Downstaging for hepatocellular cancer: harm or benefit?
Introduction
Hepatocellular carcinoma (HCC) is the commonest primary liver cancer and the 5th most prevalent cancer in males. Worldwide, it is the third most common cause of cancer death (1). Its incidence in the US has more than tripled since 1980 and liver cancer death rates have increased by almost 3% since 2000 (2). It is an important indication for liver transplantation, as the latter can remove the tumor, whist also curing the underlying liver disease. However, more than 70% of cases present at an advanced stage and are unsuitable for curative interventions (resection or transplantation) either due to tumor burden or poor liver function (3).
The most commonly used staging systems for HCC include the Barcelona Clinic Liver Cancer (BCLC) (4) and the American Joint Committee on Cancer (AJCC) tumor/node/metastasis (TNM) classification. The United Network for Organ Sharing (UNOS) has incorporated the Milan criteria into T1 and T2 in a modified staging system for HCC.
Listing criteria for transplantation include:
Milan criteria (5)/UNOS T2 stage (a single tumor ≤5 cm diameter or up to 3 tumors all ≤3 cm);
University of California, San Francisco (UCSF) (a single lesion ≤6.5 cm in diameter or 2 lesions ≤4.5 cm with total tumor diameter ≤8 cm) (6);
Extended Milan 2009—in the UK, NHS Blood and Transplant (NHSBT) uses these criteria (a single tumor ≤5 cm diameter or up to 5 tumors all ≤3 cm, or a single tumor >5 and ≤7 cm diameter where there has been no evidence of tumor progression (volume increase by <20%) and no extra-hepatic spread and no new nodule formation over a 6-month period);
‘Up to 7 criteria’ 2009 [the sum of the size of the largest tumor (in cm) and the number of tumors ≤7]. Metroticket Investigator Study Group (7);
Duvoux criteria/alpha-fetoprotein (AFP) model (which takes into account largest tumor size, number of nodes and AFP—a score of 2 or less allows listing) (8).
‘Downstaging’ is the process of applying locoregional therapy to tumors currently outside of accepted transplant criteria, with the aim of reducing tumor burden to allow transplantation. This is used because it is recognized that there may be a large number of cases who may benefit from transplantation, but do not necessarily meet the stringent Milan (or other) criteria. This process has raised some concerns about higher risks of recurrence when transplanting patients who did not originally meet listing criteria.
In this review, we critically evaluate available data and evidence on downstaging HCC.
Loco-regional therapies for downstaging HCC
Locoregional treatments, or ‘liver-directed therapies (LDT)’ include transarterial (‘bland’) embolization (TAE), transarterial chemoembolization (TACE, including drug-eluting beads, DEB-TACE), transcatheter arterial chemoinfusion (TACI), radio frequency ablation (RFA), microwave ablation and percutaneous ethanol injection (PEI), as well as transarterial radio embolization (TARE) and stereotactic body radiation (9,10). Liver resection may form part of a multimodal down-staging strategy.
Locoregional treatments are used on the liver transplant waiting list as neoadjuvant (or bridging) therapy, with the intent to reduce tumor growth and prevent dropout from the list, as well as improve outcome after transplantation (11,12). The current UK criteria recommend that locoregional therapies are considered for all transplant list patients who have HCC. They are also used to down-stage tumors that currently fall outside Milan criteria (UNOS T3 or higher), with the aim of enabling listing for transplantation. This also allows time to assess the tumor response, to gauge the biological behavior of the tumor and identify those patients at greater risk of tumor progression. Duvoux et al. found that patients moving from the high-risk group for tumor recurrence, according to the AFP-model, to the low-risk group on re-assessment after down-staging had the same risk of recurrence as those patients initially classified in the low-risk group (8).
This use has been the subject of much debate, and the European Association for the Study of the Liver (EASL)/European Organization for Research and Treatment of Cancer (EORTC) 2012 clinical practice guideline (13) stated that down-staging for HCC beyond conventional criteria is not recommended and should be explored in the context of prospective studies. The Milan criteria for suitability for transplantation are well validated and widely used, and there is concern that increasing access to transplantation out-with these criteria could result in increasing rates of post-transplant HCC recurrence, and potentially represent poor use of a limited pool of donor organs. However, down staging has been of great interest recently and identified as a priority for research at consensus meetings (14,15). The International Consensus Conference on liver transplantation for HCC reported that liver transplantation after down-staging should aim to achieve a 5-year survival rate comparable to those who initially fall within criteria for transplantation and that, based on existing evidence, no recommendation can be made for preferring a specific locoregional therapy for down-staging over others (16). It is recognized that well-designed trials are needed to determine which patients would benefit from down staging.
