Laparoscopic management of neuroendocrine tumors: state-of-the-art
Pancreatic neuroendocrine tumors (PNETs) are a rare pathology, representing 2% to 4% of all pancreatic tumors (1), with an increasing rate of diagnosis over the last twenty years (2). Even if many medical treatments have been proposed (3-6), surgery plays a key role in the curative treatment of these tumors, requiring different types of surgical approaches depending on tumor location, ranging from atypical to typical pancreatic resections. Recently, the minimally invasive pancreatic approach has been considered superior in terms of intraoperative blood loss, postoperative pain, time to recovery, and length of hospital stay (7-9).
The aim of this article was to perform a systematic review of the literature, in order to evaluate the feasibility and safety of the minimally invasive treatment of PNETs.
Materials and methods
A systematic search of the literature, restricted to articles in English, was performed using MEDLINE® and PubMed® to identify studies published between January 1, 1999 and March 30, 2015, focusing on patients who underwent a laparoscopic resection of PNETs. This review protocol was developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The keywords used to perform the bibliographic search were the following: [(“laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopic”[All Fields]) AND (“neurosecretory systems”[MeSH Terms] OR “neuroendocrine”[All Fields]) AND (“pancreas”[MeSH Terms] OR “pancreas”[All Fields]) AND (“neurosecretory systems”[MeSH Terms] OR (“neurosecretory”[All Fields] AND “systems”[All Fields]) OR “neurosecretory systems”[All Fields] OR “neuroendocrine”[All Fields]) AND (“pancreas”[MeSH Terms] OR “pancreas”[All Fields])(“laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopic”[All Fields]) AND (“insulinoma”[MeSH Terms] OR “insulinoma”[All Fields]) AND (“pancreas”[MeSH Terms] OR “pancreas”[All Fields]).
Only series with more than 20 patients were included in this study to exclude any selection bias and consider the experience of high volume centers. In case of multiple studies originating from the same group, only the one with the largest number of patients was included in the review. All relevant data and articles were analyzed and extracted by two independent observers (Riccardo Memeo, Stefania Roselli) who consensually decided upon the eligibility of articles.
Inclusion and exclusion criteria
In this review, comparative studies were included in the analysis and data concerning minimally invasive surgery were extracted manually. Studies reporting data on preoperative, intraoperative, postoperative morbidity and mortality, pathological findings, and oncological outcomes were considered for analysis. Minimum and maximum values were noted for each item. Animal studies and clinical studies including less than 20 cases were excluded.
Results
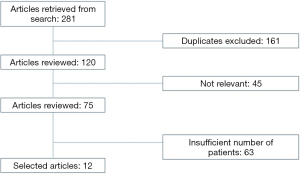
The literature search identified a total of 281 potentially relevant articles, 12 of which were chosen (10-21). A flowchart of the selected studies is shown in Figure 1. In the end, a total of 596 patients were analyzed.
Most series presented in the study were comparative studies (open versus laparoscopic surgery), except for the study by Cienfuegos et al. (15) which described a pure laparoscopic series. This comparison allowed to understand and estimate the percentage of patients who were operated on laparoscopically, with a mean value of 50% (range: 8–100%). Insulinoma, gastrinoma, and non-functioning PNETS were indications for surgery. Insulinoma was the most common indication in 66% of cases. The results were summarized in Table 1. Operative time ranged from 123.8 to 352 minutes depending on the volume and experience of the center, with a mean value of 212 minutes per procedure. Blood loss was considerably low, with a mean value of 180 mL per procedure (124.8–250 mL). The mean conversion rate was 13.7% (0–41.3%). Difficult dissection was the most common cause of conversion. As for postoperative morbidity, 33% of patients had postoperative complications. Pancreatic fistula was present in 31% of cases, with a majority of grade A pancreatic fistulas. No mortality was present in the series. The mean duration of hospital stay was 10 days (range: 5–14.4 days). R0 resection was performed in 95% of cases (range: 80.5–100%).
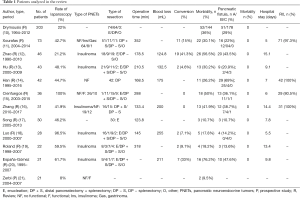
Full table
Discussion
Many studies in the literature have recently confirmed the possibility of performing a safe and feasible minimally invasive treatment of hepatobiliary cancer (22-31). This review confirms the possibility of performing laparoscopic resection for PNETs.
