Single injection dual phase CBCT technique ameliorates results of trans-arterial chemoembolization for hepatocellular cancer
Introduction
According to international guidelines catheter based treatment represent the standard of care for both intermediate and advanced hepatocellular carcinoma (HCC) (1). Different aim could be pursued with transarterial therapies: intermediate stage benefits of local tumor debulking in order to keep patient within an active list for orthotopic liver transplantation (bridging to transplantation); whereas when dealing with advanced stage HCC, catheter based approach could in selected cases downstage total tumor burden in order to perform potential curative approach (major resection) (2-4).
Cone-beam CT (CBCT) is a volumetric imaging modality, provided by all different Angio Suite manufacturer, obtained by a single rotation of the C-arm flat panel detector around the patient. The acquisition is performed directly on the Angio Suite table, without need of patient repositioning, with or without contrast media administration. Immediate processing of acquired dataset lead to visualization, on dedicated workstation, of CT-like images, that ensure perfect cross-sectional comparison with standard contrast enhanced multidetector computer tomography (MDCT) or MRI on which all procedures are nowadays planned. Since its first usage within the neuro-interventional field (5,6), growing evidence of its adjunctive diagnostic value was reported even for peripheral applications: to assist below the knee revascularization procedures (7); as guidance for combined hybrid procedure (embolization + percutaneous ablation) for single or multiple lesions (8); to intraprocedural aid detection and correction of type Ia endoleak after EVAR procedures (9); to assist portal vein embolization procedures (10).
The application of this newly introduced imaging technique to the field of liver transcatheter oncological treatments, has significantly changed everyday practice. In fact, by aiding detection of the vasculature path to be catheterized to perform the embolization (detection of intrahepatic feeder) (11-13), by demonstrating the presence of extra hepatic feeders (14) as well as the presence of occult nodules missed at standard second line imaging had dramatically improved the outcome of treatment (15). Moreover, the routinely usage of this imaging modality, permits reduction of the total radiation dose for both operator and patient and reduction of the total amount of contrast media administered (16).
The aim of this review is to present all clinical and procedural advantages of the introduction of CBCT technique as guidance for all liver oncological catheter based procedures.
CBCT technique
CBCT imaging can be performed only with new generation flat panel angiographers featuring dedicated imaging reconstruction software, as provided by different manufacturers (DynaCT, Siemens; XperCT, Philips; Innova CT, GE Healthcare). The acquisition is performed, without need of patient repositioning, either before or after the embolization procedure. In a single orbit the C-arm rotates 178–220° around the patient, whit acquisition times ranging between 5 and 20 seconds, acquiring images every 0.39–0.52° with a pitch of 200–800 micron at 15–60 frame/second. The acquired rotational angiography displayed on the conventional monitor is immediately processed in order to create volumetric CT datasets (images range 24–35 cm, matrix size 512 cm × 512 cm ×387 cm, for a total amount of images around 400–420, with a matrix and isotropic resolution of 0.49 mm with a FOV ranging between 12–40 cm). The high resolution of these images permits 3D reconstructions (including multiplanar reformations, maximum intensity projections and volume rendering images). The acquisition is performed with continuous modulation of the tube current, allowing for estimated dose around 3–10 mSv for a single CBCT.
