemokine receptor type 2 and 5 (CCR2/5) inhibitor. It was initially developed for HIV infection because of the role of CCR5 in HIV entry; it also improves cognitive function in virally suppressed HIV-infected patients (32). Because CCR2/5 are also involved in macrophage recruitment, the drug is also evaluated for fibrotic NASH. In experimental NASH models, cenicriviroc consistently demonstrates beneficial effects on fibrosis (33).
In the phase 2 CENTAUR Study, cenicriviroc failed the primary outcome of a 2-point improvement in the NAFLD activity score and no worsening of fibrosis at year 1 (34). Nevertheless, it met the key secondary endpoint of fibrosis improvement without worsening of NASH (20% in the cenicriviroc group vs. 10% in the placebo group). Only a small proportion of patients had resolution of NASH and there was no significant difference between groups (8% in the cenicriviroc group vs. 6% in the placebo group). The dissociation between NASH and fibrosis improvement is puzzling. One possible explanation is that the current assessment of histological necro-inflammation is crude and does not distinguish among different inflammatory cells. Besides, the bar for NASH resolution (no hepatocyte ballooning and no or mild lobular inflammation) may be too high, and it would be difficult to demonstrate a difference when few patients can achieve this endpoint.
The CENTAUR study is unique in having 2 years of follow-up with three liver biopsies at yearly intervals. During the second year, patients on cenicriviroc continued with the active drug, whereas those on placebo were further randomized to receive cenicriviroc or placebo (35). There was no difference in fibrosis improvement at year 2 (26% among patients who took cenicriviroc and 22% among those on placebo throughout 2 years). On the other hand, there was a trend that patients on cenicriviroc for 2 years were more likely to have a ≥2-stage improvement in fibrosis (10% vs. 3% in the placebo group). Furthermore, patients on cenicriviroc were less likely to have fibrosis progression during the second year if they had improvement at year 1. Because of the small numbers in each subgroup and patient dropout during the second year, the study is inconclusive. The findings should be confirmed in the ongoing phase 3 AURORA study.
Selonsertib
Selonsertib is an apoptosis signal-regulating kinase 1 (ASK1) inhibitor. ASK1 is upregulated in NASH patients and correlates with the fibrosis stage. In animal models, inhibition of ASK1 as well as molecules upstream and downstream of the pathway improved NASH and hepatic lipid metabolism (36,37). Despite encouraging results from a small phase 2 study (38), selonsertib failed to improve NASH or fibrosis in the phase 3 STELLAR Study (https://www.gilead.com/news-and-press/press-room/press-releases/2019/4/gilead-announces-topline-data-from-phase-3-stellar3-study-of-selonsertib-in-bridging-fibrosis-f3-due-to-nonalcoholic-steatohepatitis-nash, accessed on 25 April 2019).
Thyroid hormone receptor (THR)-beta agonists
Several decades ago, clinicians have already tried to use thyroid hormone or its derivatives for weight reduction. However, induced hyperthyroidism carries too much toxicity and cannot be recommended. THRs can be divided into two main subtypes (THR-α and THR-β), and heart rate is primarily controlled by THR-α (39). Selective stimulation of THR-β can reduce body weight, cholesterol and hepatic steatosis (40-42). In two phase 2 studies, resmetirom (MGL-3196) and VK2809 showed improvement in liver fat by MRI-PDFF, atherogenic lipids and liver enzymes (43,44). No significant change in heart rate or blood pressure was observed, but the long-term cardiovascular safety should be evaluated. In March 2019, Madrigal Pharmaceuticals announced the initiation of a phase 3 study to test the use of resmetirom in patients with fibrotic NASH (https://www.globenewswire.com/news-release/2019/03/28/1781097/0/en/Madrigal-Pharmaceuticals-Initiates-Phase-3-Multinational-Double-Blind-Randomized-Placebo-Controlled-Study-of-MGL-3196-resmetirom-in-Patients-With-Non-Alcoholic-Steatohepatitis-NASH.html, accessed on 25 April 2019).
