Gastrointestinal stromal tumor of the esophagus: current issues of diagnosis, surgery and drug therapy
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms arising from the digestive tract, with an annual incidence of 7 to 20 per million (1-3). GISTs are positive for c-KIT (CD117) or CD34, and they account for less than 1% of all gastrointestinal tumors. Intestinal cells of Cajal (ICCs) are known to be precursors of GISTs (4). Surgical resection is considered to be the only potentially curative treatment for localized GISTs at present (5).
GISTs often arise in the stomach and small intestine, while esophageal GISTs are extremely rare (6-12). Due to their rarity, clinicopathological data on esophageal GISTs are extremely limited, with only individual case reports or case series with small numbers.
The rarity of esophageal GISTs results in a lack of clear recommendations concerning their optimal surgical management (13). As esophageal segmental and wedge resections are not usually performed due to the anatomical peculiarity of the esophagus, the surgical options are limited to the highly invasive esophagectomy or the much less invasive surgical tumor enucleation (6,14).
When an esophageal submucosal tumor is found, it is sometimes difficult to determine preoperatively whether the tumor is benign or malignant by imaging examinations, such as endoscopic ultrasound (EUS), computed tomography (CT), and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Fine-needle aspiration biopsy (FNAB) under EUS provides very important information preoperatively, but it is considered a controversial technique due to the risk of tumor rupture and seeding (15). The difficulty in preoperative diagnosis makes it difficult for surgeons to select the surgical method.
Imatinib, a Bcr-Abl tyrosine kinase inhibitor (TKI), has been shown to have high efficacy in the metastatic and adjuvant settings, and the use of imatinib in neoadjuvant setting has been attempted. However, due to the rarity of esophageal GISTs, there is limited literature available regarding neoadjuvant administration of imatinib in patients with esophageal GISTs (13,16-19). Since controlled clinical trials for esophageal GISTs are not available, the best surgical procedure and the impact of adjuvant or neoadjuvant imatinib therapy have not been established.
This article provides updates on esophageal GISTs, focusing particularly on preoperative diagnosis and surgical treatment.
Epidemiology and clinical presentation
GISTs occur predominantly in the stomach (60–70%), small intestine (20–30%), and colorectum (5–10%) (6-11). Esophageal GISTs are extremely uncommon, accounting for fewer than 5% of all GISTs (9,12).
Leiomyomas are the most common mesenchymal tumors of the esophagus, and GISTs account for about 25% of mesenchymal esophageal tumors (10).
The clinical features of esophageal GISTs are not well-known. Lott et al. summarized 55 cases of esophageal GIST and reported that in comparison to gastric GISTs, esophageal GISTs occurred significantly more frequently in men, as well as in patients younger than 60 years at diagnosis (2).
The most common location for esophageal GISTs is the lower esophagus, followed by the middle esophagus, and GISTs in the upper esophagus are rare (2,20,21). Radenkovic et al. found that ICCs were abundant in the lower esophagus, less numerous in the middle region, and rare in the upper part (22). The reported distribution of ICCs was in accordance with the distribution of esophageal GISTs (20).
Esophageal GISTs were often found accidentally on esophagoscopy or barium esophagography (15). As GISTs grow in the esophagus, patients present with various symptoms. Dysphagia (36–51%) is the most frequent symptom, followed by weight loss (20%), chest pain (8–15%), and bleeding (1–10%) (2,20,21).
Diagnosis of esophageal GISTs
When a submucosal tumor is found in the esophagus, the differential diagnosis of an esophageal GIST includes both malignant and benign tumors, including leiomyoma, hemangioma, schwannoma, leiomyosarcoma, and papillary epithelioma (1). It is unfortunately difficult to distinguish esophageal leiomyoma from GIST prior to resection, because the two types of tumors appear similar on CT and EUS (23).
