Resection for hepatocellular cancer: overpassing old barriers
Introduction
Hepatocellular cancer (HCC) is the fifth most common cancer and the second most frequent cause of tumour-related death, representing one of the major global health problems (1). As a consequence, HCC management is a topic of ongoing debate. In 2000, the European Association for the Study of the Liver (EASL) proposed a diagnostic/therapeutic algorithm internationally recognised as a useful protocol for the HCC management (2). According to this algorithm, liver resection (LR) represented one of the few therapeutic interventions identified for the treatment of HCC-on-cirrhosis. However, only an “ideal patient” was considered suitable for such an approach. Specifically, the EASL 2000 Guidelines defined the following criteria for LR:
- A solitary small (<2.0 cm) tumour;
- Very well-preserved liver function (i.e., Child-Pugh A status and bilirubin <2.5 mg/dL);
- Absence of portal hypertension (PHT) (i.e., hepatic vein-to-portal system gradient ≤10 mmHg or platelet count ≥100,000/mL);
- Performance Status (PST) zero.
In recent years, great evolutions have been observed in the management of HCC, pushing on the “old barriers” proposed by the EASL 2000 Guidelines. As an example, a large revision of the EASL Guidelines has been done in 2018 (1). The EASL 2018 Guidelines confirmed the previous concept of “ideal patient”, especially in the case of LR performed in non-experienced centres. However, the more recent guidelines underlined the fact that LR patients should present excellent results also in the presence of one or more risk factors when the resection is done in experienced centres (1,3-5). Such a shred of evidence has been the direct consequence of an accurate balance of the relative weight of each prognosis determinant. Interestingly enough, patients with risk factors can achieve excellent survivals after LR even if the more recent guidelines continue to direct them only towards alternative palliative treatments (1,3-5).
As a consequence, the present review aims at investigating the specific role of LR in the setting of HCC. We intend to report the studies able to demonstrate that LR is no longer a treatment suitable only for highly selected patients, but also for patients selectively presenting one-to-more negative factors. The following specific variables have been intensely investigated: age; single vs. multiple diseases; the dimension of the nodule; hyperbilirubinemia; clinically relevant PHT; Child-Pugh status; macrovascular invasion; laparoscopic approach.
The concept of age
Progressive ageing of the population is observed in several Western and Eastern countries. However, this progressive age increase does not represent an absolute contraindication to surgical approaches. In the specific setting of LR for HCC, the first studies demonstrating the feasibility of LR in aged patients were first published in the nineties (5,6).
According to the EASL Guidelines, age does not represent a contraindication per se, if adequate PST and no significant co-morbidities are confirmed (1).
In particular, post-surgical survivals compared in age-matched LR groups suggested that this procedure can be safely offered in >70-year-old patients, being exposed to a smaller loss of their lifespan in comparison with their younger counterparts (1).
A study from Italy performed on 919 HCC-on-cirrhosis consecutive patients undergoing LR showed that postoperative mortality and 3-year survival rates were similar among age quartiles (≤60, 60–66, 67–70 and >70 years) (7).
Borzio et al. reported that post-LR outcome was mostly influenced by liver function and tumour stage rather than by age. Interestingly, the contemporaneous presence of advanced age and tumour stage, requiring a more extensive resection, was connected with lower results even when performed in experienced high-volume centres (8). Studies coming from Japan, Korea, China and Taiwan confirmed these pieces of evidence also in an Eastern setting (9-12).
Tumor dimension and number of nodules
In the absence of other risk factors, an HCC presenting with multiple nodules or with a lesion overpassing 5.0 cm of maximum diameter is not an absolute contraindication for surgical intervention (13-27) (Table 1). In particular, an HCC meeting the Milan Criteria (≤3 nodules, each ≤3 cm in size) could be approached with LR, if eligibility for liver transplantation is suboptimal or excluded (1).
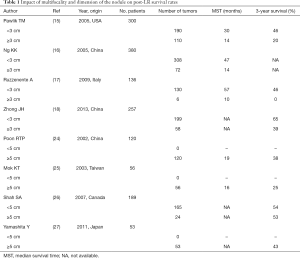
Full table
A study from Toronto showed that LR for multifocal lesions consented to achieve excellent overall survivals (3-year: 59%). Moreover, multifocality was not an independent risk factor for patient death or post-resection recurrence (13). Another study by Ishizawa et al. confirmed these results, with the presence of multiple tumours failing to be an independent risk factor for post-operative survival. Interestingly, in these series, LR showed to provide a survival benefit in patients with multiple tumours even in the presence of PHT (14).