In our review, 12 studies were included in the analysis. Most of them were comparative studies based on open and laparoscopic procedures, and only one represented a series of pure laparoscopic cases. In order to obtain strong data on the subject, series with less than 20 cases were excluded from the analysis. Even if different pathologies were treated in the series (functioning and non-functioning lesions, insulinoma, gastrinoma), patients were included in the review in order to strictly analyze the technical feasibility of the procedure without considering long-term results specific to each pathology. Accordingly, the recurrence rate and oncological results were not considered in the analysis. Given the results of the analysis, operative time and blood loss were comparable to data described in the literature. The conversion rate varies depending on centers. However, the rate of conversion seems reasonable and comparable to previously described data. Conversion was necessary due to difficulties in dissection, and could be considered mandatory in order to perform safe procedures. The morbidity rate varies according to the definition of postoperative complications. The data should be analyzed carefully according to the different classifications provided by authors. The rate of pancreatic fistula was also similar to the data described in the literature for open resections (32), considering that soft pancreatic tissue with a small pancreatic duct seemed to have an increased risk of fistula (33). Given the possibility of atypical resection or enucleation, the rate of fistula could be considered acceptable, as previously described by Drymousis et al. (10). Length of hospital stay, as previously demonstrated in the meta-analysis by Tamburrino et al. (32), is considered inferior as compared to open surgery, due to reduced postoperative pain and earlier resumption of food intake.
Our review had several biases. The retrospective characteristic of most studies could well impact the analyzed data. We did not have any detailed information on tumor characteristics and localization and we did not have any sufficient data to understand technical difficulties which had impacted length of stay and conversion rate for instance. No data were provided concerning the type of pancreatic resection. However, different classifications of postoperative complications were integrated into the analysis.
In conclusion, this review demonstrated the feasibility of pancreatic resection for PNETs, with a safe postoperative course and comparable intraoperative results. Future radiochemotherapies (RCTs) are required in order to better identify patients and pathologies which could benefit from this minimally invasive approach for pancreatic lesions.
Acknowledgements
None.
Footnote
Conflicts of Interest: P Pessaux is orator for INTEGRA and member of the board of MERCK. The other authors have no conflicts of interest to declare.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Kuo EJ, Salem RR. Population-Level Analysis of Pancreatic Neuroendocrine Tumors 2 cm or Less in Size. Ann Surg Oncol 2013;20:2815-21. [Crossref] [PubMed]
- Steward MJ, Warbey VS, Malhotra A, et al. Neuroendocrine tumors: role of interventional radiology in therapy. Radiographics 2008;28:1131-45. [Crossref] [PubMed]
- Eriksson B, Annibale B, Bajetta E, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Chemotherapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2009;90:214-9. [Crossref] [PubMed]
- Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010;28:69-76. [Crossref] [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [Crossref] [PubMed]
- Limongelli P, Belli A, Russo G, et al. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6. [Crossref] [PubMed]
- Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs Open Distal Pancreatectomy. Arch Surg 2010;145:616. [Crossref] [PubMed]
- Song KB, Kim SC, Park JB, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc 2011;25:3364-72. [Crossref] [PubMed]
- Drymousis P, Raptis DA, Spalding D, et al. Laparoscopic versus open pancreas resection for pancreatic neuroendocrine tumours: a systematic review and meta-analysis. HPB (Oxford) 2014;16:397-406. [Crossref] [PubMed]
- Xourafas D, Tavakkoli A, Clancy TE, et al. Distal Pancreatic Resection for Neuroendocrine Tumors: Is Laparoscopic Really Better than Open? J Gastrointest Surg 2015;19:831-40. [Crossref] [PubMed]