FDG-PET
Recently, the use of FDG-PET for GISTs has been reported. The maximum standardized uptake value (SUVmax) on FDG-PET is considered to correlate with the degree of malignancy of GIST, but the definitive diagnosis is difficult (1,24,25). Dendy et al. reported that esophageal leiomyomas also showed a wide range of SUVmax values, from 3.8 to 13.4 (23). The PET-avidity of benign tumors limits the role of FDG-PET in the differential diagnosis of submucosal tumors of the esophagus. On the other hand, FDG-PET is known to be useful for evaluating postoperative recurrence and the response of GISTs to chemotherapy (1,24,25).
Magnetic resonance imaging (MRI)
Recent studies have shown the utility of diffusion-weighted imaging (DWI) with the apparent diffusion coefficient (ADC) in differential diagnosis between uterine leiomyomas and leiomyosarcomas (23,26,27). DWI with the ADC might be useful as a new modality in the preoperative diagnosis of esophageal submucosal tumors.
EUS and FNAB
The main purpose of EUS is to observe the size, shape, and intratumoral character of tumors and their relationships within the layers of the bowel wall (28). Unfortunately, distinguishing GISTs from leiomyomas by EUS findings is not generally possible (23).
Pre-therapeutic histological and genetic diagnosis is essential for TKI treatment for GISTs (2). Ultrasound-guided FNAB or core biopsy is reported to be a secure procedure that enables differential diagnosis of mesenchymal tumors including GIST (14,29). In the preoperative situation, use of biopsy or FNAB is under debate (6). FNAB is often avoided with submucosal lesions because scarring could make enucleation more difficult, and there is a risk of tumor dissemination by capsule destruction (15,21,23). On the other hand, some have reported that the indications for preoperative biopsies are tumors above 2 cm in size with observed enlargement and/or intended neoadjuvant TKI treatment (2,6,14,29). In fact, FNAB seems to be performed frequently in clinical practice, especially for larger tumors. According to the NCCN Task Force Report, biopsy may not be necessary if the tumor is easily resectable and preoperative therapy is not required (3).
Pathological diagnosis and gene expression profiling
GISTs can be pathologically classified into three types: spindle cell, epithelioid cell, and mixed cell types (30). An immunohistochemical panel including KIT (CD117), DOG1, CD34, smooth muscle actin (SMA), desmin, and S100 protein is used for distinguishing GISTs from other tumors (31-33).
Frozen section examination is often used for intraoperative pathological diagnosis to guide the resection, but it may not be able to provide a definitive diagnosis because of the histologic similarities between GISTs and other spindle cell tumors (23).
The current risk stratification systems are based on tumor size, mitotic activity, tumor rupture, and tumor location (34-37). However, when these systems were established, only a few esophageal GISTs were included in risk assessment, and the accuracy of these systems for determining the prognosis of patients with esophageal GISTs is unknown (13).
Concerning the mutation status of esophageal GISTs, Kang et al. reported that most KIT mutations were detected in exon 11, the mutation spectrum of esophageal GISTs resembled that of gastric GISTs in their case series, and all cases with recurrent disease demonstrated KIT exon 11 deletions affecting codons 557 and/or 558 (31).
Surgical therapy for esophageal GISTs
The rarity of esophageal GISTs results in a lack of clear recommendations concerning their optimal surgical management (13). For localized GISTs, complete surgical resection is the treatment of choice (15). There have been a few reports regarding positive lymph node metastasis in esophageal GISTs, but GISTs rarely metastasize to lymph nodes, and routine lymphadenectomy is not recommended (38,39).
Although gastric and intestinal GISTs can be removed with segmental or wedge resections, resections for esophageal GISTs are essentially limited to enucleation or highly invasive esophagectomy due to the anatomical peculiarity of the esophagus (14). Which surgical procedure should be performed for esophageal GISTs is still under debate (2,6,40,41). With regard to postoperative morbidity and mortality, tumor enucleation seems a better option, particularly in patients with comorbidities (2,6,14,40). Generally, enucleation of esophageal GISTs is permitted for smaller tumors (2–5 cm in size), whereas esophagectomy is recommended for GISTs above 9 cm in size (2,6,14,42). The oncological outcomes of these two procedures are reported to be similar with proper patient selection (6,13,14,42-44).