Several studies have compared the post-LR results with the large span of therapeutic alternatives usually proposed for multifocal HCC (18-20). For example, LR achieved competitive survivals when compared with trans-arterial chemo-embolization (TACE) (18,19).
As for the dimension of the primary lesion, several studies demonstrated that survival benefit is observed also resecting large (>5.0 cm) tumours (21,22).
As an example, Vitale et al. examined 2,090 HCC patients treated with LR, loco-regional therapies, and best supportive care, always showing a net survival benefit in favour of LR across all the stages of tumour presentation (23).
Liver function
EASL 2018 Guidelines asserted that Child-Pugh stage remains the most practised method for measuring liver function. Child-Pugh stage A is considered the limit for performing a LR within safe limits. However, other more recent parameters such as the model for end-stage liver disease (MELD), indocyanine green (ICG) kinetics, fibrosis grade using a liver stiffness measurement (LSM), albumin/bilirubin ratio, and cholinesterase/bilirubin ratio, have shown a significant role in improving patient selection, especially in those with borderline liver function (1).
Several West and East experiences showed a significant risk of post-hepatectomy liver failure predicted by LSM above 12–15 kPa (28-30). Nishio et al. reported that LSM could also be used to estimate a safe liver remnant volume (31).
A retention rate of ICG at 15 minutes (ICGR15) can be measured at the bedside with non-invasive pulse dye densitometry devices. Various cut-offs of ICGR15 can be part of the decision making an algorithm for LR procedures in cirrhotic patients, limiting resection and segmentectomy to patients with ICGR15 below 20–25% and 30–35%, respectively (32).
As for the different Barcelona Clinic Liver Cancer (BCLC) classes, several studies demonstrated that the proposed classification failed to predict post-resection survivals. As an example, Torzilli et al. reported that qualified surgical centres must offer LR once technical feasibility and hepatic functional reserve allows the surgical approach. Specifically, half of the cases (n=1,034) undergoing LR in their series were found to be at a stage considered unsuitable for hepatectomy. Interestingly, when BCLC B–C patients were considered, post-LR 3-year overall survival rates were 62% concerning the expected survivals of 10–40% (33).
Vitale et al. performed an analysis on 2,090 HCC-on-cirrhosis cases, reporting that LR was associated with a significant net benefit over loco-regional treatments. Surprisingly, the observed benefit was present not only in the early stage (BCLC A) but also in intermediate stages (B–C). Importantly, this benefit persisted after a robust adjustment for adverse factors, including clinically significant PHT (23).
These findings may radically change the prognostic evaluation and management of HCC patients, suggesting that the BCLC stage does not influence the prognostic impact of different therapeutic approaches, and that LR should be preferred when technically feasible and clinically appropriate. On the opposite, when a stratification for MELD score was done, a net survival benefit after LR was observed concerning the other therapies when the MELD was 6–9. On the opposite, in the case of MELD >9, the net survival benefit was negligible or negative (23).
Clinically relevant PHT and hyperbilirubinemia
Although clinically relevant PHT and hyperbilirubinemia are significant prognostic factors affecting survival in both surgical and medical patients with HCC-on-cirrhosis (14,34), their relevance as independent determinants of post-surgical outcomes has been questioned. As limited resection in patients with preserved liver function and moderate PHT/hyperbilirubinemia yields competitive survival outcomes, their role in the decision making for eligibility to resection of HCC should always be balanced with the extent of hepatectomy and liver function indicators, such as the MELD score and the availability and predicted effectiveness of alternative HCC therapies (1).
As reported by the ITA.LI.CA Group, LR may be extended to patients with either clinically significant PHT or slight hyperbilirubinemia (<2.0 mg/dL) without compromising outcomes. Median survivals were similar in patients with hyperbilirubinemia alone (125 months) or PHT alone (100 months) concerning ideal candidates (93 months). On the opposite, PTH + hyperbilirubinemia had a significantly worse prognosis (85 months) (35).
Roayaie et al. reported an international study based on 10,135 HCC patients treated worldwide, in which four different groups (ideal candidates resected, ideal candidates not resected, nonideal candidates resected and nonideal candidates not resected) were compared. PHT alone (presence of either varices, splenomegaly, or platelet count <100,000/lL) or total bilirubin >1.0 mg/dL alone were not associated with an appreciable decrease in survival after LR. However, the contemporaneous presence of both was detrimental. Thus, the study concluded that there might be modest room for expansion of the resection criteria. As an example, a slight expansion able to include moderate PHT cases (platelet count >50,000/lL, no ascites) would increase the pool of ideal candidates by approximately 60%. Similarly, an expansion able to include patients with mild elevation of bilirubin (<2 mg/dL) would allow for approximately 25% more patients to undergo resection without any loss in long-term outcome (36).