- Setoyama T, Natsugoe S, Okumura H, et al. alpha-catenin is a significant prognostic factor than E-cadherin in esophageal squamous cell carcinoma. J Surg Oncol 2007;95:148-55. [Crossref] [PubMed]
- Hu M, Zhao G, Luo Y, Liu R. Laparoscopic versus open treatment for benign pancreatic insulinomas: An analysis of 89 cases. Surg Endosc 2011;25:3831-7. [Crossref] [PubMed]
- Han SH, Han IW, Heo JS, et al. Laparoscopic versus open di Laparoscopic versus open distal pancreatectomy for nonfunctioning pancreatic neuroendocrine tumors: a large single-center study stal pancreatectomy for nonfunctioning pancreatic neuroendocrine tumors: a large single-center study. Surg Endosc 2017. [Epub ahead of print]. [Crossref]
- Cienfuegos JA, Salguero J, Núñez-Córdoba JM, et al. Short- and long-term outcomes of laparoscopic organ-sparing resection in pancreatic neuroendocrine tumors: a single-center experience. Surg Endosc 2017;31:3847-57. [Crossref] [PubMed]
- Zhang J, Jin J, Chen S, et al. Minimally invasive distal pancreatectomy for PNETs: Laparoscopic or robotic approach? Oncotarget 2017;8:33872-83. [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Enucleation for benign or low-grade malignant lesions of the pancreas: Single-center experience with 65 consecutive patients. Surgery 2015;158:1203-10. [Crossref] [PubMed]
- Luo Y, Liu R, Hu MG, et al. Laparoscopic Surgery for Pancreatic Insulinomas: A Single-Institution Experience of 29 Cases. J Gastrointest Surg 2009;13:945-50. [Crossref] [PubMed]
- Roland CL, Lo CY, Miller BS, et al. Surgical approach and perioperative complications determine short-term outcomes in patients with insulinoma: results of a bi-institutional study. Ann Surg Oncol 2008;15:3532-7. [Crossref] [PubMed]
- España-Gómez MN, Velázquez-Fernández D, Bezaury P, et al. Pancreatic insulinoma: a surgical experience. World J Surg 2009;33:1966-70. [Crossref] [PubMed]
- Zerbi A, Capitanio V, Boninsegna L, et al. Surgical treatment of pancreatic endocrine tumours in Italy: Results of a prospective multicentre study of 262 cases. Langenbecks Arch Surg 2011;396:313-21. [Crossref] [PubMed]
- Memeo R, Sangiuolo F, de Blasi V, et al. Robotic pancreaticoduodenectomy and distal pancreatectomy: State of the art. J Visc Surg 2016;153:353-9. [Crossref] [PubMed]
- Untereiner X, Cagnet A, Memeo R, et al. Short-term and middle-term evaluation of laparoscopic hepatectomies compared with open hepatectomies: a propensity score matching analysis. World J Gastrointest Surg 2016;8:643-50. [Crossref] [PubMed]
- Memeo R, De Blasi V, Adam R, et al. Parenchymal-sparing hepatectomies (PSH) for bilobar colorectal liver metastases are associated with a lower morbidity and similar oncological results: a propensity score matching analysis. HPB (Oxford) 2016;18:781-90. [Crossref] [PubMed]
- Memeo R, De Blasi V, Perotto O, et al. Robotic Lymphadenectomy During Pancreatoduodenectomy with First Superior Mesenteric Artery Dissection. Ann Surg Oncol 2016;23:968. [Crossref] [PubMed]
- Dehlawi A, Memeo R, DE, Blasi V, et al. Robotic hepatectomies: advances and perspectives. Minerva Chir 2016;71:407-14. [PubMed]
- Diana M, Schiraldi L, Liu YY, et al. High intensity focused ultrasound (HIFU) applied to hepato-bilio-pancreatic and the digestive system-current state of the art and future perspectives. Hepatobiliary Surg Nutr 2016;5:329-44. [Crossref] [PubMed]
- Ntourakis D, Memeo R, Soler L, et al. Augmented Reality Guidance for the Resection of Missing Colorectal Liver Metastases: An Initial Experience. World J Surg 2016;40:419-26. [Crossref] [PubMed]
- Sánchez-Cabús S, Pittau G, Gelli M, et al. Laparoscopic Pancreaticoduodenectomy: Hybrid Surgical Technique. J Am Coll Surg 2015;220:e7-11. [Crossref] [PubMed]
- de’Angelis N, Memeo R, Calderaro J, et al. Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB (Oxford) 2014;16:783-8. [Crossref] [PubMed]
- Memeo R, de’Angelis N, Compagnon P, et al. Laparoscopic vs. Open Liver Resection for Hepatocellular Carcinoma of Cirrhotic Liver: A Case-Control Study. World J Surg 2014;38:2919-26. [Crossref] [PubMed]
- Tamburrino D, Partelli S, Renzi C, et al. Systematic review and meta-analysis on laparoscopic pancreatic resections for neuroendocrine neoplasms (PNENs). Expert Rev Gastroenterol Hepatol 2017;11:65-73. [Crossref] [PubMed]
- Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013;216:1-14. [Crossref] [PubMed]
Cite this article as: Memeo R, Roselli S, Lupo L, Cherkaoui Z, Pessaux P. Laparoscopic management of neuroendocrine tumors: state-of-the-art. Transl Gastroenterol Hepatol 2017;2:109.