Recently, thoracoscopic esophagectomy and enucleation have been successfully performed for esophageal GISTs (13,25,45). As minimally invasive esophagectomy has been performed widely for esophageal cancer, this technique can be applied for esophageal GISTs. The less invasive surgery might expand the indications for surgery, especially for smaller tumors and poor risk patients.
Neoadjuvant therapy for esophageal GISTs
There is only a little evidence based on clinical trials concerning neoadjuvant imatinib therapy for GISTs (46). In theory, downsizing of GIST by preoperative administration of imatinib seems attractive to reduce the extent of resection, especially in patients with GISTs of the esophagus, duodenum, and rectum, because wide resection may result in loss of function and greatly affect postoperative quality of life in these patients.
Concerning the duration of preoperative administration of imatinib, it has been reported to range from a few days to more than 1 year (13,47-49). The optimal duration of preoperative imatinib is considered to be as long as 6 to 12 months to obtain a maximal response prior to surgery (3). However, caution is needed during preoperative imatinib therapy, because it has a risk of rupture or bleeding due to tumor necrosis and cystic changes (1,46).
Kang et al. suggested that neoadjuvant imatinib treatment can be considered in patients with high mitotic rates and/or larger tumor sizes to obtain negative microscopic margins (R0 resection) and to reduce the risk of intraoperative complications, including tumor rupture (31).
There is limited literature available regarding neoadjuvant administration of imatinib in patients with esophageal GISTs, and the usefulness of neoadjuvant imatinib has been reported (13,16,18,19,50,51).
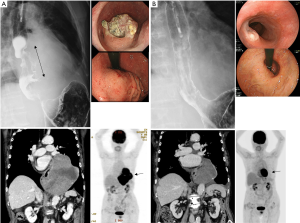
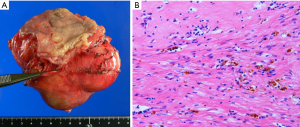
We treated a patient with a large esophageal GIST with neoadjuvant imatinib followed by surgical resection (Figures 1,2). An 86-year-old woman was diagnosed with a submucosal tumor of the lower esophagus just above the esophagogastric junction by upper gastrointestinal endoscopy. The maximum diameter on CT was 150 mm, and EUS-FNAB showed a spindle cell tumor with c-KIT (++), CD34 (+++), SMA (+), and S-100 protein (−). Based on the pathological findings, GIST of the esophagus was diagnosed. Gene mutation analysis showed KIT exon 11 deletion. She received imatinib therapy (400 mg/day) because of her high age and because she did not want surgical resection. After 3 months of imatinib therapy, she developed severe edema of the lower extremities and an eruption as adverse effects of imatinib. The tumor decreased to 87 mm on CT, and the dysphagia disappeared. Since her general condition was such that she could tolerate surgery, she underwent lower esophagectomy and proximal gastrectomy by left thoracotomy and laparotomy. Esophagogastrostomy was performed in the posterior mediastinum. Her postoperative course was excellent, and she was discharged on the 18th postoperative day. Pathological examination showed that about 90% of the tumor had disappeared with preoperative imatinib therapy.
Adjuvant therapy for esophageal GISTs
Adjuvant imatinib therapy following resection of GISTs has been shown to prevent recurrences and prolong survival in many clinical studies (52,53). However, esophageal GISTs were not included in these studies, and more comparative studies are needed to determine the effectiveness of adjuvant imatinib therapy (1).
Clinical outcome of esophageal GISTs
Clinical outcomes of esophageal GISTs from large case series studies were summarized in Table 1.

Full table
Nakano et al. summarized the clinical outcomes of 153 patients with esophageal GISTs reported in the literature (21). Recurrence occurred in 23 of 139 patients (16.5%) after surgery, and metastatic disease was more common than local recurrence (18 vs. 5 patients). They also reported that the average time to recurrence was 40 months, and the 5-year disease-free survival (DFS) and overall survival (OS) were 57% and 89%, respectively. They emphasized the need for long-term follow-up, because recurrence occurred even 5 years after surgery, unlike esophageal cancer.
