Splenic hilar dissection in the treatment of proximal advanced gastric cancer: what is an adequate strategy?
What is the best way to remove the splenic hilar lymph nodes (LNs)? Is splenectomy or splenic hilar LNs dissection really necessary for proximal advanced gastric cancer? These issues have been questionable for long time in the treatment strategy of proximal gastric cancer.
The incidences of LN metastasis around the splenic hilum (station No.10 according to the classification of Japanese Gastric Cancer Association) (1) in the patients with advanced gastric cancer involving the proximal stomach have been reported as 9–26% in the previous literatures (2-5). In the past, splenectomy was thought to be an ideal and indisputable mean to completely remove No.10 LNs. However, survival benefit of splenectomy for lymphadenectomy had been controversial. Furthermore, many investigators demonstrated that positive No.10 LN metastasis is a definitely unfavorable prognostic factor for the patients, which encouraged the importance of multidisciplinary therapy (2,6). Additionally, splenectomy has probabilities to cause several adverse events to the patient, loss of immunologic function and increased morbidity rate. In this context, clinical significances of splenectomy or splenic hilar dissection have been under debate.
History of splenectomy for splenic hilar lymphadenectomy
Lymphatic stream from the proximal stomach to the splenic hilum was demonstrated by classical dying method (7). Theoretically, there are two therapeutic significances in performing splenectomy. One is for complete lymphadenectomy of the splenic hilum, and the other is removal of the splenogastric ligament with neighboring adipose tissue to control local cancer cell spreading. Previously in Japan, pancreatosplenectomy had been advocated to accomplish complete removal of No.10 LNs or LNs along the splenic artery (station No.11). Next, pancreas-preserving total gastrectomy with splenectomy instead of such an extended procedure has been proved to have equivalent long-term outcomes with lower surgical morbidity rate, to be recognized as a standard procedure (8). In pancreas-preserving splenectomy, division point of the splenic artery was a crucial debatable issue. First, it was recommended to be divided at relatively proximal site of the splenic artery, commonly 5–6 cm from its root, to completely clear No.11p and 11d LNs, expecting compensated blood supply from the transverse pancreatic artery. However, gradually the proper division position has become thought to be more distal site to maintain blood supply or drainage at the preserved pancreas. Furthermore, the importance of preserving the pancreatic caudal vessels has also been emphasized considering the desirable conditions of the pancreas tail to prevent pancreatic leakage. Recently, preservation of the splenic artery and vein as long as possible is a main stream when performing splenectomy. Nowadays, pancreatosplenectomy is required only when the tumor directly invades the pancreas or metastatic bulky LNs exist at the splenic hilum, aiming at curative R0 resection. In spite of these efforts and measures, surgical morbidity rate after total gastrectomy with splenectomy has been reported as 20–45%, and mortality rate as 3–12% (9-11). These high incidences might be associated with undesirable condition of the pancreas tail after dissection as well as effects from its full mobilization from the retroperitoneal bed. It is pointed out that these associated morbidity and mortality are less recognized at well-experienced specialized center hospital. Actually, in western countries, splenectomy has been recommended to be avoided if possible (12). Another concern after splenectomy is reduction of immunologic function, which may be related with immunocompromised or cancer growing. As an alternative, spleen-preserving splenic hilar dissection has been applied in some experienced surgeons, which seems theoretically an ideal procedure. This procedure seems rational, but technical difficulties or oncological validity was raised as problematic points. Nevertheless, in the latest Japanese treatment guideline, splenectomy has been recommended as a radical surgery for proximal advanced gastric cancer regardless of the circumferential tumor location (13).
Current scientific evidences
A randomized controlled trial conducted by Japan Clinical Oncology Group (JCOG) was the largest that ever and also the most robust with a sufficient statistic power (14,15), comparing splenectomy versus non-splenectomy, though two other randomized controlled trials had been already published in single-institution setting with less sample-size (16,17). In JCOG0110, eligible criteria were proximal advanced gastric adenocarcinoma of cT2–4/N0–2 not invading the greater curvature line, and finally 505 patients were accrued (16). Splenectomy group had higher morbidity, but the 5-year survivals were not different in the two groups (75.1% and 76.4%; P=0.025). It concluded that splenectomy is associated with significantly increased invasiveness to the patients; and not necessary unless the tumor invades the greater curvature line (17). JCOG0110 was a well-designed clinical research, and provided us a clear insight. However, still, some clinical questions remain for us. In JCOG0110, only six patients (2.4%) in the splenectomy group had histological metastasis in No.10 LNs. Probably, the possible reasons are that its eligibility criteria excluded the patients with higher-possibility of No.10 metastasis. Interestingly, contrary to our expectations, splenectomy decreases survival in deeper tumors or patients with node metastasis. If so, is splenectomy necessary for the tumor invading the greater curvature, which is high risk subpopulation of No.10 metastasis? Can spleen-preserving splenic hilar dissection procedure replace splenectomy or these patients?
We have published a retrospective data of 421 patients’ outcomes after total gastrectomy with splenectomy for proximal advanced gastric cancer (2). The incidence of No.10 metastasis was 15.9% in patients with tumors invading the greater curvature, and the index to estimate benefit from No.10 LN dissection was 5.6, which relative high value indicated a certain survival benefit. Interestingly, the index was relatively high in patients aged <65 years, within pT3, and with type 4 tumors. These retrospective data indicates the clinical significance of the splenic hilar dissection for tumor invading the greater curvature line, however additional prospective studies are needed to reach final conclusions.