Aramchol
Aramchol is a conjugate of fatty acid and bile acid. It inhibits steroyl-CoA desaturase-1, a key enzyme converting saturated fatty acids into monounsaturated fatty acids (45). In the phase 2 ARREST Study, Aramchol at both 400 and 600 mg daily achieved the primary endpoint of greater absolute liver fat reduction over placebo by proton-magnetic resonance spectroscopy at 1 year (46). Although the study was underpowered for histological assessment, a dose-response trend towards NASH resolution and fibrosis improvement was seen. The improvement was consistent with an earlier study testing Aramchol for 3 months in NAFLD patients in Israel (47).
Engineered fibroblast growth factors
As discussed above, FGF19 stimulates FGFR4 in the liver and induces a variety of metabolic effects. NGM282, an engineered FGF19 analog, reduced liver fat by MRI-PDFF in a 12-week phase 2 study (48). A proportion of 74–79% of patients receiving NGM282 had a ≥5% absolute reduction in liver fat content. In another study of 43 patients, NGM282 at 1 mg and 3 mg daily led to a ≥2-point improvement in NAFLD activity score without worsening of fibrosis in 50% and 63%, and fibrosis improvement without worsening of NASH in 25% and 42%, respectively, though the study using histological endpoints did not include a control group (43). Probably through a similar mechanism as FXR agonists, NGM282 also increases total and LDL-cholesterol, but the dyslipidemia is readily controlled with statin (49).
FGF21 is produced by the liver, adipose tissue and pancreas and has effects on food intake, insulin sensitivity, browning of adipose tissue and energy expenditure (50). Pegbelfermin (BMS-986036), a pegylated FGF21 analog, showed significant reduction in liver fat by MRI-PDFF (absolute liver fat −6.8% in patients receiving pegbelfermin 10 mg daily, −5.2% in those receiving pegbelfermin 20 mg weekly, and −1.3% in those receiving placebo) in a 16-week phase 2 study (51).
FGF family members have broad mitogenic activities. While both NGM282 and pegbelfermin are non-mitogenic analogs of FGFs, future studies should evaluate their long-term safety. The need for subcutaneous injections may also affect treatment uptake and adherence.
Acetyl-CoA carboxylase (ACC) inhibitors
ACC is a key enzyme in hepatic de novo lipogenesis (52). Suppression of ACC prevents the conversion of acetyl-CoA into malonyl-CoA. In the mitochondria, suppression the production of malonyl-CoA also activates carnitine palmitoyl-transferase I and thus enhances fatty acid oxidation. In short-term studies in patients with NASH, GS-0976, an ACC inhibitor, reduces liver fat content by MRI-PDFF and fibrosis markers (53,54). One consistent metabolic side effect of ACC inhibition is hypertriglyceridemia, presumably secondary to the reduction in polyunsaturated fatty acids and the resultant upregulation of sterol regulatory element-binding protein 1. In both animal and human studies, the hypertriglyceridemia is readily controlled with fibrates (55).
Interestingly, because intracellular fat is conducive to cancer development, ACC inhibitors appear to suppress HCC and some other cancers in cell and animal experiments (56,57). As HCC and extrahepatic cancers are important causes of death in NASH patients, the clinical significance of these preclinical observation warrants further evaluation.
ALD
AH is a unique syndrome, which presents features of acute and chronic liver failure and has a potential of high short-term mortality of about as high as up to 40% within a month from its clinical presentation (58-60). As alluded to earlier, the current pharmacological treatment for severe AH is suboptimal and only 2–3% of the pool of patients with severe AH are eligible for early liver transplantation (61,62). Further, corticosteroids the only available current treatment option for severe AH provides limited survival benefit for short-term period of one month and beyond that underlying cirrhosis status and patient behavior in regards to alcohol consumption determine the long-term outcome (9,63). Clearly, there is a need for newer therapeutic targets for management of patients with severe AH, and we will discuss these in this section.