Macro-vascular invasion
According to the EASL 2018 guidelines, cases with HCC-related portal vein thrombosis (PVT) present an advanced stage not amenable to curative treatments (1). However, it is known that patient prognosis is directly affected by the extension of PVT, especially in the presence of elevated alpha-fetoprotein and large tumours. In fact, PVT can be graded as PV1 (segmentary), PV2 (secondary order branch), PV3 (first-order branch), and PV4 (main trunk/contralateral branch) (37).
Several studies investigated the role of PVT in terms of impact on survival rates after LR (Table 2) (23,33,36,38-54).
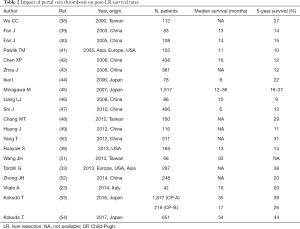
Full table
A propensity matched-cohorts analysis by the Liver Cancer Study Group of Japan demonstrated that LR should be considered as the gold-standard treatment, as long as the PVT is limited to grades PV1–3, the liver function is preserved, and a no-R2 resection is achievable (53). Similarly, an international study showed a remarkable prognosis after LR for patients with HCC and PVT (54).
The surgical indications for PVT invading the main trunk or contralateral branch (PV4) are controversial (55). In the Japanese experience, the survival benefit after LR in the PV4 group was not statistically significant, and the R2 resection rate was relatively high. Considering that complete resection is extremely difficult in PV4 patients, the surgical indication in these cases requires further investigation (54). Neoadjuvant and adjuvant treatment including sorafenib and radiotherapy together with LR may be a promising treatment strategy for PV4 patients (56).
Nonetheless, no prospective comparison of LR vs. systemic treatments or radioembolization has ever been reported. Thus, it is not clear if the remarkable survivals observed in resected PVT cases were related to a super-selection of the investigated population. Therefore, LR should be suggested only in no-PV4 extension. However, such an approach should be considered only as an option to be tested within research settings, and not as a standard of practice (1).
The role of laparoscopic surgery
The first mini-invasive cholecystectomy was reported in 1987 (57). After this initial experience, the mini-invasive approach rapidly became a reality also in the setting of liver surgery. The first mini-invasive liver surgery (MILS) approaches were reported in 1996 (58,59). After the first pioneering MILS cases (60), several series reported structured case-series with results often favouring laparoscopy respect to open surgery (61-63). As reported in the Consensus Conferences of Louisville 2008, Morioka 2014 and Southampton 2017, growing evidence exists that MILS approach is feasible for a great number of liver diseases (64-66). As an example, today laparoscopy is considered the standard for performing a left lateral sectionectomy (67).
However, retrospective MILS studies showing better outcomes concerning open series should be interpreted with caution, because of the propensity to perform these interventions only in super-selected patients (1). Concerning size, several studies questioned the superiority of laparoscopic anatomical LR in HCC >2.0 cm (22,68-71). As for the liver function, limited resections conducted through laparoscopic/robotic techniques in large-volume centres should be performed in patients with borderline conditions (i.e., Child B7, moderate PHT, bilirubin <2.0 mg/dL) (72,73).
As reported by the Southampton Consensus guidelines, meta-analyses and large propensity score-matched studies comparing open versus laparoscopic LR for HCC, MILS was associated with reduced blood loss, transfusion rate, postoperative ascites, liver failure and hospital stay with comparable operation times, disease-free margin, and recurrence rates (66,73,74). In a recent series, this evidence has also been confirmed in cases requiring a major resection (75). In case of minor resections, a laparoscopic approach was found to be the only independent factor able to reduce the complication rates in resected HCC patients.
According to the Southampton guidelines, subcategories of “high-risk” patients, such as the elderly and patients with high body mass index, were no longer considered as contra-indications to LR (66). When comparing MILS performed in cirrhotic versus no-cirrhotic cases, no differences were observed regarding operative time, blood loss, intraoperative complications, hospital stay, and morbidity (76). On the opposite, a laparoscopic approach appears to reduce the incidence of postoperative ascites, liver failure, and overall morbidity (77,78).