Feng et al. also summarized 135 cases of reported esophageal GISTs (20). They reported that 5-year DFS and disease-specific survival (DSS) were 65.1% and 65.9%, respectively. The most common site of distant metastasis was liver, followed by lung, thoracic cavity, pleura, peritoneum, and subcutaneous. On multivariate analysis, tumor size was the only independent predictor of the prognosis of esophageal GISTs. In addition, they compared the prognosis of esophageal GISTs with gastric GISTs after matching of tumor size, mitotic index, and adjuvant imatinib therapy. DFS and DSS were significantly lower for esophageal GISTs than for gastric GISTs.
Lott et al. analyzed 55 cases of esophageal GISTs and compared their prognosis with gastric GISTs (2). Esophageal GISTs were generally classified more frequently as high-risk GISTs, and 5-year DSS, DFS, and OS were 50.9%, 65.3%, and 48.3%, respectively. Esophageal GISTs showed a significantly worse prognosis than gastric GISTs.
Kukar et al. also compared 29 esophageal GISTs with 2,658 gastric GISTs from the SEER database (54). On univariate analysis, 5-year DSS was worse for esophageal GISTs (in both all patients and the resected group), but this was not significant when adjusted for covariates.
Kang et al. performed clinicopathological and molecular analyses of 27 esophageal GIST cases (31). Surgery was performed in 25 patients (10 esophagectomy and 15 enucleation), and large tumor size (≥10 cm), high mitotic rate (>5/5 mm2), presence of a deletion mutation in KIT exon11 involving codons 557–558, and a positive microscopic margin were associated with recurrence and metastasis.
Conclusions
Esophageal GISTs are rare (fewer than 5% of all GISTs), which results in a lack of evidence concerning their optimal management.
When esophageal submucosal tumors are found, distinguishing GIST from leiomyoma, hemangioma, schwannoma, leiomyosarcoma, and papillary epithelioma is important to select treatment. Unfortunately, the differential diagnosis of an esophageal GIST is not easy. FNAB under EUS-guidance gives a definite diagnosis, but there is a risk of tumor dissemination by capsule destruction and scarring of the esophageal mucosa, which might make enucleation difficult, in the preoperative situation. However, FNAB may be indicated in tumors above 2 cm in size with observed enlargement and for whom neoadjuvant TKI treatment is intended. FDG-PET is a useful modality because SUVmax is reported to correlate with the degree of malignancy of GISTs, but it seems difficult to distinguish GISTs from other esophageal mesenchymal tumors including leiomyomas, which also show a wide range of SUVmax values. MRI might be a promising modality for the differential diagnosis of esophageal mesenchymal tumors.
Surgical resection is the only potentially curative treatment for localized GISTs. Unlike for gastric and intestinal GISTs, the surgical methods for esophageal GISTs are essentially limited to enucleation or highly invasive esophagectomy. Routine lymphadenectomy is not recommended, because GISTs rarely metastasize to lymph nodes. It is difficult to choose between enucleation and esophagectomy in individual patients with esophageal GISTs; enucleation may be permitted for smaller tumors (2–5 cm in size) or poor risk patients with comorbidities, whereas esophagectomy may be recommended for larger GISTs above 5 cm in size and very high-risk lesions with a high mitotic rate.
Use of imatinib preoperatively and/or postoperatively is a promising strategy. The purpose of neoadjuvant imatinib administration is downsizing of the GIST to reduce the extent of resection and to reduce the risk of intraoperative complications, including tumor rupture. Since reports of the efficacy of neoadjuvant/adjuvant imatinib treatment for esophageal GISTs are limited to case series or case reports, evaluation of its efficacy still needs to be addressed.
In conclusion, as mentioned above, because of the rarity of esophageal GIST, its properties, malignancy, imaging diagnosis, optimal surgical method, and the efficacy of neoadjuvant/adjuvant therapy are poorly understood. More clinicopathological data and clinical trials involving esophageal GISTs are expected.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang FB, Shi HC, Shu YS, et al. Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World J Gastroenterol 2015;21:5630-4. [Crossref] [PubMed]
- Lott S, Schmieder M, Mayer B, et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res 2014;5:333-43. [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4.