The major advantages of this technique derive from its ability to combine contrast media injection with three-dimensional CT-like images, overcoming the classical limit of bi-dimensional proper digital subtraction angiography (DSA) acquisition. The contrast media intra-arterial injection maximizes the discrepancy measured in term of contrast to noise ratio (CNR) and signal to noise ratio (SNR) between the healthy liver and HCC nodules. In detail first proper DSA acquisitions (posteroanterior and right anterior oblique projections) are performed in order to verify the appropriate flow and volume of the power injector (e.g., small vasculature 2.7–3 mL/s, total volume 12 mL; hypertrophied vasculature 4 mL/s, total volume 20 mL); injection could be performed either with via the standard 4–5 Fr angiographic catheter positioned in the proper hepatic artery (suggested PSI limit 900), yielding to a complete liver parenchima enhancement, or via a superselective positioned microcatheter (suggested PSI limit 600) visualizing just the segment/lobe of interest. Once the velocity of injection is identified (based on DSA findings), single injection dual phase CBCT is performed by setting appropriate injection duration and acquisition delay, with diluted contrast media (ratio 1:3). In fact, the injection duration should cover the acquisition time (around 7 seconds of rotation) and the 8 seconds of delay needed to gain optimal liver parenchyma enhancement, yielding to total injection duration of 15 seconds. Delayed phase CBCT is acquired within 30 seconds from the first acquisition. By employing these technical specifications (duration of injection and delay of acquisition) first CBCT acquisition combine the liver vasculature visualization with appropriate parenchyma/nodule enhancement. The added value of the second CBCT acquisition is represented by the potentiality of characterizing lesion by depicting their behaviour (e.g., lesion’s washout). Figure 1 summarizes the technical detail of the dual-phase technique.
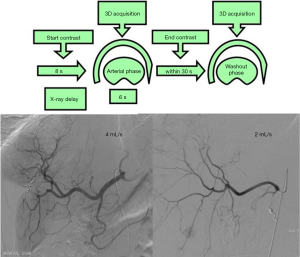
Comparison with MDCT/MDCT arteriography
As compared to conventional MDCT acquisitions, CBCT present some significant limitations in terms of image quality, mostly related to the increased photon scatter and increase image noise generated by the small FOV. Notwithstanding these limitations, recently published papers demonstrate that the clinical performance of CBCT (in terms of tumor identification, vessel delineation and soft tissue evaluation) isn’t significantly impaired as compared to that of conventional CT (Figure 2).
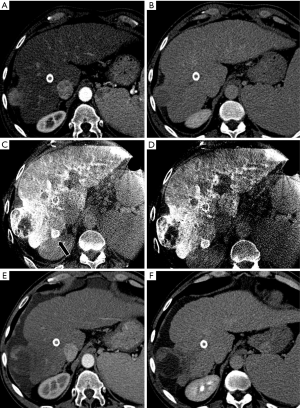
Regarding the major constrain of the limited FOV dimension, is worth to underline that most of patients usually fits even with this limited field of view. In several particular settings, such as hypertrophied left liver lobe, obese patients this could became a major limitation. In order to solve this technical drawback: “open arc cone-beam CT” has been recently reported, by modifying the axis of rotation of the c-arm around the patient in order to image all the live segments within a single acquisition (17).
Despite these over mentioned technical limits in clinical practice CBCT can permit to shows additional HCC if compared with MDCT; in fact, recent published paper reported higher sensitivity in detection of nodules. In particular Mayer et al. (18) demonstrated a specificity of 99% and of 85% in tumor detection despite the gold standard of reference is not clear being the standard of reference the blind reading of the pre-procedural MDCT; other authors demonstrate the superiority of CBCT in the detection accuracy of nodules smaller than 10 mm and the higher sensitivity in nodule smaller than 20 mm whereas no differences were observable in detection of nodules greater than 10 mm (19); although the paper of Higashihara et al. (20) demonstrate no significant differences between the mean PPV for MDCT and CBCT among 10 mm group despite the number of total HCC detected was greater in CBCT group; it’s necessary to mention that in these last two paper the standard of reference was considered as the accumulation of lipiodol observable at CT imaging 1 week after the procedure.