Conclusions
LR for the treatment of HCC-on-cirrhosis is a safe and effective procedure not only in “ideal cases”, but also for selected patients presenting some of the well-known risk factors of poor clinical course. The presence of one or more of these factors does not represent an absolute contraindication for LR but such cases should be evaluated in the context of a multidisciplinary team. Further studies investigating the “borderline” cases are required, mainly looking at the possible decisive role of laparoscopy in this setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Bolondi L, Cillo U, Colombo M, et al. Italian Association for the Study of the Liver (AISF). Position paper of the Italian Association for the Study of the Liver (AISF): The multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 2013;45:712-23. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol 1999;94:2460-6. [Crossref] [PubMed]
- Takenaka K, Shimada M, Higashi H, et al. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg 1994;129:846-50. [Crossref] [PubMed]
- Cucchetti A, Sposito C, Pinna AD, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg 2016;103:e93-9. [Crossref] [PubMed]
- Borzio M, Dionigi E, Parisi G, et al. Management of hepatocellular carcinoma in the elderly. World J Hepatol 2015;7:1521-9. [Crossref] [PubMed]
- Ueno M, Hayami S, Tani M, et al. Recent trends in hepatectomy for elderly patients with hepatocellular carcinoma. Surg Today 2014;44:1651-9. [Crossref] [PubMed]
- Kang SD, Kim JW, Jwa YJ, et al. Treatment of hepatocellular carcinoma in elderly patients. Hepatogastroenterology 2014;61:2001-8. [PubMed]
- Wang HQ, Yang J, Yan LN, et al. Liver resection in hepatitis B related-hepatocellular carcinoma: clinical outcomes and safety in elderly patients. World J Gastroenterol 2014;20:6620-5. [Crossref] [PubMed]
- Liu PH, Hsu CY, Lee YH, et al. Uncompromised treatment efficacy in elderly patients with hepatocellular carcinoma: a propensity score analysis. Medicine (Baltimore) 2014;93. [Crossref] [PubMed]
- Kim PT, Jang JH, Atenafu EG, et al. Outcomes after hepatic resection and subsequent multimodal treatment of recurrence for multifocal hepatocellular carcinoma. Br J Surg 2013;100:1516-22. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-16. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg 2005;140:450-7. [Crossref] [PubMed]
- Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol 2005;12:364-73. [Crossref] [PubMed]
- Ruzzenente A, Capra F, Pachera S, et al. Is Liver Resection Justified in Advanced Hepatocellular Carcinoma? Results of an Observational Study in 464 Patients. J Gastrointest Surg 2009;13:1313-20. [Crossref] [PubMed]
- Zhong JH, De Xiang B, Gong WF, et al. Comparison of Long-Term Survival of Patients with BCLC Stage B Hepatocellular Carcinoma after Liver Resection or Transarterial Chemoembolization. PLoS One 2013;8. [Crossref] [PubMed]
- Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015;41:236-42. [Crossref] [PubMed]
- Koh YX, Tan HL, Lye WK, et al. Systematic review of the outcomes of surgical resection for intermediate and advanced Barcelona Clinic Liver Cancer stage hepatocellular carcinoma: A critical appraisal of the evidence. World J Hepatol 2018;10:433-47. [Crossref] [PubMed]
- Levi Sandri GB, Spoletini G, Vennarecci G, et al. Laparoscopic liver resection for large HCC: short- and long-term outcomes in relation to tumor size. Surg Endosc 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Poon RTP, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg 2002;194:592-602. [Crossref] [PubMed]
- Mok KT, Wang BW, Lo GH, et al. Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surg 2003;197:730-8. [Crossref] [PubMed]
- Shah SA, Wei AC, Cleary SP, et al. Prognosis and results after resection of very large (> or 1/410 cm) hepatocellular carcinoma. J Gastrointest Surg 2007;11:589-95. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Shirabe K, et al. Outcomes of Hepatic Resection for Huge Hepatocellular Carcinoma (I10 cm in Diameter). J Surg Oncol 2011;104:292-8. [Crossref] [PubMed]
- Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2012;256:706-12. [Crossref] [PubMed]
- Llop E, Berzigotti A, Reig M, et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol 2012;56:103-8. [Crossref] [PubMed]
- Wong JS, Wong GL, Chan AW, et al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg 2013;257:922-8. [Crossref] [PubMed]
- Nishio T, Taura K, Koyama Y, et al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery 2016;159:399-408. [Crossref] [PubMed]
- Zipprich A, Kuss O, Rogowski S, et al. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut 2010;59:963-8. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526-36. [Crossref] [PubMed]
- Trevisani F, Bucci L, Garuti F, et al. Is it time to extend criteria for hepatic resection in the treatment of hepatocellular carcinoma? Hepatology 2016;64:2257-8. [Crossref] [PubMed]
- Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015;62:440-51. [Crossref] [PubMed]
- Shi J, Lai ECH, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010;17:2073-80. [Crossref] [PubMed]
