N staging system: tumor-node-metastasis and future perspectives
The tumor-node-metastasis (TNM) staging system is the most widely used classification for the assessment of the extent of disease. Since its first application in 1974 (1), it has been updated and revised by the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) on the basis of evidences and improvements in understanding and predicting of cancer related survival, increasing its accuracy in stratification of prognosis of gastric cancer patients.
The current edition (seventh edition), published in 2009, overcame the previous one (sixth edition, 2002) (2,3). The revision of both T and N parameter, introduced in the latest edition, should have a greater influence on both staging and surgery, especially in the era of multimodal treatment. Actually, changes in the definition of the extent of tumor (T) suggested just some variations in clinical practice.
On the other hand, lymph node staging still presents several controversies, and alternative system for definition of nodal extent have been proposed; however, most of these proposals seem an attempt to minimize the impact of extent of lymphadenectomy.
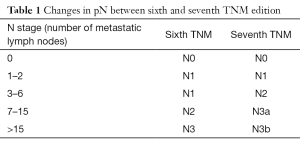
In the seventh TNM staging system, cut offs for definition of N parameter have been changed: N1 is now defined as the presence of 1 or 2 metastatic lymph nodes; involvement of 3 to 6 nodes is classified as N2, while N3 defines the presence of 7–15 (N3a) or more than 15 (N3b) metastatic lymph nodes (4).
Metastatic lymph nodes are a well-known relevant prognostic factor for patients undergoing curative surgery for gastric cancer. The definition of lymph node status aroused a great interest in last decades, leading to the proposal of many different systems for its classification; an accurate assessment of the N parameter still represents an oncological challenge nowadays.
However, latest revision of TNM, supported by studies that reported previous cut offs to be inadequate for stratifying prognosis of gastric cancer patients, seems to be rather influenced by the need to supply to a lack of homogeneity in lymphadenectomy in surgically treated patients.
In fact, if a potential advantage in survival and a more accurate pathological staging have been reported in patients underwent extended (D2) lymphadenectomy, many surgeons in Europe and in the United States are still reluctant to routinely perform extended lymph node dissection. In this context, the need of an “ideal” N staging system, as a reliable prognostic factor independently from the type of lymphadenectomy becomes more urgent. The thresholds for definition of N status introduced in latest TNM edition appear to be more a compromise between the independence from extent of lymphadenectomy and the classical numeric criterion, initially introduced by some Japanese authors, with no fully satisfactory results in their first uses (5,6).
Moreover, in last staging system the minimum number of required nodes reached 16. Once again, this proposal seems to derive from the need to minimize non-homogeneity in the extent of lymphadenectomy.
Indeed, differently from JGCA classification, which represents a comprehensive guidance for clinical practice, TNM classification does not provide “guidelines” for a correct management, if we exclude the recommendation of removing this minimum number of lymph nodes to obtain an accurate staging. In previous editions, TNM suggested that 15 lymph nodes at least had to be removed in order to reach an accurate staging of disease; latest TNM edition instead states that 16 nodes or more have to be ordinarily included in the histological examination of the specimen. It seems like this modification of the cut off is due more to the figure of 16 introduced for N3b rather than deriving from “numeric controversies” in literature. In fact, also pN0 definition in latest TNM edition is clear, confirming that even if the minimum number of retrieved nodes is not met, but all of the examined nodes are negative, the tumor has to be classified as pN0, so that it seems that the retrieval of at least 16 nodes is more a recommendation than a requirement for excluding lymph node involvement (pN0). Hence, this indulgence may be extended to evaluation of other pN categories as well; this trend is emerging from current policy of deleting pNx categories and from the lowering of the cut offs used to establish the N parameter. Since the numeric criterion cannot be applied radiologically and intraoperatively, the use of a number alone to determine pN status continues to hinder a tailored treatment before examination of surgical specimen.
This implies that lymph node dissection cannot purse a “minimum number” of harvested nodes, as this does not correspond to performing an extended lymphadenectomy (D2) according to eastern guidelines (7).
Consequently, last TNM edition may be more biased from stage migration phenomenon because of reduced pN thresholds and small ranges of different pN categories (8) (Table 1). For example, Ahn et al., analyzing survival rates of 9,998 surgically treated gastric cancer patients, reported stage migration phenomenon to occur in more than 20% of patients (9).