Tumor characteristics associated with progression include vascular invasion and poor differentiation. Since microvascular invasion and tumor grade can only be reliably determined by assessing the explant, surrogate markers of more aggressive tumors have been used, including tumor size and number (17,18). However, tumor size and number are not always accurate in predicting the behavior of the tumor, and bio-markers, including alpha-fetoprotein and des-gamma-carboxy prothrombin (DCP), are also used to aid in predicting tumor aggressiveness. These both correlate with post- treatment prognoses (19-23). It has been suggested that the biological aggressiveness of the tumor may also be determined by response to locoregional therapies over a specified period (24-27).
Types of locoregional therapy
TAE involves transarterial embolization with particles, including gelatin sponge particles (gel foam), polyvinyl alcohol, microspheres or drug eluting beads. The occlusion of the arterial supply results in tumor hypoxia and necrosis.
TACE is the technique of infusing chemotherapy before embolization of particles. TACE has been used in the majority of published studies and often several sessions of TACE are needed for down staging. TACE is not a standardized procedure—studies vary in protocol in terms of the embolic and chemotherapeutic agents used, different particle size in embolization, different arterial selectivity and variable surveillance protocols, time between sessions and indication for repeating therapy. Chemotherapeutic agents used include doxorubicin, cisplatin, mitomycin C and 5-fluorouracil. Embolization can be lobar, segmental or subsegmental. TACE should be applied no more than 3–4 times per year, since shorter intervals can cause decompensation of liver disease. Super-selective TACE is more effective at inducing tumor necrosis and minimizes the ischemic insult to the remainder of the liver. It therefore may be employed if liver synthetic function is borderline (13,28). Although TACE is widely used, there is considerable heterogeneity in the studies that have used it (29). TACE is no better than TAE according to existing evidence (30).
Embolization with DEB allows a steady local drug administration, however DEB-TACE was not more effective than conventional TACE in terms of tumor response in a randomized controlled trial. Its benefit though, is in limiting systemic exposure to the toxic effects of chemotherapeutic agents, and has been shown to significantly reduce the incidence of hepatitis and alopecia (31-35). A phase II/III trial of 3-weekly cisplatin-based sequential TACE showed a higher response rate than TAE alone but without a survival benefit (36).
TACI is a variant of TACE that is not at present widely-used. TACI delivers high concentrations of chemotherapeutic drugs in a super-selective manner, without solid particle embolization. This affords it a better safety profile and can be used in patients with more advanced liver disease without the same risks of decompensation (37).
HCC is radiosensitive and radio labelled particles delivered to the tumor transarterial limits exposure to the surrounding liver and allows higher dose intensity. TARE with Iodine-131 or Yttrium-90 glass beads has shown effectiveness in several studies, and is particularly useful in patients with portal vein thrombosis and no TACE option (38-42).
Percutaneous techniques are effective in treating smaller lesions and can be curative when used for patients with early stage disease who are unsuitable for resection or transplantation. Tumor cells are damaged by thermal techniques (radiofrequency, microwave, laser) or injection of alcohol or acetic acid (43). PEI is inferior to RFA in treating tumors greater than 2 cm in size (44) but equally effective for tumors less than 2 cm, with a low rate of adverse effects (45,46). It may also be used in cases where RFA is not technically feasible. It is performed under ultrasound guidance and requires several sessions of treatment. RFA is equally effective in lesions less than 2 cm but with the need for fewer sessions (47-49). RFA is more expensive than PEI and has a higher rate of adverse effects. Both carry the risk of tumor seeding.
A recent Cochrane meta-analysis on treatment of early or very-early HCC (BCLC stage A) concluded that mortality rate was higher for percutaneous acetic acid injection [hazard ratio (HR) 1.77, 95% CI: 1.12–2.79; 125 participants; 1 trial] and percutaneous alcohol injection (HR 1.49, 95% CI: 1.18–1.88; 882 participants; 5 trials; I2=57%) compared with RFA for patients not eligible for liver resection (50).
Inclusion and exclusion criteria for downstaging
There is no clear upper limit in terms of tumor size or number for eligibility for down-staging. The UCSF protocol uses upper limits of one tumor ≤8 cm, two or three tumors each ≤5 cm and the sum of the maximal tumor diameters ≤8 cm, and four or five tumors each ≤3 cm and the sum of the maximal tumor diameters ≤8 cm (51).
A US national HCC conference proposed eligibility criteria that were a modification of the UCSF criteria: one tumor <8 cm, two or three tumors each <5 cm and the sum of the maximal tumor diameters <8 cm, and excluding four or five tumors (15). Macroscopic vascular invasion or metastasis and poor liver function are usually exclusion criteria. Tumor rupture and an AFP >10,000 IU/mL are absolute contraindications to transplantation. The same conference proposed that transplantation should not be performed in those with AFP >1,000 ng/mL unless it decreases to <500 ng/mL with LDT (15).
