Lateral cavo-caval shunt: an alternative veno-venous bypass in liver surgery
Introduction
During liver transplantation (LT), inferior vena cava (IVC) conservation with “piggy-back” reconstruction (1) or latero-lateral anastomosis (2) has replaced the historic vena cava resection procedure (3) as it proved to be safer and provide better outcomes (4). Nevertheless, in some specific cases, the IVC must be resected. In those cases, especially if the complete caval clamping is not tolerate, an extra corporal veno-venous bypass (VVB) is mandatory. However, VVB could be responsible of severe adverse effects like pulmonary embolism, lung injury or coagulation disorder (5). Also, in extreme liver surgery cases the IVC may be resected.
Here we described an alternative method allowing the preservation of both IVC and portal blood flow without extracorporeal VVB, combining a cavo-caval shunt (with a prosthetic vascular graft) and a temporary porto-caval shunt (TPCS).
Case presentation
A 60-year-old woman presented with a huge intrahepatic cholangiocarcinoma. The tumor was considered unresectable due to infiltration of all vascular structures (i.e., the portal vein, the hepatic artery and the 3 hepatic veins). The retrohepatic vena cava also had a very close contact with the tumor and was probably infiltrated (Figure 1). There was no extra hepatic tumoral localization and the patient’s clinical status was normal.
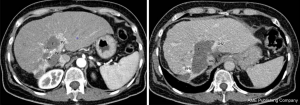
After discussion in multidisciplinary meeting, a neo-adjuvant treatment was decided associating systemic chemotherapy regimen (gemcitabine and cisplatin) and Yttrium-90 radioembolization (6). Despite a significant down staging, the tumor remained unresectable due to persistent vascular infiltration. Orthotropic liver transplantation (OLT) was then discussed as it may be the only potential curative treatment as we already reported (7). Due to persistent contact with IVC and as Yttrium-90 radioembolization is known to be responsible of inflammatory adherences between the retrohepatic IVC and the liver (8), the resection of the IVC was mandatory in order to achieve a safe R0 resection.
The OLT was performed with a graft discarded by all other transplantation team due to advanced donor’s age. The procedure started, like usual with liver pedicle dissection. A TPCS was then performed in order to preserve outflow of the splanchnic territory. The liver was partly mobilized with section of the right and left triangular ligament.
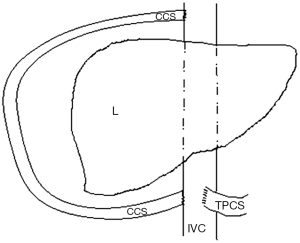
The suprahepatic IVC was dissected and controlled below and above the liver. This step required a dissection of the cavo-atrial junction in order to get approximately 5 cm of free supra-hepatic IVC. A prosthetic vascular graft (GORE-TEX®) was then sutured on the lateral right flank of the IVC in order to perform a cavo-caval shunt between the infra-hepatic and the supra-hepatic IVC. The upper anastomosis was performed firstly, approximately 2 cm above the confluence of the hepatic veins with a partial lateral-clamping of the right flank of the IVC. The lower anastomosis was performed approximately 1 cm above the TPCS with a partial lateral-clamping of the right flank of the IVC (Figure 2).
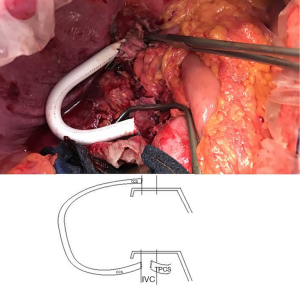
The shunt was opened and the retrohepatic IVC could be safely cross-clamped and resected with the attached liver (Figure 3). The liver graft could then be implanted orthotopically with an end-to-end caval anastomosis between the graft’s and the recipient’s IVC. After removing the TPCS, an end-to-end portal anastomosis was performed. The caval anastomosis was then unclamped and after ensuring of the absence of blood leakage, the shunt was sectioned with vascular stapler. After a total cold ischemic time of 7 hours, the liver was revascularized. No reperfusion syndrome was observed. The procedure was pursued with the arterial and the biliary anastomosis. Eventually, no transfusion was required.