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. [Crossref] [PubMed]
- Lee HJ, Park SI, Kim DK, et al. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009;87:1569-71. [Crossref] [PubMed]
- Abraham SC, Krasinskas AM, Hofstetter WL, et al. "Seedling" mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol 2007;31:1629-35. [Crossref] [PubMed]
- Ji F, Wang ZW, Wang LJ, et al. Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography. J Gastroenterol Hepatol 2008;23:e318-24. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211-22. [Crossref] [PubMed]
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet 2007;369:1731-41. [Crossref] [PubMed]
- Neofytou K, Costa Neves M, Giakoustidis A, et al. Effective Downsizing of a Large Oesophageal Gastrointestinal Stromal Tumour with Neoadjuvant Imatinib Enabling an Uncomplicated and without Tumour Rupture Laparoscopic-Assisted Ivor-Lewis Oesophagectomy. Case Rep Oncol Med 2015;2015:165736. [Crossref] [PubMed]
- Blum MG, Bilimoria KY, Wayne JD, et al. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2007;84:1717-23. [Crossref] [PubMed]
- Nemeth K, Williams C, Rashid M, et al. Oesophageal GIST-A rare breed case report and review of the literature. Int J Surg Case Rep 2015;10:256-9. [Crossref] [PubMed]
- Yanagawa S, Tanabe K, Suzuki T, et al. A large esophageal gastrointestinal stromal tumor that was successfully resected after neoadjuvant imatinib treatment: case report. World J Surg Oncol 2014;12:47. [Crossref] [PubMed]
- Tirumani SH, Shinagare AB, Jagannathan JP, et al. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol 2014;40:420-8. [Crossref] [PubMed]
- Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45. [Crossref] [PubMed]
- Shinagare AB, Zukotynski KA, Krajewski KM, et al. Esophageal gastrointestinal stromal tumor: report of 7 patients. Cancer Imaging 2012;12:100-8. [Crossref] [PubMed]
- Feng F, Tian Y, Liu Z, et al. Clinicopathologic Features and Clinical Outcomes of Esophageal Gastrointestinal Stromal Tumor: Evaluation of a Pooled Case Series. Medicine (Baltimore) 2016;95:e2446. [Crossref] [PubMed]
- Nakano A, Akutsu Y, Shuto K, et al. Giant esophageal gastrointestinal stromal tumor: report of a case. Surg Today 2015;45:247-52. [Crossref] [PubMed]
- Radenkovic G, Ilic I, Zivanovic D, et al. C-kit-immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res 2010;340:427-36. [Crossref] [PubMed]
- Dendy M, Johnson K, Boffa DJ. Spectrum of FDG uptake in large (>10 cm) esophageal leiomyomas. J Thorac Dis 2015;7:E648-51. [PubMed]
- Park JW, Cho CH, Jeong DS, et al. Role of F-fluoro-2-deoxyglucose Positron Emission Tomography in Gastric GIST: Predicting Malignant Potential Pre-operatively. J Gastric Cancer 2011;11:173-9. [Crossref] [PubMed]
- Isaka T, Kanzaki M, Onuki T. Long-term survival after thoracoscopic enucleation of a gastrointestinal stromal tumor arising from the esophagus. J Surg Case Rep 2015;2015:rju155. [Crossref] [PubMed]
- Tasaki A, Asatani MO, Umezu H, et al. Differential diagnosis of uterine smooth muscle tumors using diffusion-weighted imaging: correlations with the apparent diffusion coefficient and cell density. Abdom Imaging 2015;40:1742-52. [Crossref] [PubMed]
- Sato K, Yuasa N, Fujita M, et al. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol 2014;210:368.e1-368.e8. [Crossref] [PubMed]
- Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy for the molecular diagnosis of gastrointestinal stromal tumors: shifting treatment options. J Gastrointestin Liver Dis 2008;17:131-3. [PubMed]
- Stelow EB, Stanley MW, Mallery S, et al. Endoscopic ultrasound-guided fine-needle aspiration findings of gastrointestinal leiomyomas and gastrointestinal stromal tumors. Am J Clin Pathol 2003;119:703-8. [Crossref] [PubMed]