- Wu CC, Hseih S, Ho WM, et al. Surgical treatment for recurrent hepatocellular carcinoma with tumor thrombi in right atrium: using cardiopulmonary bypass and deep hypothermic circulatory arrest. J Surg Oncol 2000;74:227-31. [Crossref] [PubMed]
- Fan J, Wu ZQ, Zhou J, et al. Hepatocellular carcinoma associated with tumor thrombosis in the portal vein: the effects of different treatments. Hepatobiliary Pancreat Dis Int 2003;2:513-9. [PubMed]
- Fan J, Zhou J, Wu ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215-9. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: Results of a multicenter study. Surgery 2005;137:403-10. [Crossref] [PubMed]
- Chen XP, Qiu FZ, Wu ZD, et al. Long-term outcome of resection of large hepatocellular carcinoma. Br J Surg 2006;93:600-6. [Crossref] [PubMed]
- Zhou J, Fan J, Tang ZY, et al. Time dependency of factors influencing survival of hepatocellular carcinoma patients with portal vein tumor thrombosis after surgery. Zhonghua Yi Xue Za Zhi 2006;86:3005-8. [PubMed]
- Ikai I, Takayasu K, Omata M, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol 2006;41:884-92. [Crossref] [PubMed]
- Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006;12:7561-7. [Crossref] [PubMed]
- Liang LJ, Hu WJ, Yin XY, et al. Adjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomy. World J Surg 2008;32:627-31. [Crossref] [PubMed]
- Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010;17:2073-80. [Crossref] [PubMed]
- Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery 2012;152:809-20. [Crossref] [PubMed]
- Huang J, Hernandez-Alejandro R, Croome KP, et al. Hepatic resection for huge (>15 cm) multinodular HCC with macrovascular invasion. J Surg Res 2012;178:743-50. [Crossref] [PubMed]
- Yang T, Lin C, Zhai J, et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol 2012;138:1121-9. [Crossref] [PubMed]
- Wang JH, Kuo YH, Wang CC, et al. Surgical resection improves the survival of selected hepatocellular carcinoma patients in Barcelona clinic liver cancer stage C. Dig Liver Dis 2013;45:510-5. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver Cancer Study Group of Japan. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology 2017;66:510-7. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Kermiche-Rahali S, Di Fiore A, Drieux F, et al. Complete pathological regression of hepatocellular carcinoma with portal vein thrombosis treated with sorafenib. World J Surg Oncol 2013;11:171. [Crossref] [PubMed]
- Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopic cholecystectomy. Am J Surg 1991;161:385-7. [Crossref] [PubMed]
- Azagra JS, Goergen M, Gilbart E, et al. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc 1996;10:758-61. [Crossref] [PubMed]
- Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery 1996;120:468-75. [Crossref] [PubMed]
- Hüscher CG, Lirici MM, Chiodini S, et al. Current position of advanced laparoscopic surgery of the liver. J R Coll Surg Edinb 1997;42:219-25. [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Descottes B, Lachachi F, Sodji M, et al. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg 2000;232:641-5. [Crossref] [PubMed]
- Shimada M, Hashizume M, Maehara S, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc 2001;15:541-4. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second International Consensus Conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [PubMed]
- Giovanardi Lai Q. Minimally invasive right hepatectomy for living liver donation: a systematic review of the literature. Laparosc Surg 2018;2:17. [Crossref]
- Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 2008;143:469-75. [Crossref] [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: Systematic review and meta-analysis. World J Gastroenterol 2014;20:8274. [Crossref] [PubMed]
- Franken C, Lau B, Putchakayala K, et al. Comparison of short term outcomes in laparoscopic vs. open hepatectomy. JAMA Surg 2014;149:941-6. [Crossref] [PubMed]
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Lim C, Osseis M, Lahat E, et al. Safety of laparoscopic hepatectomy in patients with hepatocellular carcinoma and portal hypertension: interim analysis of an open prospective study. Surg Endosc 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Cipriani F, Fantini C, Ratti F, et al. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc 2018;32:617-26. [Crossref] [PubMed]
- Xiong JJ, Altaf K, Javed MA, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-68. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-7. [Crossref] [PubMed]
- Shehta A, Han HS, Yoon YS, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc 2016;30:638-48. [Crossref] [PubMed]
- Zhang Y, Huang J, Chen XM, et al. A comparison of laparoscopic versus open left hemihepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 2016;26:146-9. [Crossref] [PubMed]
- Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015;22:342-52. [Crossref] [PubMed]
Cite this article as: Giovanardi F, Lai Q, Bertacco A, Vitale A. Resection for hepatocellular cancer: overpassing old barriers. Transl Gastroenterol Hepatol 2018;3:64.


