Long-term adjuvant treatment of gastrointestinal stromal tumors (GIST) with imatinib—a comment and reflection on the PERSIST-5 study
Gastrointestinal stromal tumors (GIST) are the only solid tumors where adjuvant treatment is considered for more than one year. This is even more striking as it is now discussed whether adjuvant treatment should be done 3 or 5 years.
In cell culture, mesenchymal cells grow slowly and are less responsive to chemotherapeutic agents than i.e. epithelial cells. For GIST, the most common mesenchymal tumors, it has been shown that they are neither well responsive to chemotherapy, nor to radiotherapy – and it has not been completely elucidated why this is the case (1).
In 2001, Joensuu, et al. published the break-through case report for small-molecule therapy in solid tumors (2). Only three years earlier, Hirota et al. showed gain-of-function mutations within the KIT gene in GIST, with constitutive activation of the KIT receptor (3). Similarly, it was recent knowledge that CML is driven by the translocation of part of the breakpoint-cluster-region (BCR) with ABL. ABL carries a domain which transfers phosphate groups to tyrosine residues. The translocation thus leads to a proto-oncogenic tyrosine kinase fusion protein (4). As a consequence, the Scandinavian center had been participating in a study showing that CML patients respond to a novel tyrosine kinase inhibitor (ST1571/imatinib) (5,6). Thus, the heavily pretreated female patient (mesna, doxorubicin, ifosfamide, dacarbazine and -interferon) with metastatic GIST of the stomach (KIT exon 11 mutation), received this tyrosine-kinase inhibitor. The tumor showed an impressive rapid response and already after 4 weeks, no abnormal uptake was seen in the PET-CT (2).
Even though the initial hype that small molecular therapy would be as successful in other tumors did not prevail, for GIST with KIT or PDGFRA mutations, it still holds. Needless to say that this therapy is not cytotoxic. Indeed, on the verge of autophagy it initiates apoptosis (7). A hyaline stroma is generated—though it is not clear how—in which dormant GIST cells can be embedded (8). If any tumor cell prevails within the body, recurrences even after more than 10 years can be seen (9).
The initial question was thus: Who needs tyrosine-kinase inhibitor treatment to prevent recurrences, and at which dose. The assumed effect of adjuvant treatment being the eradication of microscopic disease (10). Whilst the second question was and still is adjuvant treatment duration after surgery.
From classification to treatment
In 2002, Fletcher published the first classification for the prediction of aggressive behavior in GIST (11). This classification proposes four risk categories for tumor recurrence (i.e., very low risk, low risk, intermediate risk and high risk) according to tumor size and mitotic count (per 50 high power fields, HPF). In the same issue, Miettinen and Lasota published their criteria for three categories of GIST malignancy (probably benign, uncertain or low malignant potential and probably malignant) (12). In addition to size and mitotic count, gastric and intestinal origin were differentiated (12). From 2006, the Miettinen classification (so-called AFIP criteria) included stomach, duodenum, small intestine, and rectum location of the primary (13). It adopted the nomenclature very low risk, low risk, intermediate and high risk GIST from the earlier Fletcher classification. Interestingly, the new UICC/TNM classification again only differentiates between gastric and non-gastric locations (14) (Mitoses are described for 5 mm2 instead of per 50 HPF for standardization). Whilst the UICC/TNM classification remains descriptive, the Miettinen classification (13) has been validated for the decision of adjuvant treatment.
Very low risk and low risk GIST do not need adjuvant therapy after surgical resection of the primary, as their risk of recurrence is negligible (however not 0) (13). For intermediate risk, Miettinen et al. described a recurrence risk of 10–24%, and for high risk GIST the risk of recurrence is 34–90% without adjuvant treatment (13). It would thus be tempting to differentiate this group into high risk and very high risk in the future.
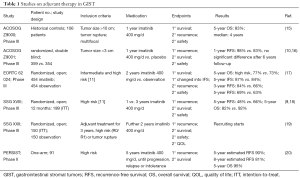
Recurrence can be postponed—or prevented, when adjuvant treatment is given. Table 1 gives an overview of the studies on adjuvant treatment. The initial American College of Surgeons Oncology Group (ACOSOG) Z9000-Study (ClinicalTrials.gov Identifier NCT 00025246) tested 1 year adjuvant imatinib treatment (400 mg) in tumors with a tumor diameter >10 cm, intraperitoneal tumor rupture, or up to four peritoneal implants. Needless to say, that these inclusion criteria are currently obsolete for adjuvant treatment. In this study, patients would be treated until recurrence or progression. Even though tumors were advanced in this study, one year of imatinib treatment was beneficial. The 5-year recurrence-free survival (RFS) rate was 40%, with a median of 4 years. The 5-year overall-survival rate was 83% (15). This study also showed that KIT Exon 11 mutations respond significantly better to 400 mg imatinib than KIT Exon 9 mutations (15), which was later proven in metastatic GIST (21).
