Enlarged selection criteria for hepatocellular cancer: is the upper limit needed?
Introduction
Milan criteria (MC) are so far the safest and still the most commonly used way to select early-stage hepatocellular carcinoma (HCC) patients for transplantation. However, in a world facing a constant increase of HCC cases (1), the pressure to enlarge the pool of donors and to extend these criteria is extremely strong. As a result, in the last decade, the use of marginal donors (steatotic and/or older grafts) in association to less restrictive criteria, has certainly reduced the mortality on waiting list (and in the first few years after transplantation) (2,3), but it has been associated to higher rates of tumor recurrence after transplantation (4,5). Being organ shortage a real problem, defining where is the border between an acceptable ‘waste’ and the misuse of a limited resource seems to be mandatory.
Criteria selection for liver transplantation (LT)
Allocation policies
LT for HCC is particularly attractive for its ability to remove at the same time the macroscopic lesions, the undetected micro-disease and the underlying cirrhosis, when present. On the other hand, hepatic resection is still indicated for HCC patients having well-compensated cirrhosis [model for end-stage liver disease (MELD) ≤9] (6) and no evidence of portal hypertension, along with an operable liver. In the population of patients without chronic liver disease, resection can yield to 5-year overall survival rates around 50% (7), which looks barely acceptable.
Indication boundaries are constantly evolving, and several studies have compared LT and hepatic resection outcomes, trying to define the best management strategy. Chapman et al. (8) for example, demonstrated that LT can grant an overall and disease-free survival advantage over resection, among patients who qualify for both treatment modalities. This is due to the fact that by transplantation, all the underlying pathologies are treated at once. On the other hand, Vitale et al. recommended transplantation as second line treatment for patients with very early stage HCC, in case of recurrence or for parenchymal insufficiency after liver resection (9). This last approach seems to be the most pragmatic, since it allows to spare organs and immunosuppression-related side effects (and costs) on one side, while it identifies the borderline patients who are promptly rescued by transplantation on the other.
LT is the best treatment option not only for early-stage HCC, but for a wide range of pathologies, like chronic and acute hepatitis, or genetically determined metabolic and developmental liver diseases (10). The success of this treatment determines a high demand for grafts, which translates into a chronic organ shortage worldwide. To face this problem, the transplant community has adopted quite strict criteria, in order to prioritize the patients on the waiting list for a transplantation, based on their clinic conditions. MELD is the fairest tool to assess the urgency of a transplantation in cirrhotic patients, based on their risk of mortality in the short period (11). HCC patients get MELD exceptions points, in order to compensate the time-related risk of developing an incurable disease. Exception points are constantly revised based on the outcomes of both HCC and non-HCC patients, aiming to play fair on both sides (12). It was in fact noticed that, based on the initial scoring, the transplantation rate of HCC patients was too high, which was ‘unfair’ towards non-HCC patients, and unconcerned about tumor aggressiveness (because cancer patients were transplanted too early in their follow-up). In some of the last revisions it was decided to award the exception points six months after the diagnosis (and not right from the start), in order to indirectly assess tumor biology based on its evolution during this reasonably short follow-up (13). Allocation policies when dealing with HCC patients still vary considerably depending on the country (or the area) (14).
MC: are they still valid?
In 1996, Mazzaferro et al. (15) taught the world how to select HCC patients for LT. In their series, the 5-year survival rate was >70%, being the conditions to fulfil: a solitary HCC nodule ≤5 cm or up to 3 nodules, each one ≤3 cm, along with the absence of macroscopic vascular invasion or spread disease.
The MC have been corroborated by numerous transplant units all over the world and finally adopted by the United Network for Organ Sharing as the gold standard for selecting patients with early-stage HCC for LT.
Although undoubtedly safe to select very good candidates for LT, MC are now considered too strict and unable to identify all the possible suitable patients. In this sense, many extended criteria have been proposed (5).
Beyond MC
Schwartz et al. have clearly identified the limitations that reside in the MC patient selection (16). According to their analysis, the fact they only rely on the tumor’s (macro)morphological characteristics may lead to misdiagnosis, due to the limitations connected to the imaging technique. Second, tumor aggressiveness is not taken into account. Third, such criteria are too limitative in terms of nodules number and size. Fourth, MC used as a drop-out tool are responsible for an excessive removal of patients from the waiting list.
MC’s limitations were also evident to other transplant experts, fueling their motivation to develop more compliant criteria, to which we refer as ‘extended’. The most commonly used being University of California San Francisco (UCSF) criteria (17), Up-to-7 criteria (18), total tumor volume/alpha-fetoprotein (TTV/AFP) criteria (19), extended Toronto criteria (ETC) (4), and Kyoto criteria (20). Table 1 summarizes the characteristics of MC and the most commonly used extended criteria, focusing on their propensity to consider tumor biology and grading.