The International Consensus Conference on liver transplantation for HCC produced the recommendation that criteria for successful downstaging should include tumor size and number of viable tumors, noting that there is currently no well-defined upper limit for size and number of lesions as eligibility criteria for downstaging, although vascular invasion and extrahepatic disease are contraindications. AFP levels before and after downstaging may add additional information, although there is no agreement on a threshold (16).
Assessment of response and definitions of down-staging success or failure
Response to locoregional therapies should be assessed by the modified Response Evaluation Criteria in Solid Tumors (mRECIST), which assesses both change in tumor volume and in arterial enhancement. The tumor dimensions are assessed by CT or MRI and the maximum size of only viable tumors is taken into account (24). The EASL criteria also assess only viable tumor: the estimation of the reduction in viable tumor volume re cogniseed by non-enhanced areas by spiral CT (52). The World Health Organization (WHO) also sets criteria for down-staging success, which, like the mRECIST criteria, are based on the entire lesion and not just the viable areas (50% reduction of the product of the perpendicular diameters of largest lesion). The new dimensions of the tumor(s) are classified according to the UNOS TNM staging to assess whether down staging to within listing criteria has occurred. The definition of down staging is usually to within Milan criteria.
Failure of LDT can be represented by metastasis and vascular invasion, and progression to beyond eligibility criteria for downstaging. If tumor progression occurs but the active tumor burden remains within down-staging entry criteria, transplantation should be suspended and repeat LDT should be undertaken until the endpoint of down-staging is achieved for the patients to be eligible again for transplantation (53).
To address the heterogeneity of reporting of trials into locoregional therapy for HCC, Parikh proposed minimum criteria reporting for future studies in this area (54). Yao proposed a standardized downstaging protocol, including eligibility criteria and criteria for successful downstaging or downstaging failure (55).
Risks of treatment
The disadvantages of these treatments include potential risks related to their delivery. Post embolization syndrome is the most common adverse effect of TACE/TAE: a self-limiting illness up to 48 hours post-procedure characterized by abdominal pain, fever and elevation of liver enzymes, observed in 60% of patients (56). Side effects of TACE also include symptoms related to the chemotherapy: nausea, vomiting, marrow suppression, renal dysfunction (43). Other TAE and TACE-induced adverse events include bile duct damage, liver abscess in necrotic tumor, ischemic cholecystitis and decompensation of cirrhosis with ascites, worsening of synthetic function, and potentially death (9). The risk of decompensation is dependent on liver function pre-procedure and therefore patient selection in studies of LDT has a significant effect on reported adverse outcomes. In the presence of ascites, TACE-associated liver failure occurred in 17% and the mortality rate was high at 1 year (57). Since the protocol for TAE/TACE varies by center, decisions about arterial selectivity for embolization will also affect outcome. A systematic review reported a median treatment related mortality of 2.4% in 37 trials including 2,878 patients (35).
Tumor seeding along the needle track may occur with percutaneous therapies. The risk of adverse effects with RFA is up to 10%, including pleural effusion and peritoneal bleeding, and procedure-related mortality is 0–0.3% (58,59).
The most common treatment side effects of TARE were fatigue and transient nonspecific flu-like symptoms lasting 7–10 days, observed in 60% of patients.
Finally, the ultimate potential harm with down staging is that of increasing post-transplant HCC recurrence, which may represent poor use of a limited pool of donor organs.
Existing evidence of downstaging protocols
It has been a challenge to make a judgement on outcomes in down staging therapy because of reported differences in treatment protocol and variability in survival data reporting. The majority of studies have been single-center, with small cohorts. A summary of the results of 14 studies discussed below is found in Table 1.
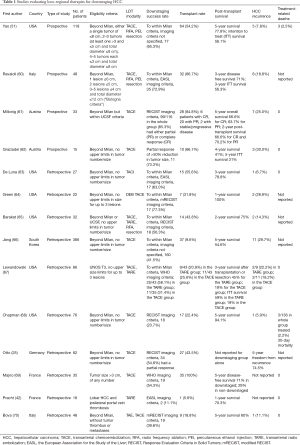
Full table
A prospective study by Yao et al., San Francisco, California (51) compared 118 patients who underwent down staging to within T2 criteria. Patients were consecutively enrolled in the down staging protocol from March 2002 to January 2012. Eligibility criteria were tumors beyond Milan criteria and up to 8 cm as follows: either a single tumor of ≤8 cm, two to three tumors at least one >3 and ≤5 cm and total diameter ≤8 cm, four to five lesions each ≤3 cm and total diameter ≤8 cm. Modalities used were TACE (doxorubicin, cisplatin and mitomycin C), RFA, PEI and resection. Patients had CT or MRI one month after each LDT and a minimum of three monthly. There was a minimum observation period of 3 months between LDT and listing for transplantation. Data for the control group of 488 patients meeting T2 criteria without requiring down staging were collected retrospectively.