Full table
Imatinib for adjuvant treatment was approved based on the ACOSOG Z9001 Study (ClinicalTrials.gov Identifier NCT 00041197) in 2008 in the US and in 2009 in Europe. In this study, eligible patients had complete resection of a primary GIST of at least 3 cm in size. The median follow-up was 19.7 months. Adjuvant treatment for one year led to a RFS of 98% (placebo 83%) after one year, the overall survival (OS) not being reached (99.2%; placebo 99.7%).
In the French Phase-III-Intergroup-Study of the European Organization for Research and Treatment of Cancer (EORTC, ClinicalTrials.gov Identifier NCT 00103168) patients with intermediate and high-risk GIST were treated for 2 years with imatinib (400 mg) (17,22). The median follow-up was 4.7 years. Five-year RFS was 69% versus 63% in the observation arm. Five-year OS was 100% versus 99% for observation only. The authors argue that the small differences rather represent the heterogeneous groups with a short follow-up time and the cross-over design of the study, allowing patients assigned to the placebo arm to receive imatinib upon recurrence.
Both studies, the French EORTC 62024-study and the Scandinavian-German SSG XVIII study were posted in 2005. The SSG XVIII study (Clinicaltrials.gov Identifier NCT 00116935) compared 12 vs. 36 months adjuvant imatinib treatment (400 mg) in high risk GIST. Until October 2006, when the protocol was amended, patients with operable intra-abdominal GIST metastases could be entered into the study. Tumor spillage into the abdominal cavity at the time of surgery or spontaneous tumor rupture was also included (18). These manifestations would now be considered and treated according to metastatic GIST (9). The main result of this study was the prolonged RFS in the prolonged treatment arm with a 5-year RFS of 65.6% versus 47.9%, respectively. This translated for the first time into a significantly longer OS of 92.0% in the 3-year arm versus 81.7% in the 1-year treatment arm. Now, it was apparent that the benefit for RFS of +17.1% in the 3-year treatment arm vanished after another two years (18). In other words, the slopes were shifted two years in time, even though ‘tumor rupture’ was present to a similar extend in both groups. To date, it is not clear, whether microscopic tumor spillage with serosa involvement or vascular involvement (V1), repressed immune defense of the body, tumor cell hibernation or a combination promote late recurrence. From a clinical point-of-view, the question is, whether another two years of treatment would eradicate the remaining GIST cells, so that late recurrence would be negligible. To this aim, two further studies have been designed. The American PERSIST-5 study and the Scandinavian-German SSG XXII study.
The SSG XXII study (Clinicaltrials.gov Identifier NCT02413736) examines patients with high risk GIST who had been treated with imatinib for 3 years. These patients will be randomized into another two years of imatinib versus the current procedure of follow-up care. High risk of recurrence is defined as gastric GIST with a mitotic count of >10/50 high power fields (HPF) or non-gastric GIST with a mitotic count >5/50 HPF or tumor rupture (https://clinicaltrials.gov/ct2/show/record/NCT02413736). The study is open, no data are available yet.
The American PERIST-5 study (Clinicaltrials.gov Identifier NCT00867113) has now been completed (20). It is a phase II, single-arm study which included patients with primary GIST ≥2 cm and a mitotic rate of ≥5/50 HPF and GIST of non-gastric primary of ≥5 cm. The primary endpoint was to evaluate long term use of adjuvant imatinib in patients with high risk for GIST recurrence (https://clinicaltrials.gov/ct2/show/NCT00867113). Ninety one patients were enrolled and received imatinib 400 mg/d (median age 60 years, range 30–90 years). Median tumor size was 6.5 cm (range, 2.3–30 cm), 55% were of gastric origin. Median treatment duration was 55.7 months (range, 0.5–75 months). 46 patients (50.5%) completed the treatment. The 5-year RFS was 90% and the 5-year OS was 95%. In 6 patients (7.7%) GIST recurred after adjuvant treatment, whilst one patient died from GIST whilst under treatment. He had an imatinib resistant mutation (PDGFRA D842V). Treatment was discontinued mostly for patients’ choice (20%) or side effects (18%). The most common side-effects were nausea, diarrhea, fatigue, muscle cramps, vomiting and periorbital edema (20).
The authors conclude that five years adjuvant imatinib treatment was effective in preventing recurrence in patients with imatinib-sensitive mutations. They also summarized that most recurrences occurred after imatinib-discontinuation (20).