Liver injury from alcohol is due to indirect effects of alcohol on the intestinal microbiome and mucosal permeability translocation bacterial endotoxin (“gut-liver axis”) (64), and direct effects of alcohol through its metabolite acetaldehyde. Both these effects result in inflammatory signaling with cytokine cascade via activation of toll-like receptor 4 (TLR-4) on the hepatic macrophages, resulting in necro-apoptosis of hepatocytes and macrophages with consequent oxidative stress and generation of reactive oxygen species. Further, the end result of the initiated pathophysiology depends upon the ability of the liver to regenerate and regain mitochondrial health. Cross talk between hepatocytes, macrophages, and stellate cells with changes in nitric oxide in the hepatic microcirculation from inflammatory signaling leads to laying down of collagen and liver fibrosis with portal hypertension.
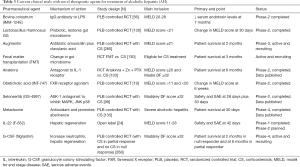
Many therapeutic targets are actively undergoing clinical trials in the US within the NIAAA funded consortia and in Europe. These targets will be reviewed in four different sections including (I) gut-Liver axis; (II) hepatic inflammation; (III) oxidative stress; and (IV) hepatic regeneration (Table 3) (58,59,65).
Full table
Targeting gut-liver axis
Bacterial lipopolysaccharide (LPS) and endotoxin translocate across the intestinal mucosa to initiate the inflammatory cascade via activation of TLR-4 on hepatic macrophages. Purified hyper immune bovine colostrum (IMM-124E) contains antibodies to neutralize the LPS, and has been studied in a phase 2 randomized clinical trial in combination with corticosteroids on 56 patients with severe AH (MELD score 20–28). The drug is administered orally in a powder form in a dose of 2400 mg/d in two divided doses, with the primary endpoint of the serum LPS levels.
Other pathophysiological changes in the gut in subjects with AH include bacterial overgrowth, alteration in the microbiome with increase of harmful bacteria such as Actinobacteria and Firmicutes, along with decrease in beneficial ones like Bacteroides (66). These changes along with inflammatory cytokine cascade activation increase risk of infections in patients with AH, especially those with high bacterial DNA load undergoing treatment with corticosteroids (67,68). Currently, two phase 2 randomized clinical trials are ongoing, one within the NIAAA consortium using probiotic Lactobacillus rhamnosus GG among moderate AH patients (MELD score 11–20), with primary endpoint of change in the MELD score (NCT01922895), and another one from Lille France using antibiotic, Augmentin (amoxicillin plus clavulanic acid) in combination with corticosteroids among severe AH patients (NCT02281929). Both these trials are expected to be completed this year and results are awaited regarding the benefit in the treatment of AH patients.
Like in many other diseases, fecal matter transplantation from healthy donors has been studied as a treatment option for AH patients. In one study, eight severe AH steroid ineligible males, fecal matter transplantation reduced disease severity, liver disease complications, and patient mortality compared to 18 matched patients receiving standard of care for severe AH (69). Larger studies from India are ongoing to further examine the efficacy and safety of this approach against standard of care in the treatment of severe AH (NCT03091010, NCT02458079, NCT03827772).
Targeting inflammation
Once the TLR-4 receptors are activated in the pathology of AH, further inflammatory signaling is primarily mediated by interleukin-1 (IL-1) (70). Based on the efficacy in rheumatoid arthritis (71,72), IL-1 receptor antagonist (anakinra) evaluated 103 severe AH patients in a phase 2 randomized clinical trial within the NIAAA consortium. The active arm included 53 patients treated with IL-1RA given as subcutaneous injection combined with oral administration of zinc and pentoxifylline, and is being compared to 50 corticosteroid therapy with methylprednisolone 32 mg IV (NCT01809132). The results showed similar survival at 30 days, but trend for improved survival at 3 and 6 months (69.7% vs. 55.2%, P=0.28 and 66.8% vs. 52.8%, P=0.26 respectively). MELD was the best predictor of survival, with significantly improved survival among patients with MELD 21–25 compared to 26–31.