Downstaging, as defined by reaching Milan criteria, was successful in 77 (65.3%) patients, and 64 (54.2%) were eventually transplanted. Post-transplant 5-year survival was 78% and intention to treat survival was 56%, compared to 63% in the group who initially met T2 listing criteria (P=NS). HCC recurrence was 7.5% (5 patients). Factors predicting dropout in the downstaging group included pretreatment alpha-fetoprotein >1,000 ng/mL (multivariate HR: 2.42) and Child’s B versus Child’s A cirrhosis (multivariate HR: 2.19). Downstaging failed in 41 patients (34.7%)—because of tumor progression in 33, due to death without transplantation in 5 and for other reasons in 3. The cumulative dropout rate was higher in this group than the control group. There were three deaths due to decompensation after LDT (treatment-related mortality 2.5%). This study’s strengths were its prospective nature, relatively large sample size, median 3.8 years post-transplant follow-up and the control group of patients meeting Milan criteria, allowing comparison. It also reported eligibility and exclusion criteria and gave full details of the UCS downstaging protocol. Explants were also analyzed and showed a low rate of unfavorable histological tumor characteristics, supporting the role of down staging to select more favorable tumor biology.
In a prospective study by Ravaioli et al., from Italy (60), 48 patients had attempted downstaging. Patients were included from January 2003 to January 2006. Eligibility criteria were HCC beyond Milan as follows: 1 lesion ≤6 cm, 2 lesions ≤5 cm, 3–5 lesions ≤4 cm and total diameter ≤12 cm (“Bologna criteria”). Those with AFP level >400 ng/mL were excluded. Although the initial protocol included single nodules up to 8 cm, the cohort did not in the end include any patient with a single nodule >6 cm. 14.6% of patients in the downstaged group met the UCSF criteria for transplantation. The modalities used were TACE, RFA, PEI, or resection used alone or in combination. A minimum observation period of 3 months was used between downstaging and listing. Successful downstaging to within Milan criteria occurred in 33 (69%) patients and 32 (67%) underwent transplantation. Three-year disease-free survival was 71%, comparable to the cohort initially meeting Milan criteria (n=129), and 3-year intention to treat survival was 56.3% (62.8% in the Milan group). Tumor recurrence developed in 6 patients (18.8%; compared to 13.8% of those initially within criteria). Treatment-related morbidity or mortality after LDT was not reported. Five out of 48 (10.4%) cases did not complete the protocol, mainly due to tumor progression, which also resulted in removal from the waiting list in all cases. The number of cases excluded for tumor progression before transplantation was significantly higher in the down-staging group: 13 (27.1%) versus 15 cases (11.6%), (P<0.05), despite a comparable waiting time before dropout (371 versus 290 days). The rate of deaths on the list due to decompensation was not statistically different (9% vs. 4%). This study had a smaller cohort of patients and shorter follow-up but its strengths were its prospective study design, clearly stated inclusion criteria and inclusion of a comparison group within Milan criteria.
In a prospective study by Millonig from Austria (61), 33 patients with HCC beyond Milan but within UCSF criteria had attempted down-staging with TACE using epirubicin and lipiodol. Thirty patients were transplanted (transplantation rate 91%). Response was assessed by RECIST criteria and 85% had either partial or complete response. Six patients with complete response were transplanted and had a 5-year survival of 67%, 22 patients with partial response were transplanted, and had a 5-year survival of 64%. Two had stable or progressive disease and 5-year survival was 25%. In this study, TACE did not confer a survival benefit in the group exceeding Milan criteria, even if it led to tumor regression pre-transplantation. Conversely, response to LDT did confer a survival benefit to the patients within Milan criteria on the waiting list for transplantation. In contrast to the previous studies, the authors found that Milan stage significantly affected survival and recurrence rates post-transplantation, when compared to UCSF-expanded criteria, and was a better predictor of poorer outcomes than response to TACE. Recurrence rate was 25% (7 patients) (recurrence 7.6% in the Milan group). Treatment-related mortality is not reported specifically but side effects of TACE in this study were pain, fever, nausea, and inguinal hematoma. Two patients developed a hepatic abscess after TACE, which required antibiotic treatment alone in one patient and required drainage plus antibiotic treatment in the other.
In a prospective study by Graziadei et al., Austria, (62) 15 patients with HCC beyond Milan criteria, with no upper limits in tumor size or number, underwent TACE therapy between January 1997 and December 2001. Epirubicin and lipiodol were used. Response was defined as a 50% reduction in tumor size and this was achieved in 67%. Patients who responded to the first TACE session were listed for transplantation and TACE was repeated 6–8 weekly until complete response was achieved or organ became available. Ten (67%) were transplanted and the 4-year post-transplant survival was 41%, 5-year intention-to-treat survival was 31%. Recurrence developed in 30% (3 patients). TACE was generally well-tolerated but one patient in the down staging group developed a liver abscess, requiring antibiotic therapy, and one patient in the Milan group required surgical drainage for hepatic abscess. This study found that downstaged patients had significantly worse survival and recurrence outcomes than the group within Milan criteria, however the numbers studied were small which may limit the conclusions that can be drawn.