The postoperative outcome was uneventful with normal graft function recovery. The length of stay in ICU lasted 3 days and the patient was discharged from the hospital at postoperative day 12 and is still doing well.
Discussion
During LT with IVC resection, a VVB is usually required in order to maintain hemodynamic stability (9) as well as abdominal organs function (10) and result in decreasing the early postoperative mortality (9). Here, we present a simple alternative procedure which avoids the use of an extracorporeal VVB while never interrupting the caval flow. Indeed, the association of a TPCS and a cavo-caval shunt using a prosthetic graft implanted laterally on the right flank of the IVC above and below the native liver allow both caval and portal blood flow to be maintained. No complications were observed during the procedure and no transfusion was required. Moreover, the flexibility of the prosthetic graft and its lateral implantation allow easy graft implantation and revascularization while maintaining the shunt. When it is no longer necessary, the shunt could be easily removed with a vascular stapler.
To our knowledge, this technique has never been reported and, in our opinion, could present several advantages compared to the classical VVB. Firstly, it avoids the necessity of a pump which is a costly procedure and usually required a specialized nurse for machine manipulation and surveillance. Secondly, it theoretically avoids occurrence of VVB related adverse events like acute pulmonary embolism (11,12), coagulation disorders or postoperative renal failure (13). Thirdly, it avoids potential additional incision required for cannulation of the femoral and axillary veins which could be responsible of wound infection or neurological damage (14). In our case, we did not observed specific adverse effects related to this procedure, however we must remain cautious as we only performed this procedure once.
Conclusions
In conclusion, when IVC resection is mandatory during liver surgery or transplantation, a temporary lateral cavo-caval shunt seem to be a safe method allowing maintenance of the caval flow and could be an alternative of the classical extracorporeal VVB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg 1989;210:649-52. [Crossref] [PubMed]
- Belghiti J, Panis Y, Sauvanet A, et al. A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet 1992;175:270-2. [PubMed]
- Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg 1968;168:392-415. [Crossref] [PubMed]
- Hoffmann K, Weigand MA, Hillebrand N, et al. Is veno-venous bypass still needed during liver transplantation? A review of the literature. Clin Transplant 2009;23:1-8. [Crossref] [PubMed]
- Sakai T, Matsusaki T, Marsh JW, et al. Comparison of surgical methods in liver transplantation: retrohepatic caval resection with venovenous bypass (VVB) versus piggyback (PB) with VVB versus PB without VVB. Transpl Int 2010;23:1247-58. [Crossref] [PubMed]
- Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol 2015;22:3102-8. [Crossref] [PubMed]
- Rayar M, Levi Sandri GB, Houssel-Debry P, et al. Multimodal Therapy including Yttrium-90 Radioembolization as a Bridging Therapy to Liver Transplantation for a Huge and Locally Advanced Intrahepatic Cholangiocarcinoma. J Gastrointestin Liver Dis 2016;25:401-4. [PubMed]
- Ettorre GM, Levi Sandri GB, Laurenzi A, et al. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J Surg 2017;41:241-9. [Crossref] [PubMed]
- Shaw BW Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg 1984;200:524-34. [Crossref] [PubMed]
- Ringe B, Bornscheuer A, Blumhardt G, et al. Experience with veno-venous bypass in human liver transplantation. Transplant Proc 1987;19:2416. [PubMed]
- Khoury GF, Mann ME, Porot MJ, et al. Air embolism associated with veno-venous bypass during orthotopic liver transplantation. Anesthesiology 1987;67:848-51. [Crossref] [PubMed]
- Navalgund AA, Kang Y, Sarner JB, et al. Massive pulmonary thromboembolism during liver transplantation. Anesth Analg 1988;67:400-2. [Crossref] [PubMed]
- Jovine E, Mazziotti A, Grazi GL, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int 1997;10:109-12. [Crossref] [PubMed]
- Katirji MB. Brachial plexus injury following liver transplantation. Neurology 1989;39:736-8. [Crossref] [PubMed]
Cite this article as: Rayar M, Levi Sandri GB, Blondeau M, Lakehal M, Desfourneaux V, Sulpice L, Meunier B, Boudjema K. Lateral cavo-caval shunt: an alternative veno-venous bypass in liver surgery. Transl Gastroenterol Hepatol 2018;3:23.