To our knowledge to date there is only one paper focused on comparing CBCT with the hybrid CT/Angiography suite (21). The paper does not provide a comparison of clinical results but analyses advantages or disadvantages of every system. In particular, advantages provided by using CT instead of flat-panel C-arm regards the larger FOV with the certainty of includes the entire liver in the acquisition and the less evidence of motion or beam hardening artifacts; on the other hand, the patient radiation exposure is higher (22) and the cost of the system that is 1.5–2 times more expensive than CBCT. Despite these advantages of hybrid CT/angiography suite, this technology has not been widespread across countries, mainly due to the high equipment costs. For these reasons comparison between techniques is not possible. The other proposed technique, mainly reported by Asiatic literature (23-25), consist in a MDCT acquisition after having positioned one or two arterial catheters within the hepatic and superior mesenteric arteries, named MDCT-angiography. Despite the known advantages of higher image quality when utilizing the MDCT-angiography, these techniques need patient repositioning, higher radiation dose and finally did not permit any catheter repositioning. Moreover, in the reported series the total volume of contrast media administered throughout the examination in up to ten times more than then one suggested by our single injection dual phase imaging protocol.
Intraprocedural implications
The routine usage of CBCT during chemoembolization lead to several advantages: detection of occult nodules, better feeding vessels depiction, identification of extrahepatic parasitic feeder, sparing of non-target feeder, prediction of treatment outcome, reduction of both contrast media and radiation dose.
Regarding nodule detection, there’s a wide evidence that CBCT in course of TACE has the ability to demonstrate nodules not visualized at pre-operative MDCT and at standard DSA. Small HCC detectability, at DSA, depends on the size of the tumor and of its vascularization; more in particular Miyayama et al. (26) demonstrated that the 95% of tumor 15) demonstrated how CBCT was capable to depict an occult nodule rate of 11.5% not visualized at pre-procedural MDCT. The short interlapsed time between intraprocedural CBCT and pre-procedural MDCT underline the potentiality of this novel imaging modality in depicting an aggressive subset of patients that prompt strictly imaging surveillance, aggressive management, prioritization in those patient within an active list for transplantation.
Another pivotal point of strength of this technique relies in the ability of assist the embolization procedure identifying tumor feeding arteries as well as extrahepatic parasitic feeder presence (Figure 3). In fact has been reported that the number of feeders visualized is higher in CBCT that in arteriography alone; in particular Iwazawa et al. (27) reported a CBCT sensitivity, specificity and accuracy of 96.9%, 97.0% and 96.9% respectively, significant higher than those reported for angiography (77.2%, 73% and 74.5% respectively); other authors confirms these results reporting the number visualized of tumor feeders during CBCT and DSA that was 4±1.7 and 3.3±1.4 respectively with a significative statistical evidence between the two imaging techniques (28). Recently an “automated tumor feeder detector” has been developed and some authors (29) evaluate the technical performance of this software; in particular Iwazawa et al. (30) reported that the sensitivity of this software was significantly higher than the conventional assessment using DSA (87.7% vs. 71.8%, Pet al. demonstrated how CBCT guidance aid to spare cystic artery when it fed the tumor (31).
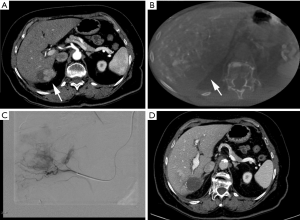
Extrahepatic feeder presence could be supposed, especially in patients undergoing several catheters based treatment or with lesion located in certain liver areas (subcapsular, liver dome, gallbladder bed), in cases were CBCT had shown incomplete nodule enhancement (16).
Moreover, CBCT usage has been correlated with better procedural outcome: Wang et al. (32) demonstrated a strong correlation between lipiodol deposition on CBCT and tumor necrosis (cm3) assessed with enhanced MRI.
Lastly, the routine employment of this novel imaging modality by acquiring a CT-like 3D-volume, permitting multiplanar reconstruction, aid in the detection of the best projection to facilitate catheterization thus sparing fluoroscopy time as well as contrast media administration.