A retrospective cohort study by De Luna et al., Stanford, California (63) included 27 patients who had attempted down-staging with transarterial chemoinfusion (TACI) between January 1995 and March 2008. The eligibility criteria were tumors beyond Milan criteria but there were no upper limits in tumor number and size. They were compared to a group of 95 patients meeting Milan criteria. The chemotherapeutic agent consisted of an emulsion of Ethiodol, cisplatin and doxorubicin dissolved in the contrast medium. After infusion, if there was still arterial feeding of the tumor, gelfoam was introduced via the catheter. If there was still evidence of viable tumor at a 3-month follow-up CT, another session of TACI was offered. Downstaging to within Milan criteria was achieved in 17 patients (63%). Fifteen patients (55.6%) were transplanted and the 3-year post-transplant survival of the group of down-staged patients was 78.8%. This was not a statistically significant difference to the 82.4% survival of those meeting Milan criteria. HCC recurrence was observed in just one patient (recurrence rate 7%). The rate of serious complications was 5.6%. Symptoms included nausea, vomiting, fever, variceal bleeding and encephalopathy. One patient required surgery for a right groin hematoma and pseudoaneurysm. The main drawbacks of this study were its retrospective design and small cohort size from a single centre.
In a single-centre retrospective cohort study by Green et al., Colorado, (64) 22 patients had down-staging with DEB TACE (doxorubicin-eluting beads). Eligibility criteria were beyond Milan, UNOS T3; no upper size limits for up to three lesions. Patients were included between September 2008 and December 2011. Seventeen out of 22 (77%) were successfully down-staged, as assessed by mRECIST criteria and 7 (32%) were transplanted. HCC recurrence was 29% (2 patients). Treatment related mortality or morbidity was not reported. This study lacked longer-term post-transplantation follow-up data on mortality and recurrence (median post-transplantation follow-up 26.1 months).
In a retrospective cohort study by Barakat et al., Houston, Texas (65), 32 patients underwent down-staging with TACE, TARE and RFA. They were included from June 2003 to April 2006. Inclusion criteria were beyond Milan and UCSF criteria, with no upper limit in size or number of lesions. The study protocol was for patients to receive TACE with Ethiodol and doxorubicin, and this was then followed by either RFA or TARE with radioactive Yttrium-90 resin microspheres. The type of LDT used and number of sessions was individual specific, but in general TACE followed by RFA was preferred when there were fewer and smaller lesions, and TACE followed by TARE was used when HCC was multifocal or larger than 6 cm, or as third line after the other therapies. RFA was percutaneous, laparoscopic or open. For those with preserved liver function, resection was performed. The majority of patients received multimodal treatment. Additional sessions of LDT were offered if there were signs of progression or recurrence during surveillance. Down-staging success, to within UNOS T2 criteria, was achieved in 18 patients (56%) and 14 (44%) were transplanted. Two-year survival was 75% and HCC recurrence was 14% (2 patients, at median 35 months follow-up). A total of 55.5% of those successfully down-staged had HCCs outside the proposed UCSF criteria for downstaging. Four patients (28.5%) with infiltrative tumors died of progressive liver failure during LDT and the authors suggested caution with aggressive treatments for these types of tumors. Treatment-related morbidity and mortality were not reported for the whole group.
In a retrospective cohort study by Jang et al., Seoul, Korea (66), 386 patients with preserved hepatic synthetic function had attempted down-staging with TACE with no upper limits in tumor number and size for tumors beyond the Milan criteria. Patients were consecutively enrolled between June 2000 and December 2007. The treatment was lipiodol plus epirubicin and/or cisplatin, without gelfoam embolization and was repeated at one to two monthly intervals as needed, until complete necrosis of all lesions was seen. One hundred and sixty (41.5%) were successfully down-staged to within Milan criteria and 37 (9.6%) were transplanted. Five-year survival was 54.6% and 11 (29.7%) had recurrent HCC. This study, like others, suggested that there is an upper limit in tumor size, above which, no benefit can be gained from down staging before liver transplantation. In this study, tumor size >7 cm, incomplete necrosis after TACE and AFP >100 ng/mL were predictors of poorer outcome. As there is a scarcity of deceased donor livers in Korea, living donor transplantation was utilized in the majority. This meant that most recipients were transplanted soon after successful down-staging, without an observation period, and that the results may not be applicable to deceased donor transplantation. Although the down staging cohort was large, the number of patients transplanted was small, reducing the power of the conclusions drawn from follow-up analysis.