FXR, nuclear receptors expressed in the liver and small bowel mucosa, regulate lipid and bile acid metabolism (73), and provide anti-inflammatory hepato-protective effects, and decrease nitric oxide levels in microvasculature of the liver with consequent improvement in portal hypertension (74,75). A phase 2 placebo controlled randomized clinical trial (NCT02039219) using an FXR agonist obeticholic acid in a dose of 10 mg daily for six weeks has been completed in patients with moderate AH (MELD score 12–19). Results of this study are awaited.
Caspases and apoptosis signal regulating kinase-1 9ASK-1) enzymes mediate inflammation and apoptosis or necrosis (76). Emricasan, a pan caspase inhibitor was evaluated in a clinical trial in the NIAAA consortium on five patients with severe AH, however, the trial was terminated prematurely due to poor pharmacokinetic data and excessively high blood levels of the caspase inhibitory compound. Phase 1 dose finding studies are needed before considering further clinical trials with this drug or consider changing the study population to moderate AH (77).
Selonsertib (GS-4997), an oral inhibitor of ASK-1 has been evaluated in a phase 2 clinical trial as an adjuvant therapy to prednisolone among 102 (99 biopsy confirmed) severe AH patients (mDF 32–60 or MELD >29). Comparing 48 treated patients to 51 receiving placebo, there was no mortality benefit at 28 days (4.3% vs. 4%, P=0.99). There was so difference on Lille response and infection rates (77.1% vs. 86.3%, P=0.30 and 33.3% vs. 25.5%, P=0.25 respectively). In fact, mortality at two months tended to be higher with adjuvant selonsertib vs. prednisolone alone (20.5% vs. 6.1%, P=0.061).
Extracorporeal liver assist device (ELAD) using hepatoblastoma C3A cells provides anti-inflammatory effects. However, a randomized placebo-controlled trial on 203 severe AH patients (96 in ELAD arm) failed to show survival benefit (78).
Targeting hepatic regeneration
Liver has remarkable regenerative capacity, and the capacity of liver to regenerate in severe AH is a predictor and biomarker for disease severity, responsiveness to corticosteroids, and patient survival (79). IL-22 of anti-inflammatory IL-10 family released from peripheral immune cells is a major cytokine to mediate regeneration and provide hepatoprotection via its antioxidant, antimicrobial, and anti-fibrotic properties (80,81). Hepatic regeneration is also promoted by neutrophils infiltrating the hepatic parenchyma (82,83), and the liver progenitor cells/bone marrow derived stem cells (84).
Recently, an open label clinical trial (NCT02655510) using recombinant IL-22 protein (F652) on 24 moderately severe AH patients (MELD score 11–28) showed good safety profile and determined the dose for proposed randomized placebo controlled study. The primary aim of this study will be 90-day survival among moderate AH patients (MELD score 11–20).
Growth factors such as granulocyte colony stimulating factor (G-CSF) has shown beneficial effects in randomized controlled clinical trials mainly from Asian continent, with increase in CD34 positive hematopoietic stem cells in the liver parenchyma, increase in neutrophil blood count, improvement in MELD score, reduced risk of infections and sepsis, and improved overall survival (85-87). Randomized controlled trials from the West are needed.
Targeting oxidative stress
Oxidative stress and generation of reactive oxygen species is multifactorial process in patients with AH (88). However, antioxidants in general have not shown survival benefit in AH patients (89). Largest randomized placebo controlled clinical trial with N-acetylcysteine (NAC) on 174 patients with severe AH, NAC infusion as an adjuvant therapy to corticosteroids tended to improve short-term mortality at one month, without any survival benefit in long-term at six months. There were reduced risks for infections and hepatorenal syndrome in the treatment arm however, compared to placebo treated patients (90). NAC also failed to show any survival benefit when used as add on therapy to G-CSF (91). Metadoxine, an antioxidant has shown some benefit at three and at six months when used as adjuvant to corticosteroids or to pentoxifylline (92). This agent also has some properties to reduce alcohol craving and improve abstinence rates. Whether this effect or the antioxidant effect is useful in the clinical practice is not clearly known. It remains to be seen whether mitochondrial targeted antioxidants like mitoquinone will have better efficacy in the treatment of AH.