Conclusions
CBCT represent the novel standard of care of imaging guidance for catheter-based treatments. Its usage leads to several advantages over the pre-procedural MDCT. This technique is performed directly in the Angio Suite without patient repositioning. Thanks to these unique features it’s able to ameliorate procedural results by depicting occult nodules, tumor feeding vessels (intra- and extra-hepatic), best projection to aid catheterization.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Levi Sandri GB, Ettorre GM, Colasanti M, et al. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr 2017;6:44-8. [Crossref] [PubMed]
- Levi Sandri GB, Lai Q, Lucatelli P. The forgotten place of radioembolization for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis. Liver Int 2017;37:469-70. [Crossref] [PubMed]
- Ettorre GM, Levi Sandri GB, Laurenzi A, et al. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J Surg 2017;41:241-9. [Crossref] [PubMed]
- Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199-205. [PubMed]
- Missler U, Hundt C, Wiesmann M, et al. Three-dimensional reconstructed rotational digital subtraction angiography in planning treatment of intracranial aneurysms. Eur Radiol 2000;10:564-8. [Crossref] [PubMed]
- Jens S, Lucatelli P, Koelemay MJ, et al. Three-dimensional rotational angiography of the foot in critical limb ischemia: a new dimension in revascularization strategy. Cardiovasc Intervent Radiol 2013;36:797-802. [Crossref] [PubMed]
- Wang ZJ, Wang MQ, Duan F, et al. Clinical application of transcatheter arterial chemoembolization combined with synchronous C-arm cone-beam CT guided radiofrequency ablation in treatment of large hepatocellular carcinoma. Asian Pac J Cancer Prev 2013;14:1649-54. [Crossref] [PubMed]
- Biasi L, Ali T, Hinchliffe R, et al. Intraoperative DynaCT detection and immediate correction of a type Ia endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol 2009;32:535-8. [Crossref] [PubMed]
- Kapoor BS, Esparaz A, Levitin A, et al. Nonvascular and portal vein applications of cone-beam computed tomography: current status. Tech Vasc Interv Radiol 2013;16:150-60. [Crossref] [PubMed]
- Minami Y, Yagyu Y, Murakami T, et al. Tracking Navigation Imaging of Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma Using Three-Dimensional Cone-Beam CT Angiography. Liver Cancer 2014;3:53-61. [Crossref] [PubMed]
- Lee IJ, Chung JW, Yin YH, et al. Cone-Beam Computed Tomography (CBCT) Hepatic Arteriography in Chemoembolization for Hepatocellular Carcinoma: Performance Depicting Tumors and Tumor Feeders. Cardiovasc Intervent Radiol 2015;38:1218-30. [Crossref] [PubMed]
- Deschamps F, Solomon SB, Thornton RH, et al. Computed analysis of three-dimensional cone-beam computed tomography angiography for determination of tumor-feeding vessels during chemoembolization of liver tumor: a pilot study. Cardiovasc Intervent Radiol 2010;33:1235-42. [Crossref] [PubMed]
- Miyayama S, Yamashiro M, Nagai K, et al. Efficacy of automated tumor-feeder detection software using cone-beam computed tomography technology in transarterial embolization through extrahepatic collateral vessels for malignant hepatic tumors. Hepatol Res 2016;46:166-73. [Crossref] [PubMed]
- Lucatelli P, Argiro R, Ginanni Corradini S, et al. Comparison of Image Quality and Diagnostic Performance of Cone-Beam CT during Drug-Eluting Embolic Transarterial Chemoembolization and Multidetector CT in the Detection of Hepatocellular Carcinoma. J Vasc Interv Radiol 2017;28:978-86. [Crossref] [PubMed]
- Lucatelli P, Corona M, Argiro R, et al. Impact of 3D Rotational Angiography on Liver Embolization Procedures: Review of Technique and Applications. Cardiovasc Intervent Radiol 2015;38:523-35. [Crossref] [PubMed]