Full table
Actually, according to statistics of comparative studies, latest TNM for cancer of the stomach provided a more detailed classification of prognosis than the sixth one (9,10), but in attempt to solve the above mentioned problems, ratio of metastatic lymph nodes (LNR, defined as the number of involved nodes related to the number of all of the harvested nodes) has been proposed as a superior staging method for prediction of survival, allowing avoidance of stage migration phenomenon. Most studies concluded that LNR is superior to the conventional numeric criterion used in the TNM system in stratifying prognosis of gastric cancer patients (11-17). About that, Wang et al., comparing the latest TNM to a staging system including LNR, observed that LNR staging shows superiority to the seventh edition of pN staging in a series of 1,343 D2 gastrectomies (18). At least a correction of TNM with LNR, or other alternative node staging system, such as LODDs (logarithm of the ratio between the probability of a lymph node to be positive and the probability of a lymph node to be negative when only one node is retrieved) (19) or N score (another prognostic model that considers the differential impact of the number of examined nodes among pN0 and pN+ patients and the possible nonlinear interaction between total number of examined nodes and total number of pathologic lymph nodes) (20) have been proposed in cases where less than 15 nodes were dissected. However it seems that most of the attempts are made to justify a suboptimal surgery about lymphadenectomy, rather than to increase prognostic power of pathological lymph node staging (21,22).
In conclusion, AJCC/UICC TNM staging system does not seem to offer the best prognostic stratification according to lymph node status in gastric cancer patients if compared with LNR, N score and LODDs. Its main advantage of being easily applicable and reproducible is contrasted by a suboptimal stratification in patients with less than 15 nodes. Surgeons and pathologists have to make a great attempt to minimize this defect of the staging system, improving their surgical performance and their examination of lymph nodes in the surgical specimen, waiting for a further TNM edition where the pure numeric criterion could be improved by other reasonable factors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- International Union Against Cancer (UICC). TNM Classification of malignant tumours. 2nd ed. Geneva: UICC, 1974.
- Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th ed. New York: Wiley, 2002.
- Sobin LH, Gospodarowicz MK, Wittekind C. editors. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell, 2009.
- Sobin LH, Gospodarowicz MK, Wittekind C. UICC: TNM Classification of Malignant Tumors, 7th ed. Oxford: Wiley-Blackwell, 2010.
- Deng J, Liang H, Sun D, et al. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol 2010;17:1259-66. [Crossref] [PubMed]
- Ha TK, Kim HJ, Kwon SJ. Does the new UICC/AJCC TNM staging system (7th Edition) improve assessing prognosis in gastric cancer compared to the old system (6th Edition)? J Korean Gastric Cancer Assoc 2009;9:159-66.
- Rausei S, Dionigi G, Sano T, et al. Updates on surgical management of advanced gastric cancer: new evidence and trends. Insights from the First International Course on Upper Gastrointestinal Surgery--Varese (Italy), December 2, 2011. Ann Surg Oncol 2013;20:3942-7. [Crossref] [PubMed]
- Rausei S, Boni L, Rovera F, et al. Locally advanced gastric cancer: a new definition to standardise. J Clin Pathol 2013;66:164-5. [Crossref] [PubMed]
- Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010;116:5592-8. [Crossref] [PubMed]
- Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer 2011;14:166-71. [Crossref] [PubMed]
- Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg 2012;255:478-85. [Crossref] [PubMed]
- Wang X, Appleby DH, Zhang X, et al. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br J Surg 2013;100:505-14. [Crossref] [PubMed]
- Zhang BY, Yuan J, Cui ZS, et al. Evaluation of the prognostic value of the metastatic lymph node ratio for gastric cancer. Am J Surg 2014;207:555-65. [Crossref] [PubMed]
- Zhou Y, Zhang J, Cao S, et al. The evaluation of metastatic lymph node ratio staging system in gastric cancer. Gastric Cancer 2013;16:309-17. [Crossref] [PubMed]
- Wong J, Rahman S, Saeed N, et al. Prognostic impact of lymph node retrieval and ratio in gastric cancer: a U.S. single center experience. J Gastrointest Surg 2013;17:2059-66. [Crossref] [PubMed]
- Nelen SD, van Steenbergen LN, Dassen AE, et al. The lymph node ratio as a prognostic factor for gastric cancer. Acta Oncol 2013;52:1751-9. [Crossref] [PubMed]
- Medina-Franco H, Cabrera-Mendoza F, Almaguer-Rosales S, et al. Lymph node ratio as a predictor of survival in gastric carcinoma. Am Surg 2013;79:284-9. [PubMed]
- Wang W, Xu DZ, Li YF, et al. Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection--results of a single-institution study of 1343 Chinese patients. Ann Oncol 2011;22:2049-56.
- Sun Z, Xu Y. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer 2010;116:2571-80. [Crossref] [PubMed]
- Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543-52. [Crossref] [PubMed]
- Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Ruspi L, Galli F, Frattini F, et al. “perfect” lymph node staging system requires a “perfect” surgery. Transl Gastroenterol Hepatol 2016;1:10. [Crossref]
Cite this article as: Galli F, Ruspi L, Marzorati A, Lavazza M, Di Rocco G, Boni L, Dionigi G, Rausei S. N staging system: tumor-node-metastasis and future perspectives. Transl Gastroenterol Hepatol 2017;2:4.