Summary
NAFLD and ALD are two front line commonest liver diseases in the world, especially since the introduction of highly effective and safe direct acting antiviral therapy for hepatitis C virus infection. Currently, there are no FDA approved medical therapies for both these diseases, and the current standard of care is only limited to control of the risk factor, i.e. weight loss with metabolic risk factors control for NAFLD and alcohol abstinence for AH. The field is ripe with many new targets actively being studied as basis for FDA approval for the respective diseases. Given similarities in the pathogenesis between the two diseases, many pharmacological therapies are being studied in both the diseases such as obeticholic acid, IgG to LPS, probiotics, selonsertib, ASK-1 inhibitor, and anti-inflammatory drug cenicriviroc. Although, role of combination therapy has been recognized in NAFLD, with pharmaceutical industries initiating drug trials using combination therapy, whether the similar approach would also be needed for AH will be seen with test of time. Nevertheless, there is light at the end of tunnel with many potential pharmacological therapies in pipeline which could be FDA approved in the near future for the treatment of NAFLD or AH.
Acknowledgments
None.
Footnote
Conflicts of Interest: VW Wong has served as a consultant or advisory board member for 3V-BIO, AbbVie, Allergan, Echosens, Gilead Sciences, Janssen, Perspectum Diagnostics, Pfizer and Terns; and a speaker for Bristol-Myers Squibb, Echosens, Gilead Sciences and Merck. He has also received research grants from Gilead Sciences. AK Singal has served as advisory board member for Gilead Sciences, Alnylam Pharmaceuticals, Recordati Pharma, American Porphyria Foundation, Chronic Liver Disease Foundation, and Medscape Gastroenterology.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019;69:2672-82. [Crossref] [PubMed]
- Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643-54.e1-9; quiz e39-40.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-65. [Crossref] [PubMed]
- Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2013;59:536-42. [Crossref] [PubMed]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
- Masarone M, Rosato V, Dallio M, et al. Epidemiology and Natural History of Alcoholic Liver Disease. Rev Recent Clin Trials 2016;11:167-74. [Crossref] [PubMed]
- Madureira-Lima J, Galea S. Alcohol control policies and alcohol consumption: an international comparison of 167 countries. J Epidemiol Community Health 2018;72:54-60. [Crossref] [PubMed]
- Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol 2018;16:1356-8. [Crossref] [PubMed]
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH): a 2 x 2 factorial randomised controlled trial. Health Technol Assess 2015;19:1-104. [Crossref] [PubMed]
- Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409-15. [Crossref] [PubMed]
- Vilar-Gomez E, Calzadilla-Bertot L, Wong VW, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155:443-57.e17. [Crossref] [PubMed]
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-85. [Crossref] [PubMed]
- Vilar-Gomez E, Vuppalanchi R, Gawrieh S, et al. Vitamin E Improves Transplant-Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology 2018. [Epub ahead of print]. [PubMed]
- European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J Gastroenterol Hepatol 2018;33:86-98. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57. [Crossref] [PubMed]
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-90. [Crossref] [PubMed]
- Wong VW, Chitturi S, Wong GL, et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 2016;1:56-67. [Crossref] [PubMed]
- Bower G, Toma T, Harling L, et al. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg 2015;25:2280-9. [Crossref] [PubMed]
- Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 2011;53:1874-82. [Crossref] [PubMed]
- Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-54. [Crossref] [PubMed]
- Wong VW, Adams LA, de Ledinghen V, et al. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol 2018;15:461-78. [Crossref] [PubMed]
- Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017;65:350-62. [Crossref] [PubMed]
- Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 2006;116:1102-9. [Crossref] [PubMed]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956-65. [Crossref] [PubMed]
- Younossi Z, Ratziu V, Loomba R, et al. Positive results from REGENERATE: A phase 3 international, randomized, placebo-controlled study evaluating obeticholic acid treatment for NASH. J Hepatol 2019;70:e5. [Crossref]
- Pockros PJ, Fuchs M, Frieilich BL, et al. Control: A randomized, double-blind, placebo-controlled phase 2 study investigating the effects of obeticholic acid and atorvastatin treatment on lipoprotein metabolism in patients with nonalcoholic steatohepatitis. Hepatology 2018;68:951A-2A.
- Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013;36:2923-30. [Crossref] [PubMed]
- Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013;58:1941-52. [Crossref] [PubMed]
- Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150:1147-59.e5. [Crossref] [PubMed]
- Choi YJ, Song J, Johnson JD, et al. Seladelpar improves hepatic steatohepatitis and fibrosis in a diet-induced and biopsy-confirmed mouse model of NASH. Hepatology 2018;68:755A.
- D'Antoni ML, Paul RH, Mitchell BI, et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr 2018;79:108-16. [Crossref] [PubMed]
- Krenkel O, Puengel T, Govaere O, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018;67:1270-83. [Crossref] [PubMed]
- Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754-67. [Crossref] [PubMed]
- Ratziu V, Sanyal A, Francque S, et al. Cenicriviroc treatment for adults with non-alcoholic steatohepatitis: Year 2 analysis of the Phase 2b CENTAUR study. J Hepatol 2018;68:S1-2. [Crossref]
- Zhang P, Wang PX, Zhao LP, et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med 2018;24:84-94. [Crossref] [PubMed]
- Ye P, Liu J, Xu W, et al. Dual-Specificity Phosphatase 26 Protects Against Nonalcoholic Fatty Liver Disease in Mice Through Transforming Growth Factor Beta-Activated Kinase 1 Suppression. Hepatology 2019;69:1946-64. [Crossref] [PubMed]
- Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018;67:549-59. [Crossref] [PubMed]
- Wikstrom L, Johansson C, Salto C, et al. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J 1998;17:455-61. [Crossref] [PubMed]
- Grover GJ, Mellstrom K, Ye L, et al. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci U S A 2003;100:10067-72. [Crossref] [PubMed]
- Vatner DF, Weismann D, Beddow SA, et al. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab 2013;305:E89-100. [Crossref] [PubMed]
- Kelly MJ, Pietranico-Cole S, Larigan JD, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med Chem 2014;57:3912-23. [Crossref] [PubMed]
- Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Loomba R, Neutel J, Mohseni R, et al. VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled trial. J Hepatol 2019;70:e150-1. [Crossref]
- Iruarrizaga-Lejarreta M, Varela-Rey M, Fernandez-Ramos D, et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:911-27. [Crossref] [PubMed]
- Ratziu V, Ladron-De-Guevara L, Safadi R, et al. One-year results of the global phase 2b randomized placebo-controlled ARREST trial of aramchol, a stearoyl CoA desaturase inhibitor, in patients with NASH. Hepatology 2018;68:1447A-8A.
- Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014;12:2085-91.e1. [Crossref] [PubMed]
- Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174-85. [Crossref] [PubMed]
- Rinella ME, Trotter JF, Abdelmalek MF, et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol 2019;70:735-44. [Crossref] [PubMed]
- Wong VWS, Adams LA. Fibroblast growth factor 21 for non-alcoholic steatohepatitis. Lancet 2019;392:2658-60. [Crossref] [PubMed]
- Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019;392:2705-17. [Crossref] [PubMed]
- Stiede K, Miao W, Blanchette HS, et al. Acetyl-coenzyme A carboxylase inhibition reduces de novo lipogenesis in overweight male subjects: A randomized, double-blind, crossover study. Hepatology 2017;66:324-34. [Crossref] [PubMed]
- Lawitz EJ, Coste A, Poordad F, et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2018;16:1983-91.e3. [Crossref] [PubMed]
- Loomba R, Kayali Z, Noureddin M, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:1463-73.e6. [Crossref] [PubMed]
- Kim CW, Addy C, Kusunoki J, et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab 2017;26:394-406.e6. [Crossref] [PubMed]
- Lally JSV, Ghoshal S, DePeralta DK, et al. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab 2019;29:174-82.e5. [Crossref] [PubMed]
- Svensson RU, Parker SJ, Eichner LJ, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 2016;22:1108-19. [Crossref] [PubMed]
- Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014;12:555-64; quiz e31-2.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758-69. [Crossref] [PubMed]