Full table
HCC biological behavior
While UCSF and Up-to-7 criteria represent a cautious extension of MC and still consider macromorphological aspects only, the other proposed criteria try to deal with tumor biological aggressiveness. TTV/AFP criteria, developed between Edmonton and Geneva, use indeed AFP as (surrogate) biological marker of cancer violence. Higher plasma concentrations of this protein are in fact associated to increased tumor burden and enhanced risk of post-transplant recurrence (21).
The Kyoto criteria involve a less exploited molecule: des-γ-carboxyprothrombin (DCP), which has also been associated to increased tumor activity and recurrence risk (22).
Toronto, instead, has completely abandoned morphological criteria and focuses on tumor biological behavior, involving direct and indirect measures. ETC include a biopsy of the largest nodule, obtaining the most precise information available about cancer grading and general aggressiveness (differentiation, microvascular infiltration).
Future criteria will probably include ‘liquid biopsies’ (23). This emerging concept is based on the ability to detect specific tumor DNA or cancer cells RNA (exosome) in mainstream blood (and other body fluids) (24). The potential advantage over physical biopsies is dual, as the analysis is not limited to a single nodule, while the risk of dissemination is completely avoided. Exosomes specific to HCC have already been identified (25,26) and challenged on their efficacy in predicting recurrence after LT (27). The results are encouraging and the future of this technology is certainly bright.
An enlarged selection criterion for every situation
All the enlarged selection criteria listed, have been validated on large cohorts of patients. As a consequence, their principles could be applied to the ‘general population’ of HCC patients with predictable outcomes. Conversely, HCC epidemiology varies consistently depending on the region, country or continent (1). Even grater discrepancies exist in terms of organs availability and allocation policies (28).
These differences explain why, assuming that MC are too restrictive and different ones were needed, the transplant community has not unified under the same new criteria yet (5).
In countries (or regions) with low HCC incidence and very good organs availability, aiming to 10-year overall survival <40%, for example, can be acceptable. In this situation, selection criteria deserve to be sensibly enlarged, because even a ‘sub optimal’ outcome would not be considered as a waste of resources. In contrast to that, regions with high incidence of HCC and a more limited availability of organs, cannot afford to enlarge their selection criteria too much, in order to protect the potential recipients with indications different to HCC. For these reasons it is rational to think that every transplant center will keep on developing its own enlarged criteria, and that the upper limit will be set based on the local epidemiology and resources, instead of by a globally predetermined acceptable outcome.
As shown in Figure 1, every extended criterion allows to transplant more patients (green columns). The more permissive the criteria are, the higher the price to pay in terms of survival, compared to MC. UCSF criteria, which are a prudent revision of MC, enlarge the pool of recipients by 10% (17), still allowing for similar disease-free survival and slightly reduced overall survival. The ETC, more bravely, permit to include 40% more recipients. In this case the downside is represented by the disease-free survival, which drops to 30%, being the overall survival still quite high (68%) (4). The team from Toronto can afford such criteria because they were able to extend their pool of donors by the extraordinary implementation of the living donation (29) and the use of DCD grafts. Similar criteria could not be applied in many other environments, with more traditional donation strategies. On the other hand, TTV/AFP criteria (presently applied in Geneva and Edmonton) represent a more affordable compromise, because they extend the pool of recipients by 20%, reducing the overall survival by only 10% and without affecting the disease-free survival too much (19).
