Salvage liver transplant for hepatocellular carcinoma: rescues and benefits
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China, and it is the third cause leading to cancer mortality (1). Primary liver transplantation (PLT) was known as the most effective treatment for patients with HCC, which not only achieves radical tumor resection, but also cures the concurrent end-stage liver diseases, such as severe cirrhosis (2,3). However, the long waiting time for transplant, severe organ shortage, high medical cost and high perioperative risk are important difficulties confronted for PLT. Patients often fail to get a liver transplant in time, and will face tumor progression and loss of the chance for transplantation or even death (4,5).
Primary hepatic resection can achieve a 5-year overall survival rate of approximately 55–71%, remains to be a prime and more reasonable treatment with long-term survival outcomes for patients with resectable early HCC (6,7). Studies have shown that there is no obvious difference in the total survival rate after hepatic resection compared with that of liver transplantation (8,9). But due to the invasive and metastatic nature of HCC, it might face a higher risk of tumor recurrence or liver function deterioration after hepatic resection. Studies showed that almost 70% of patients would develop intrahepatic recurrence after hepatic resection within 5 years (10,11). Gratifyingly, 80% of patients with recurrent HCC after curative resection had a chance to receive liver transplant (12,13).
Advent of salvage liver transplantation (SLT)
SLT was first proposed in 2000 by Majno et al. (5), and has been performed for those patients with recurrent HCC or liver function deterioration following initial treatment with primary hepatic resection. Primary resection and SLT may be a rational way to cope with lengthening waiting lists in the current situation of donor shortage (5).
However, the indications for SLT are still controversial, and there is no sufficient research to confirm its safety and postoperative survival rate. SLT has been thought to increase the difficulty and risk of surgery due to the history of previous hepatic resection, severe adhesion in the abdominal operation area, abnormal anatomical structures and more intraoperative blood loss (14). In the early stage of development, it is reported that the mortality rate of patients with SLT was as high as 28.6%, which was much higher than that of patients with PLT (2.1%) (15). Compared with SLT, PLT has less blood loss and risks, is considered to be an ideal choice for the treatment of HCC with liver cirrhosis. Multiple surgical procedures, accompanied by adjuvant therapies such as radiotherapy will increase the difficulty of operation during SLT. Due to previous hepatectomy and severe adhesion during SLT, we suggest directly dissecting the porta-hepatitis, clamping the inferior vena cava first, then mobilizing and rapidly removing the diseased liver, so as to reduce the hemorrhage and the risk of tumor metastasis during operation.
Benefits of SLT
With the advancement of surgical techniques and the continuous accumulation of surgical experience, however, more and more studies have shown that the perioperative risk of SLT gradually decreases (Table 1). Kim et al. (16) compared 15 patients who underwent SLT after prior partial hepatectomy with 31 patients following PLT, and found that there was no difference in the incidence of surgical complications and overall survival rates between the two groups, and concluded that SLT is a feasible procedure for recurrent HCC, the operative risk of the SLT is also acceptable. A meta-analysis has shown that SLT for recurrent HCC can achieve the same short- and long-term outcomes as PLT. Therefore, SLT may be accepted as a valid treatment for patients with recurrent HCC (28). A meta-analysis of 7 studies (29) have shown that there were no significant differences in the overall survival rates of SLT and PLT, and in the incidence of sepsis and biliary complications as for postoperative complications, but there was a significantly higher incidence of bleeding with SLT (P=0.001). A synthesis of 16 studies comprising 319 patients suggests that SLT following primary hepatic resection is a highly applicable strategy with long-term survival outcomes that are comparable to upfront liver transplantation (17). A similar paper analyzed 111 patients who received SLT, including operative characteristics, survival rate, and prognostic factors, and put forward that primary liver resection can not only completely remove the tumor lesions, but also provide detailed information on the tumor before SLT, such as tumor size, tumor number, degree of differentiation, pathological type, with or without vascular invasion, and other important pathological data (18). SLT is an effective and feasible treatment for patients with HCC recurrence after primary hepatic resection.
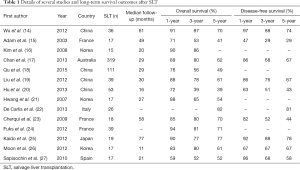
Full table
Opportunity of SLT
Furthermore, it is very important to select the opportunity of SLT. From the domestic and foreign research, patients with HCC who have severe preoperative cirrhosis, poor liver function, and younger age should choose PLT as much as possible. For those with better liver function, older age may choose to undergo primary hepatic resection, and later may receive comprehensive treatment such as transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection, and immunotherapy to delay the progression of the tumor and make preparation for SLT in the future (30). Sala et al. (31) also recommended that patients with high risk of recurrence, such as pathological microvascular infiltration and tumor microsatellite lesions, could undergo liver transplantation prior to tumor recurrence, making SLT more targeted.