- Schernthaner RE, Chapiro J, Sahu S, et al. Feasibility of a Modified Cone-Beam CT Rotation Trajectory to Improve Liver Periphery Visualization during Transarterial Chemoembolization. Radiology 2015;277:833-41. [Crossref] [PubMed]
- Meyer BC, Frericks BB, Voges M, et al. Visualization of hypervascular liver lesions During TACE: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol 2008;190:W263-9. [Crossref] [PubMed]
- Iwazawa J, Ohue S, Hashimoto N, et al. Detection of hepatocellular carcinoma: comparison of angiographic C-arm CT and MDCT. AJR Am J Roentgenol 2010;195:882-7. [Crossref] [PubMed]
- Higashihara H, Osuga K, Onishi H, et al. Diagnostic accuracy of C-arm CT during selective transcatheter angiography for hepatocellular carcinoma: comparison with intravenous contrast-enhanced, biphasic, dynamic MDCT. Eur Radiol 2012;22:872-9. [Crossref] [PubMed]
- Tanaka T, Arai Y, Inaba Y, et al. Current role of hybrid CT/angiography system compared with C-arm cone beam CT for interventional oncology. Br J Radiol 2014;87:20140126. [Crossref] [PubMed]
- Bai M, Liu B, Mu H, et al. The comparison of radiation dose between C-arm flat-detector CT (DynaCT) and multi-slice CT (MSCT): a phantom study. Eur J Radiol 2012;81:3577-80. [Crossref] [PubMed]
- Ohki T, Tateishi R, Akahane M, et al. Characteristics of hepatocellular carcinoma nodules newly detected by computed tomography during arteriography and arterial portography: preliminary report of a randomized controlled trial. Hepatol Int 2012;6:639-45. [Crossref] [PubMed]
- Joshi JV, Pandey SN, Galvankar P, et al. Prevalence of premenstrual symptoms: Preliminary analysis and brief review of management strategies. J Midlife Health 2010;1:30-4. [Crossref] [PubMed]
- Kim SR, Ando K, Mita K, et al. Superiority of CT arterioportal angiography to contrast-enhanced CT and MRI in the diagnosis of hepatocellular carcinoma in nodules smaller than 2 cm. Oncology 2007;72 Suppl 1:58-66. [Crossref] [PubMed]
- Miyayama S, Yamashiro M, Okuda M, et al. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol 2009;32:255-64. [Crossref] [PubMed]
- Iwazawa J, Ohue S, Mitani T, et al. Identifying feeding arteries during TACE of hepatic tumors: comparison of C-arm CT and digital subtraction angiography. AJR Am J Roentgenol 2009;192:1057-63. [Crossref] [PubMed]
- Meyer BC, Witschel M, Frericks BB, et al. The value of combined soft-tissue and vessel visualisation before transarterial chemoembolisation of the liver using C-arm computed tomography. Eur Radiol 2009;19:2302-9. [Crossref] [PubMed]
- Miyayama S, Yamashiro M, Ikuno M, et al. Ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinoma guided by automated tumor-feeders detection software: technical success and short-term tumor response. Abdom Imaging 2014;39:645-56. [Crossref] [PubMed]
- Iwazawa J, Ohue S, Hashimoto N, et al. Clinical utility and limitations of tumor-feeder detection software for liver cancer embolization. Eur J Radiol 2013;82:1665-71. [Crossref] [PubMed]
- Kinoshita M, Takechi K, Iwamoto S, et al. The usefulness of cone-beam computed tomography during chemoembolization of hepatocellular carcinomas fed exclusively by the cystic artery. Jpn J Radiol 2016;34:747-53. [Crossref] [PubMed]
- Wang Z, Chen R, Duran R, et al. Intraprocedural 3D Quantification of Lipiodol Deposition on Cone-Beam CT Predicts Tumor Response After Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2015;38:1548-56. [Crossref] [PubMed]
Cite this article as: Lucatelli P, Argirò R, Bascetta S, Saba L, Catalano C, Bezzi M, Levi Sandri GB. Single injection dual phase CBCT technique ameliorates results of trans-arterial chemoembolization for hepatocellular cancer. Transl Gastroenterol Hepatol 2017;2:83.