In a retrospective cohort study by Lewandowski et al., Chicago, Illinois (67), down-staging was attempted in 86 patients between January 1, 2000 and December 31, 2008. Patients had UNOS T3 HCC and no upper size limits for up to three lesions. TACE was used in 43 patients and TARE was used in another 43 patients with HCC beyond Milan criteria. This was not randomized—TARE was used in those in whom TACE was unlikely to be tolerated (more elderly patients). TACE delivered mitomycin, adriamycin and cisplatin with lipiodol, followed by embolization particles. TARE was performed using Yttrium-90 microspheres. Patients were imaged 1 month after treatment, and on 3 monthly intervals thereafter. Response was assessed using WHO criteria (50% decrease in cross-sectional diameter of target lesions from baseline) and EASL criteria (50% necrosis/avascularity in target lesions from baseline). Eight patients in the TACE cohort did not have follow-up imaging (reasons were early post treatment transplant, death or lost to follow up) and so down-staging outcomes are based on 35 patients. EASL complete response rates were 6 (17%) in the TACE group and 20 (47%) in the TARE group. A total of 58% (25/43) in the TARE group were successfully down-staged to T2 and 31% (11/35) in the TACE group achieved down-staging. A total of 26% (11/43) TACE and 21% (9/43) TARE-Y90 patients were transplanted. Three-year survival after transplantation or resection was 19% for the TACE cohort and 45% for TARE patients. Intention-to-treat survival was 19% in the TACE group and 59% in the TARE group. HCC recurred in 18% (2/11) of the TACE patients and 22% (2/9) of the TARE-Y90 patients. Post-embolization syndrome was observed in 60% of patients in the TACE group. Transient flu-like symptoms lasting 7–10 days were observed in 60% of patients in the TARE group. The study was retrospective and single-center, with non-randomized cohort arms, which could have left it open to bias. In addition, the imaging assessment of downstaging differed from the recommended mRECIST or EASL criteria: the entire lesion was measured rather than only the enhancing viable parts (WHO criteria).
In a retrospective cohort study by Chapman et al., St. Louis, Missouri (68), 76 patients with HCC between 1999 to 2006 had attempted downstaging with TACE. Eligibility criteria were tumors beyond Milan, with no upper limits in lesion size and number. The definition of response was based on RECIST criteria and this was achieved in 23.7% (18/76). Seventeen (22.4%) were transplanted and 5-year survival was 94.1%. HCC recurrence was 6% (1 patient). Three patients died from the whole cohort of 136 treated with TACE (30-day mortality 2.2%). Deaths were due to liver failure or tumor lysis syndrome. One patient required percutaneous drainage of liver abscess post-TACE.
In a retrospective study of consecutive patients by Otto et al., Germany (25), between May 1998 and May 2005, 62 patients with HCC beyond Milan criteria, with no limits in tumor size or number, underwent attempted downstaging with TACE, which was performed in 6-weekly intervals until transplantation. Mitomycin and lipiodol were used. The aim of the study was to transplant patients who responded to TACE even if their tumor initially and after downstaging exceeded the listing criteria, in order to study the oncological result of treatment. The RECIST criteria were used to assess response and 34 (55%) had a partial response to TACE and were listed. TACE continued on the waiting list and 27 (44%) patients underwent transplantation. Recurrence free 5-year survival was 74.5%. Hepatic decompensation occurred in 5 patients during downstaging (8%). In this study, freedom from recurrence was associated with response to TACE but was not related to Milan stage. The authors therefore suggested that response to TACE is a better criterion for selection for transplantation than tumor number and size as it is more predictive of recurrence.
In a retrospective cohort study by Majno et al., France (69), between January 1985 and December 1995, 35 patients with tumor size >3 cm, of any number, underwent LDT with TACE prior to transplantation. Lipiodol with doxorubicin or cisplatin was used, with gelatin sponge powder or pellets embodied afterwards. Response was defined by WHO criteria and this was achieved in 19 (54%). All 35 were transplanted and 5-year disease-free survival was 71% in those who had achieved down-staging (n=19) compared to 29% without response to TACE (n=16). TACE was generally well-tolerated with no increased rate of liver failure post-operatively, however there was one case of severe hepatocellular failure in whom TACE had been performed despite a pre-existing surgical shunt. This patient was rescued by emergency transplantation. This study demonstrated that those tumors >3 cm which respond to TACE have similar post-transplantation recurrence as smaller lesions.