- Dei Tos AP, Laurino L, Bearzi I, et al. Gastrointestinal stromal tumors: the histology report. Dig Liver Dis 2011;43 Suppl 4:S304-9. [Crossref] [PubMed]
- Kang G, Kang Y, Kim KH, et al. Gastrointestinal stromal tumours of the oesophagus: a clinicopathological and molecular analysis of 27 cases. Histopathology 2017;71:805-12. [Crossref] [PubMed]
- Yamaguchi U, Hasegawa T, Masuda T, et al. Differential diagnosis of gastrointestinal stromal tumor and other spindle cell tumors in the gastrointestinal tract based on immunohistochemical analysis. Virchows Arch 2004;445:142-50. [Crossref] [PubMed]
- Kang GH, Srivastava A, Kim YE, et al. DOG1 and PKC-θ are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol 2011;24:866-75. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Matsumoto S, Takayama T, Wakatsuki K, et al. An esophageal gastrointestinal stromal tumor with regional lymph node metastasis. Esophagus 2010;7:115-8. [Crossref]
- Masuda T, Toh Y, Kabashima A, et al. Overt lymph node metastases from a gastrointestinal stromal tumor of the esophagus. J Thorac Cardiovasc Surg 2007;134:810-1. [Crossref] [PubMed]
- Coccolini F, Catena F, Ansaloni L, et al. Esophagogastric junction gastrointestinal stromal tumor: resection vs enucleation. World J Gastroenterol 2010;16:4374-6. [Crossref] [PubMed]
- Peparini N, Carbotta G, Chirletti P. Enucleation for gastrointestinal stromal tumors at the esophagogastric junction: is this an adequate solution? World J Gastroenterol 2011;17:2159-60. [Crossref] [PubMed]
- Jiang P, Jiao Z, Han B, et al. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. Eur J Cardiothorac Surg 2010;38:223-7. [Crossref] [PubMed]
- Robb WB, Bruyere E, Amielh D, et al. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg 2015;261:117-24. [Crossref] [PubMed]
- von Rahden BH, Stein HJ, Feussner H, et al. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. Surg Endosc 2004;18:924-30. [Crossref] [PubMed]
- Koyanagi K, Nakagawa M, Ozawa S, et al. Thoracoscopic enucleation for small-sized gastrointestinal stromal tumor of the esophagus: report of two cases. Esophagus 2010;7:219-24. [Crossref]
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [Crossref] [PubMed]
- Bednarski BK, Araujo DM, Yi M, et al. Analysis of prognostic factors impacting oncologic outcomes after neoadjuvant tyrosine kinase inhibitor therapy for gastrointestinal stromal tumors. Ann Surg Oncol 2014;21:2499-505. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Tielen R, Verhoef C, van Coevorden F, et al. Surgery after treatment with imatinib and/or sunitinib in patients with metastasized gastrointestinal stromal tumors: is it worthwhile? World J Surg Oncol 2012;10:111. [Crossref] [PubMed]
- Kobayashi H, Kiguchi G, Miki A, et al. A case report of giant esophageal gastrointestinal stromal tumor surgically resected after preoperative imatinib treatment. Esophagus 2011;8:119-24. [Crossref]
- Sato H, Kanda T, Hirota S, et al. Surgical resection of gastrointestinal stromal tumor of esophagus following preoperative imatinib treatment: a case report. Esophagus 2010;7:65-9. [Crossref]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Kukar M, Kapil A, Papenfuss W, et al. Gastrointestinal stromal tumors (GISTs) at uncommon locations: a large population based analysis. J Surg Oncol 2015;111:696-701. [Crossref] [PubMed]
Cite this article as: Hihara J, Mukaida H, Hirabayashi N. Gastrointestinal stromal tumor of the esophagus: current issues of diagnosis, surgery and drug therapy. Transl Gastroenterol Hepatol 2018;3:6.