It would now be interesting to know when these recurrences occurred. Joensuu et al. have analyzed the risk of recurrence for the three main adjuvant trials: the ACOSOG Z9001 trial, the French EORTC 62024-study and the Scandinavian-German SSG XVIII trial (23). They report that recurrence occurs within the first three years from randomization. Recurrences were consistent with mitotic count and non-gastric location. Large tumor size and tumor rupture were also independently associated with recurrence. Patients with intermediate and high risk GIST who received 3 years of adjuvant imatinib had fewer recurrences than patients with shorter treatment duration (23).
Conclusions
Imatinib thus protects against recurrences in GIST with imatinib-sensitive mutations. OS seems to depend on the tumor behavior itself once it has returned, and by its response to therapy.
We should thus keep in mind that imatinib is not everything. Tumor detection, correct classification with mutation analysis and careful surgical removal are the base for treatment outcome. Even in the current study, the inclusion of R1 resections was reviewed on a case by case basis by the Study Management Committee.
Furthermore, neither tumor perforation, nor serosa penetration are classified within the different classifications (24,25). Similarly necrosis, cellularity, vascular density and appearance as single nodule or conglomerate should be stated as it might reflect aggressive behavior (grading) (26). Serosa perforation leads to peritoneal metastasis, whilst liver metastasis is rather caused by hematogenic spread. Both these manifestations behave differently, as their microenvironment differs (27). Peritoneal metastases seem to be closer related to the primary, as they can be interpreted as droplets (25). However, peritoneal metastases are generally more difficult to treat, as they are less encapsulated and systemic treatment might reach them more difficult. Interestingly, lymphatic spread can be neglected.
Five years of adjuvant imatinib treatment might definitively treat or postpone recurrences in 90% of the cases, as against 66% in the 3-year arm of the SSG XVIII trial. The 5-year overall survival was less distinct with 95% versus 92% in the 3-year arm of the SSG XVIII trial. It has to be kept in mind, that also intermediate GIST were included into the PERSIST-5 study, as against only high risk GIST in the SSG XVIII trial. Subgroup analyses are awaited.
The fact that only about 50% of the patients concluded the treatment of 5 years will be a problem in real life, if 5-year adjuvant treatment shall become an option. It should be considered in very high risk GIST, a group, which still needs to be defined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Reichardt P, Reichardt A, Cameron S. Gastrointestinale Stromatumoren. Der Gastroenterologe 2017;12:75-88. [Crossref]
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052-6. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology (Williston Park) 1999;13:169-80. [PubMed]
- Druker BJ. ST1571: a paradigm for clinical trials of molecularly targeted agents. Biomed Pharmacother 2001;55:529-30. [Crossref] [PubMed]
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031-7. [Crossref] [PubMed]
- Ravegnini G, Sammarini G, Nannini M, et al. Gastrointestinal stromal tumors (GIST): Facing cell death between autophagy and apoptosis. Autophagy 2017;13:452-63. [Crossref] [PubMed]
- Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol 2007;14:526-32. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Hall KS, et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer 2014;120:2325-33. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, El-Rifai W, Sobin HL, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002;33:478-83. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Wittekind C, Asamura H, Sobin LH. TNM Atlas, 6th Edition. Wiley-Blackwell, 2014.
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Casali PG, LeCesne A, Velasco AP, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): The EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol 2013.31. Abstract 10500.
- Joensuu H, Eriksson M, Sundby HK, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Scandinavian Sarcoma Group: Three Versus Five Years of Adjuvant Imatinib as Treatment of Patients With Operable GIST. Available online: https://clinicaltrials.gov/ct2/show/, NCT02413736, ClinicalTrials.gov Identifier: NCT02413736, 2017.
- Raut CP, Espat NJ, Maki RG, et al. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol 2017.35. Abstract 11009.
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- Casali PG, Le CA, Poveda VA, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276-83. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol 2010;3:461-71. [PubMed]
- Agaimy A, Vassos N, Wunsch PH, et al. Impact of serosal involvement/extramural growth on the risk of synchronous and metachronous peritoneal spread in gastrointestinal stromal tumors: proposal for a macroscopic classification of GIST. Int J Clin Exp Pathol 2012;5:12-22. [PubMed]
- Agaimy A, Bauer S, Beham A, et al. Gastrointestinal Stromal Tumours (GIST) - development in pathology, surgery and medical therapy. Z Gastroenterol 2015;53:235-43. [Crossref] [PubMed]
- Cameron S, Gieselmann M, Blaschke M, et al. Immune cells in primary and metastatic gastrointestinal stromal tumors (GIST). Int J Clin Exp Pathol 2014;7:3563-79. [PubMed]
Cite this article as: Cameron S. Long-term adjuvant treatment of gastrointestinal stromal tumors (GIST) with imatinib—a comment and reflection on the PERSIST-5 study. Transl Gastroenterol Hepatol 2018;3:16.