A retrospective study by Pracht et al. (42) treated patients with lobar HCC and ipsilateral portal vein thrombosis with Yttrium-90 glass microspheres with intention to downstage to resection or transplantation. Eighteen patients were included from January 2007 to December 2010 and radiological assessment of response was by EASL criteria, at 3 and 6 months. All patients had good liver synthetic function and performance status. Mean follow-up was 13.0 months (range 2.2–50.6 months). Two patients (11%) were down-staged to within transplantation criteria and a further 2 to resection. One patient was transplanted (5.6%) and had an overall survival of 50.6 months. Overall survival for the whole group at 1 year was 70.3%. Seven patients (38.9%) developed transient liver dysfunction, 6 patients decompensated with ascites and 1 hepatic encephalopathy. No deaths were attributed to the treatment. The authors concluded that TARE was an effective therapy for down staging HCC with portal vein thrombosis. The main limitations of this study were its retrospective design, small cohort and short follow-up time, which may limit the conclusions that can be drawn.
In a retrospective study by Bova et al. (70), 48 patients with HCC beyond Milan, without tumor thrombus or metastases, underwent TAE, TACE or TOCE (transarterial oily chemoembolization) between January 2004 and December 2010. Epirubicin and/or lipiodol and gelfoam were used. Nineteen (40%) were successfully down-staged, by mRECIST criteria. AFP level <100 ng/mL and 3-year calculated survival probability using the Metroticket calculator were independent predictors of successful downstaging (P<0.023 and <0.049 respectively). In the downstaged group, the 5-year survival rate was 60%. Nine (19%) underwent OLT and recurrence rate was 11% (1 patient) at median follow up of 40 months. No major complications were observed after the intra-arterial therapies. Morbidity included abdominal pain, nausea and transient fever.
A systematic review of 13 trials (950 patients) by Parikh et al. (54) showed the overall success rate of down staging therapy to be 48% but with a recurrence rate of 16%. Studies that included patients with portal vein thrombosis had the lowest success rates and excluding these brought the pooled success rate up to 54%. There was also a significantly improved success rate for those studies with a prospectively designed study protocol, compared to retrospective studies. There was no clear difference in efficacy between TACE and TARE. The highest success rates came from studies with multimodal LDT treatments, all of which included a proportion of patients undergoing liver resection for downstaging. Modality did not have an effect on recurrence rate. The review noted variability in reporting of post transplantation survival data, and so a pooled analysis could not be made. The majority of studies reported over 90% survival at 1 year but 4- or 5-year survival rates varied from 70% to over 90%. There was inconsistent reporting of inclusion criteria and heterogeneity in baseline tumor burden, downstaging protocol, waiting time and assessment of response. Only two studies included a mandatory waiting period before listing for transplantation.
Conclusions
Drawing clear conclusions about the use of locoregional therapies to down-stage HCC is made difficult by the heterogeneity of published studies so far. There are variations in inclusion criteria—tumor burden, treatment protocols, and study designs—prospective or retrospective; definitions of response and inclusion or not of mandatory waiting time after between down staging and transplantation. The protocol, modality and delivery technique used is also variable. Within these limitations, pooled analyses have suggested success in down staging in about half (48%) of patients treated, but with higher recurrence rates than patients initially within transplantation criteria (16%) (54). Studies with strict inclusion criteria and mandatory waiting time before transplantation reported better outcomes in terms of success of down staging and recurrence rate, with survival equivalent to patients who did not require down staging (5-year survival 78%) (51). This would satisfy the requirement set out by the international consensus group (16). Treatment-related morbidity, where reported, was generally mild. Treatment-related mortality was not reported in the majority of studies but, where reported, was around 2.5%.
It is likely that, in carefully selected patients, there is a role for down staging to provide the chance of transplantation and cure, with acceptable outcomes. Further multi center, well-designed studies are needed to clarify who will benefit. There should be criteria stated within transplantation programs, defining eligibility for down staging and decisions regarding each case should be discussed by the multidisciplinary team, involving hepatologists, surgeons and radiologists.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519-24. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5.