- Singal AK, Kodali S, Vucovich LA, et al. Diagnosis and Treatment of Alcoholic Hepatitis: A Systematic Review. Alcohol Clin Exp Res 2016;40:1390-402. [Crossref] [PubMed]
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790-800. [Crossref] [PubMed]
- Hasanin M, Dubay DA, McGuire BM, et al. Liver transplantation for alcoholic hepatitis: A survey of liver transplant centers. Liver Transpl 2015;21:1449-52. [Crossref] [PubMed]
- Altamirano J, Lopez-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology 2017;66:1842-53. [Crossref] [PubMed]
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015;148:30-6. [Crossref] [PubMed]
- Singal AK, Shah VH. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin North Am 2011;40:611-39. [Crossref] [PubMed]
- Bull-Otterson L, Feng W, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [Crossref] [PubMed]
- Vergis N, Atkinson SR, Knapp S, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017;152:1068-77.e4. [Crossref] [PubMed]
- Singal AK, Shah VH, Kamath PS. Infection in Severe Alcoholic Hepatitis: Yet Another Piece in the Puzzle. Gastroenterology 2017;152:938-40. [Crossref] [PubMed]
- Philips CA, Pande A, Shasthry SM, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol 2017;15:600-2. [Crossref] [PubMed]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821-32. [Crossref] [PubMed]
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev 2009.CD005121. [PubMed]
- Opal SM, Fisher CJ Jr, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997;25:1115-24. [Crossref] [PubMed]
- Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med 2015;3:5. [PubMed]
- Verbeke L, Farre R, Trebicka J, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 2014;59:2286-98. [Crossref] [PubMed]
- Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 2008;7:678-93. [Crossref] [PubMed]
- Verma VK, Li H, Wang R, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 2016;64:651-60. [Crossref] [PubMed]
- Frenette CT, Morelli G, Shiffman ML, et al. Emricasan Improves Liver Function in Patients With Cirrhosis and High Model for End-stage Liver Disease Scores Compared With Placebo. Clin Gastroenterol Hepatol 2019;17:774-83.e4. [Crossref] [PubMed]
- Thompson J, Jones N, Al-Khafaji A, et al. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl 2018;24:380-93. [Crossref] [PubMed]
- Lanthier N, Rubbia-Brandt L, Lin-Marq N, et al. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol 2015;63:609-21. [Crossref] [PubMed]
- Brand S, Dambacher J, Beigel F, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol 2007;292:G1019-28. [Crossref] [PubMed]
- Schnabl B. Liver capsule: Mechanisms of alcoholic hepatitis. Hepatology 2016;64:276. [Crossref] [PubMed]
- Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231-9.e1-6.
- Taieb J, Delarche C, Paradis V, et al. Polymorphonuclear neutrophils are a source of hepatocyte growth factor in patients with severe alcoholic hepatitis. J Hepatol 2002;36:342-8. [Crossref] [PubMed]
- Dubuquoy L, Louvet A, Lassailly G, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 2015;64:1949-60. [Crossref] [PubMed]
- Garg V, Garg H, Khan A, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012;142:505-12.e1. [Crossref] [PubMed]
- Kedarisetty CK, Anand L, Bhardwaj A, et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 2015;148:1362-70.e7. [Crossref] [PubMed]
- Singh V, Sharma AK, Narasimhan RL, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 2014;109:1417-23. [Crossref] [PubMed]
- Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231-42. [Crossref] [PubMed]
- Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int 2011;31:1432-48. [Crossref] [PubMed]
- Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011;365:1781-9. [Crossref] [PubMed]
- Singh V, Keisham A, Bhalla A, et al. Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2018;16:1650-6.e2. [Crossref] [PubMed]
- Higuera-de la Tijera F, Servin-Caamano AI, Cruz-Herrera J, et al. Treatment with metadoxine and its impact on early mortality in patients with severe alcoholic hepatitis. Ann Hepatol 2014;13:343-52. [Crossref] [PubMed]
Cite this article as: Wong VW, Singal AK. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol 2019;4:53.