Transplantation criteria for SLT
So far, there is still a lack of transplantation criteria for SLT in patients with recurrent HCC. In recent years, different transplantation centers have begun to apply indications of liver transplantation for HCC to SLT, in an attempt to continuously expand the surgical indications of SLT. Wu et al. (14) compared patients with SLT and PLT performed from 2004 to 2008, and concluded that the Milan criteria are still eligible for the 36 patients at the time of recurrence. And the 5-year overall survival rate and disease-free survival rate are not lower than those undergoing PLT. Liu et al. (19) observed the efficacy of SLT for patients with recurrent HCC who met the University of California San Francisco (UCSF) criteria, although SLT group has more intraoperative blood loss, the perioperative mortality and postoperative complications were similar in SLT and PLT groups. There was no significant difference in overall survival and recurrence rates between the two groups. Moreover, they observed that SLT for recurrent HCC beyond Milan but within UCSF criteria could achieve the same efficacy as patients who met Milan criteria. Therefore, SLT for recurrent HCC within UCSF criteria was feasible and it could achieve the same outcome as PLT. Hu et al. (20) retrospectively analyzed 53 cases of SLT patients performed from 2004 to 2012. Among them, 16 cases met the Milan criteria, 14 cases met the Hangzhou criteria. The overall survival rate of the Hangzhou criteria in the first and third year were both 70.1%, which was close to the Milan criteria group of 93.8% and 62.1% (P=0.586). Similarly, no statistical difference in the tumor-free survival rates between the two groups, which concluded that the Hangzhou criteria can safely expand the transplantable candidates of SLT.
At present, the medical community has not reached an agreement on the transplantation criteria of SLT, and more and more studies showed that even though the Milan criteria was first proposed and can effectively improve the disease-free survival rate of patients with HCC, the inclusion criteria are too strict, so that many patients who could have received liver transplantation lost surgery opportunity (32-34).And for a country with a large amount of patients with HCC like China, the Milan criteria does not seem to be suitable either. It still depends on a more rigorous design and a larger sample size of prospective, randomized, controlled clinical trials to further verify. In our center, we perform LT for HCC patients based on Hangzhou criteria, the percentage of SLT is about 22% of LT for HCC patients.
The feasibility of salvage living donor liver transplantation (LDLT)
As we know, the initial studies on SLT were almost based on deceased donor liver transplantation (DDLT). However, LDLT, as a choice of liver transplantation, can offer a great opportunity to the supply of transplantable organs, thereby reducing the patients’ waiting time and reducing the risk of being removed from the waiting group for liver transplantation due to tumor progression. If appropriate living donor liver resources are available, a significant proportion of patients with HCC tend to adopt LDLT as an initial treatment or as a treatment option when non-surgical treatment fails. So, salvage LDLT will gradually increase with the development of LDLT.
However, the feasibility of salvage LDLT for patients after hepatectomy has been controversial (21,35). Hu et al. (20) reported in a study that the 1-year, 3-year, and 5-year overall and disease-free survival rates were similar between the LDLT and DDLT in the SLT group, which indicates that salvage LDLT is a safe and good alternative treatment option because of the shortage of cadaveric donor livers. Abe et al. (36) researched 45 patients who underwent primary LDLT and 15 patients followed salvage LDLT after initial hepatic resection to investigate the efficacy of salvage LDLT after initial hepatic resection in HCC patients. The salvage LDLT group had significantly more reoperations for postoperative bleeding, nevertheless overall and recurrence-free survival rates were comparable, reported that salvage LDLT for HCC offered long-term outcomes at least as good as those of primary LDLT.
In short, salvage LDLT not only alleviates the situation of the deficiency of donor liver, but also achieves an ideal therapeutic effect, which can be regarded as an effective treatment.