- Tsochatzis EA, Fatourou EM, Triantos CK, et al. Transarterial therapies for hepatocellular carcinoma. Recent Results Cancer Res 2013;190:195-206. [Crossref] [PubMed]
- Tsochatzis EA, Germani G, Burroughs AK. Transarterial chemoembolization, transarterial chemotherapy, and intra-arterial chemotherapy for hepatocellular carcinoma treatment. Semin Oncol 2010;37:89-93. [Crossref] [PubMed]
- Tsochatzis E, Garcovich M, Marelli L, et al. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int 2013;33:944-9. [Crossref] [PubMed]
- Lai Q, Vitale A, Iesari S, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017;66:1910-9. [Crossref] [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 2010;28:3994-4005. [Crossref] [PubMed]
- Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262-78. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. [Crossref] [PubMed]
- Marelli L, Grasso A, Pleguezuelo M, et al. Tumour Size and Differentiation in Predicting Recurrence of Hepatocellular Carcinoma After Liver Transplantation: External Validation of a New Prognostic Score. Ann Surg Oncol 2008;15:3503-11. [Crossref] [PubMed]
- Gunsar F. Liver Transplantation for Hepatocellular Carcinoma Beyond the Milan Criteria. Exp Clin Transplant 2017;15:59-64. [PubMed]
- Xu DW, Wan P, Xia Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: A review. World J Gastroenterol 2016;22:3325-34. [Crossref] [PubMed]
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. [Crossref] [PubMed]
- Hakamada K, Kimura N, Miura T, et al. Des-gamma-carboxy prothrombin as an important prognostic indicator in patients with small hepatocellular carcinoma. World J Gastroenterol 2008;14:1370-7. [Crossref] [PubMed]
- Elshamy M, Aucejo F, Menon KV, et al. Hepatocellular carcinoma beyond Milan criteria: Management and transplant selection criteria. World J Hepatol 2016;8:874-80. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006;12:1260-7. [Crossref] [PubMed]
- Roberts JP, Venook A, Kerlan R, et al. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925-9. [Crossref] [PubMed]
- Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol 2011;55:814-9. [Crossref] [PubMed]
- Golfieri R, Cappelli A, Cucchetti A, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011;53:1580-9. [Crossref] [PubMed]
- Roccarina D, Majumdar A, Thorburn D, et al. Management of people with intermediate-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011649. [PubMed]
- Tsochatzis EA, Meyer T, Burroughs AK. Hepatocellular carcinoma. N Engl J Med 2012;366:92-3. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Vogl TJ, Lammer J, Lencioni R, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol 2011;197:W562-70. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]
- Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. [Crossref] [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30:6-25. [Crossref] [PubMed]
- Meyer T, Kirkwood A, Roughton M, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer 2013;108:1252-9. [Crossref] [PubMed]
- Okusaka T, Kasugai H, Shioyama Y, et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2009;51:1030-6. [Crossref] [PubMed]
- Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997;26:1156-61. [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [Crossref] [PubMed]
- Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9. [Crossref] [PubMed]
- Pracht M, Edeline J, Lenoir L, et al. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol 2013;2013:827649.
- Bruix J, Sherman M. Practice Guidelines Committee, et al. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380-8. [Crossref] [PubMed]
- Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995;197:101-8. [Crossref] [PubMed]
- Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy of hepatocellular carcinoma: analysis of 77 patients. AJR Am J Roentgenol 1990;155:1221-6. [Crossref] [PubMed]
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655-61. [Crossref] [PubMed]
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40. [Crossref] [PubMed]
- Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma ≤4 cm. Gastroenterology 2004;127:1714-23. [Crossref] [PubMed]
- Majumdar A, Roccarina D, Thorburn D, et al. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011650. [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968-77. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Yao FY, Breitenstein S, Broelsch CE, et al. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl 2011;17 Suppl 2:S109-16. [Crossref] [PubMed]
- Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl 2015;21:1142-52. [Crossref] [PubMed]
- Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology 2016;63:1014-25. [Crossref] [PubMed]
- Leung DA, Goin JE, Sickles C, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001;12:321-6. [Crossref] [PubMed]
- Hsin IF, Hsu CY, Huang HC, et al. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol 2011;45:556-62. [Crossref] [PubMed]
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441-51. [Crossref] [PubMed]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201-9. [Crossref] [PubMed]
- Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 2008;8:2547-57. [Crossref] [PubMed]
- Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2007;13:272-9. [Crossref] [PubMed]
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003;9:557-63. [Crossref] [PubMed]
- De Luna W, Sze DY, Ahmed A, et al. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant 2009;9:1158-68. [Crossref] [PubMed]
- Green TJ, Rochon PJ, Chang S, et al. Downstaging disease in patients with hepatocellular carcinoma outside of Milan criteria: strategies using drug-eluting bead chemoembolization. J Vasc Interv Radiol 2013;24:1613-22. [Crossref] [PubMed]
- Barakat O, Wood RP, Ozaki CF, et al. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl 2010;16:289-99. [PubMed]
- Jang JW, You CR, Kim CW, et al. Benefit of downsizing hepatocellular carcinoma in a liver transplant population. Aliment Pharmacol Ther 2010;31:415-23. [Crossref] [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [Crossref] [PubMed]
- Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-25. [PubMed]
- Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997;226:688-701; discussion 701-3. [Crossref] [PubMed]
- Bova V, Miraglia R, Maruzzelli L, et al. Predictive factors of downstaging of hepatocellular carcinoma beyond the Milan criteria treated with intra-arterial therapies. Cardiovasc Intervent Radiol 2013;36:433-9. [Crossref] [PubMed]
Cite this article as: Bryce K, Tsochatzis EA. Downstaging for hepatocellular cancer: harm or benefit? Transl Gastroenterol Hepatol 2017;2:106.