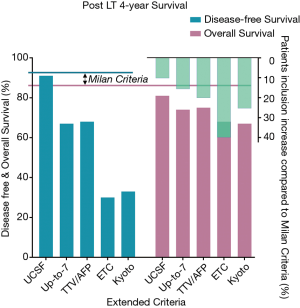
As inclusion criteria for LT of HCC patients will continue to evolve, universally setting an acceptable outcome after LT for HCC does not reflect the current situation, where cancer epidemiology and resources can be extremely variable, depending on the country or the region of interest. As a consequence, even the most extreme enlarged selection criteria should not be considered excessive if they well fit the population and the local means.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McGlynn KA, London WT. The Global Epidemiology of Hepatocellular Carcinoma: Present and Future. Clin Liver Dis 2011;15:223-43. vii-x. [Crossref] [PubMed]
- Emre S, Schwartz ME, Altaca G, et al. Safe use of hepatic allografts from donors older than 70 years. Transplantation 1996;62:62-5. [Crossref] [PubMed]
- Lué A, Solanas E, Baptista P, et al. How important is donor age in liver transplantation? World J. Gastroenterol 2016;22:4966-76. [Crossref] [PubMed]
- Sapisochin G, Goldaracena N, Laurence JM, et al. The Extended Toronto Criteria for Liver Transplantation in Patients With Hepatocellular Carcinoma: A Prospective Validation Study. Hepatology 2016;64:2077-88. [Crossref] [PubMed]
- Silva MF, Sherman M. Criteria for liver transplantation for HCC: What should the limits be? J Hepatol 2011;55:1137-47. [Crossref] [PubMed]
- Citterio D, Facciorusso A, Sposito C, et al. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. Jama Surg 2016;151:846-53. [Crossref] [PubMed]
- Belghiti J, Regimbeau JM, Durand F, et al. Resection of hepatocellular carcinoma: A European experience on 328 cases. Hepatogastroenterology 2002;49:41-6. [PubMed]
- Chapman WC, Klintmalm G, Hemming A, et al. Surgical Treatment of Hepatocellular Carcinoma in North America: Can Hepatic Resection Still Be Justified? J Am Coll Surg 2015;220:628-37. [Crossref] [PubMed]
- Vitale A, Peck-Radosavljevic M, Giannini EG, et al. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol 2017;66:412-23. [Crossref] [PubMed]
- Farkas S, Hackl C, Schlitt HJ. Overview of the Indications and Contraindications for Liver Transplantation. Cold Spring Harb Perspect Med 2014;4:a015602. [Crossref] [PubMed]
- Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol 2013;3:50-60. [Crossref] [PubMed]
- Toso C, Mazzaferro V, Bruix J, et al. Toward a Better Liver Graft Allocation That Accounts for Candidates With and Without Hepatocellular Carcinoma. Am J Transplant 2014;14:2221-7. [Crossref] [PubMed]
- Parikh ND, Singal AG. Model for end-stage liver disease exception points for treatment-responsive hepatocellular carcinoma. Clinical Liver Disease 2016;7:97-100. [Crossref]
- Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14:203-17. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Schwartz ME, D'Amico F, Vitale A, et al. Liver transplantation for hepatocellular carcinoma: Are the Milan criteria still valid? Eur J Surg Oncol 2008;34:256-62. [Crossref] [PubMed]
- Unek T, Karademir S, Arslan NC, et al. Comparison of Milan and UCSF criteria for liver transplantation to treat hepatocellular carcinoma. World J Gastroenterol 2011;17:4206-12. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total Tumour Volume and Alpha Fetoprotein for Selection of Transplant Candidates with Hepatocellular Carcinoma: A Prospective Validation. Hepatology 2015;62:158-65. [Crossref] [PubMed]
- Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053-60. [Crossref] [PubMed]
- Kojima K, Takata A, Vadnais C, et al. MicroRNA122 is a key regulator of alpha-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma. Nat Commun 2011;2:338. [Crossref] [PubMed]
- Yamashiki N, Sugawara Y, Tamura S, et al. Diagnostic accuracy of a-fetoprotein and des-γ-carboxy prothrombin in screening for hepatocellular carcinoma in liver transplant candidates. Hepatol Res 2011;41:1199-207. [Crossref] [PubMed]
- Yin CQ, Yuan CH, Qu Z, et al. Liquid Biopsy of Hepatocellular Carcinoma: Circulating Tumor-Derived Biomarkers. Dis Markers 2016;2016:1427849. [Crossref] [PubMed]
- Zhang HG, Grizzlet WE, Exosomes A. Novel Pathway of Local and Distant Intercellular Communication that Facilitates the Growth and Metastasis of Neoplastic Lesions. Am J Pathol 2014;184:28-41. [Crossref] [PubMed]
- Fornari F, Ferracin M, Trere D, et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. Plos One 2015;10:e0141448. [Crossref] [PubMed]
- Kogure T, Lin WL, Yan IK, et al. Intercellular Nanovesicle-Mediated microRNA Transfer: A Mechanism of Environmental Modulation of Hepatocellular Cancer Cell Growth. Hepatology 2011;54:1237-48. [Crossref] [PubMed]
- Sugimachi K, Matsumura T, Hirata H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Brit J Cancer 2015;112:532-8. [Crossref] [PubMed]
- Schilsky ML, Moini M. Advances in liver transplantation allocation systems. World J Gastroenterol 2016;22:2922-30. [Crossref] [PubMed]
- Sapisochin G, Goldaracena N, Laurence JM, et al. Right lobe living-donor hepatectomy-the Toronto approach, tips and tricks. Hepatobiliary Surg Nutr 2016;5:118-26. [PubMed]
Cite this article as: Peloso A, Oldani G. Enlarged selection criteria for hepatocellular cancer: is the upper limit needed? Transl Gastroenterol Hepatol 2017;2:73.