Summary
From the above review, we believe that as long as a reasonable choice of indications, adequate preoperative assessment and precise surgical operation, SLT after hepatic resection can achieve the same satisfactory clinical efficacy as PLT. In particular, under the conditions of many patients with liver cancer in China but few liver resources, SLT has unique advantages and wider prospects. As for the specific treatment to be adopted, a comprehensive assessment should be conducted based on the patient’s age, liver function, and patient’s willingness. The difficulty of SLT should also be evaluated due to complicated previous treatment. In addition, the transplantation criteria for SLT has not yet reached a consensus and still needs to be confirmed by a large number of multi-center studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Bhoori S, Sposito C, Germini A, et al. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int 2010;23:712-22. [Crossref] [PubMed]
- Rahbari NN, Mehepatic A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011;253:453-69. [Crossref] [PubMed]
- Li HY, Wei YG, Yan LN, et al. Salvage liver transplantation in the treatment of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 2012;18:2415-22. [Crossref] [PubMed]
- Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome -oriented decision analysis. Hepatology 2000;31:899-906. [Crossref] [PubMed]
- Fan ST, Mau LC, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma:a 20-year experience. Ann Surg 2011;253:745-58. [Crossref] [PubMed]
- Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996;111:720-6. [Crossref] [PubMed]
- Moon DB, Lee SG, Hwang S. Liver Transplantation for Hepatocellular Carcinoma: Single Nodule with Child-Pugh Class A Sized Less than 3 cm. Dig Dis 2007;25:320-8. [Crossref] [PubMed]
- Margarit C, Escartin A, Castells L, et al. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl 2005;11:1242-51. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Kamiyama T, Nakanishi K, Yokoo H, et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol 2009;16:1560-71. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373-82. [Crossref] [PubMed]
- Tanaka H, Kubo S, Tsukamoto T, et al. Recurrence rate and transplantability after liver resection in patients with hepatocellular carcinoma who initially met transplantation criteria. Transplant Proc 2005;37:1254-6. [Crossref] [PubMed]
- Wu L, Hu A, Tam N, et al. Salvage liver transplantation for patients with recurrent hepatocellular carcinoma after curative resection. PLoS One 2012;7. [Crossref] [PubMed]
- Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508-18; discussion 518-9. [PubMed]
- Kim BW, Park YK, Kim YB, et al. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: feasibility of the Milan criteria and operative risk. Transplant Proc 2008;40:3558-61. [Crossref] [PubMed]
- Chan DL, Alzahrani NA, Morris DL, et al. Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:31-41. [Crossref] [PubMed]
- Qu W, Zhu ZJ, Sun LY, et al. Salvage liver transplantation for hepatocellular carcinoma recurrence after primary liver resection. Clin Res Hepatol Gastroenterol 2015;39:93-7. [Crossref] [PubMed]
- Liu F, Wei Y, Wang W, et al. Salvage Liver Transplantation for Recurrent Hepatocellular Carcinoma within UCSF Criteria after Liver Resection. PLoS One 2012;7. [Crossref] [PubMed]
- Hu Z, Zhou J, Li Z, et al. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: retrospective study of the Milan and Hangzhou criteria. PLoS One 2014;9. [Crossref] [PubMed]
- Hwang S, Lee SG, Moon DB, et al. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transpl 2007;13:741-6. [Crossref] [PubMed]
- De Carlis L, Di Sandro S, Giacomoni A, et al. Liver transplantation for hepatocellular carcinoma recurrence after liver resection: why deny this chance of cure? J Clin Gastroenterol 2013;47:352-8. [Crossref] [PubMed]
- Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg 2009;250:738-46. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Kaido T, Mori A, Ogura Y, et al. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery 2012;151:55-60. [Crossref] [PubMed]
- Moon JI, Kwon CH, Joh JW, et al. Primary versus salvage living donor liver transplantation for patients with hepatocellular carcinoma: impact of microvascular invasion on survival. Transplant Proc 2012;44:487-93. [Crossref] [PubMed]
- Sapisochin G, Bilbao I, Balsells J, et al. Optimization of liver transplantation as a treatment of intrahepatic hepatocellular carcinoma recurrence after partial liver resection: experience of a single European series. World J Surg 2010;34:2146-54. [Crossref] [PubMed]
- Zhu Y, Dong J, Wang WL, et al. Short- and long-term outcomes after salvage liver transplantation versus primary liver transplantation for hepatocellular carcinoma: a meta-analysis. Transplant Proc 2013;45:3329-42. [Crossref] [PubMed]
- Hu Z, Wang W, Li Z, et al. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: a systematic review and meta-analysis. Liver Transpl 2012;18:1316-23. [Crossref] [PubMed]
- Facciuto ME, Koneru B, Rocca JP, et al. Surgical treatment of hepatocellular carcinoma beyond Milan criteria. Results of liver resection, salvage transplantation, and primary liver transplantation. Ann Surg Oncol 2008;15:1383-91. [Crossref] [PubMed]
- Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 2004;10:1294-300. [Crossref] [PubMed]
- Scatton O, Zalinski S, Terris B, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transpl 2008;14:779-88. [Crossref] [PubMed]
- Scatton O, Liddo G, Belghiti J. Liver transplantation for hepatocellular carcinoma: current topics in France. J Hepatobiliary Pancreat Sci 2010;17:567-73. [Crossref] [PubMed]
- Koschny R, Schmidt J, Ganten TM. Beyond Milan criteria--chances and risks of expanding transplantation criteria for HCC patients with liver cirrhosis. Clin Transplant 2009;23 Suppl 21:49-60. [Crossref] [PubMed]
- Lee SG. Salvage living-donor liver transplantation to previously hepatectomized hepatocellular carcinoma patients: is it a reasonable strategy? Hepatobiliary Pancreat Dis Int 2013;12:10-1. [Crossref] [PubMed]
- Abe T, Tashiro H, Teraoka Y, et al. Efficacy and Feasibility of Salvage Living Donor Liver Transplantation after Initial Liver Resection in Patients with Hepatocellular Carcinoma. Dig Surg 2016;33:8-14. [Crossref] [PubMed]
Cite this article as: Zheng S, Xie Q, Cheng J. Salvage liver transplant for hepatocellular carcinoma: rescues and benefits. Transl Gastroenterol Hepatol 2018;3:65.