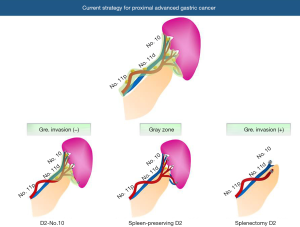
In clinical practice, now three types of procedures are available to manage LNs around the splenic hilar lesion as follows (Figure 1): (I) D2-No.10: spleen is preserved and No.10 LNs are almost left. The splenogastric ligament is divided very close to the splenic hilum to completely remove LNs of No.4sa or No.4sb station; (II) spleen-preserving D2: the branches of the splenic artery and vein in the splenic hilum are skeletonized to remove No.10 LNs. LNs at the posterior site of the splenic hilum tend to be left; (III) splenectomy D2: LNs at the splenic hilum are completely removed with the spleen. Maybe, an optimal procedure should be selected among them considering individual patient’s situation or tumor progression. However, surgeons should make utmost efforts to prevent postoperative morbidity, because many researches indicated that postoperative morbidity is correlated with worse long-term outcomes.
Future perspectives
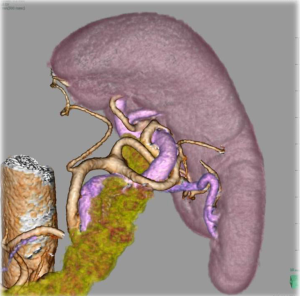
Obviously, surgical maneuver around the splenic hilum is technically difficult. In order to facilitate this procedure associated with its anatomical complexity, several new technological emergences seem promising. Laparoscopic surgery appears to be fundamentally suitable to approach this deeply located operative field without mobilization of the pancreas (18). In contrast, in open surgery, it is sometimes troublesome to approach this area because of poor visibility especially in obese patients. In addition, recently several high-performance energy devices with secure coagulation ability have been available, which enable more accurate dissection with less blood loss even in complex anatomical structures. Another problem which makes splenic hilar dissection embarrassing is the anatomical variation (19). Particularly, for spleen-preserving splenic hilar dissection, in which splenic vessels at the hilum should be skeletonized, its difficulty depends on the anatomical architecture. Li et al. reported that the difficulty of the laparoscopic spleen-preserving splenic hilar dissection depends on number of splenic lobar arteries and anatomical subtype (20). Huang et al. advocated anatomical classification of the splenic hilum with their many experiences, and reported their standardized techniques to perform laparoscopic spleen-preserving splenic hilar dissection (21). Three-dimensional (3D) laparoscopy has a potential to decrease technical difficulties in such complicated anatomical situations. Preoperative three-dimensional computed-tomography (3D-CT) anatomical reconstruction has been reported to be effective to assess diversities before surgery, which has a possibility to enhance surgical quality (22,23) (Figure 2). Robotic surgical instrument is thought to be also one of the promising devices to facilitate the procedures, because the forceps with articulating function may enable more precise dissection with optimal approaching angle as surgeons want. Actually, Son et al., has reported their excellent surgical outcome of spleen-preserving splenic hilar dissection using robotic surgical systems (24). Fluorescence endoscopic images using dying agents, such as indocyanine green, may have a potential to highlight LN in real-time around the splenic hilum, which will avoid leaving behind.
As described, scientific evidences have been accumulated regarding this issue. Nevertheless, several clinical questions still remain for us. When considering clinical studies regarding the tumors invading or limited to the greater curvature, such patients are not so common. Therefore, to answer these questions, prospective multi-institutional studies can be conducted in the future, probably global setting seems preferable.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Rulin Miao, MD [Key laboratory of Carcinogenesis and Translational Research (Ministry of education/Beijing), Gastrointestinal Tumor Center, Peking University Cancer Hospital & Institute, Beijing, China].
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Watanabe M, Kinoshita T, Enomoto N, et al. Clinical Significance of Splenic Hilar Dissection with Splenectomy in Advanced Proximal Gastric Cancer: An Analysis at a Single Institution in Japan. World J Surg 2016;40:1165-71. [Crossref] [PubMed]
- Okajima K, Isozaki H. Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 1995;19:537-40. [Crossref] [PubMed]
- Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 2001;76:89-92. [Crossref] [PubMed]
- Kosuga T, Ichikawa D, Okamoto K, et al. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer 2011;14:172-7. [Crossref] [PubMed]
- Zhu GL, Sun Z, Wang ZN, et al. Splenic hilar lymph node metastasis independently predicts poor survival for patients with gastric cancers in the upper and/or the middle third of the stomach. J Surg Oncol 2012;105:786-92. [Crossref] [PubMed]
- Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 1987;11:418-25. [Crossref] [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995;19:532-6. [Crossref] [PubMed]
- Kwon SJ. Prognostic impact of splenectomy on gastric cancer: results of the Korean Gastric Cancer Study Group. World J Surg 1997;21:837-44. [Crossref] [PubMed]
- Brady MS, Rogatko A, Dent LL, et al. Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg 1991;126:359-64. [Crossref] [PubMed]
- Griffith JP, Sue-Ling HM, Martin I, et al. Preservation of the spleen improves survival after radical surgery for gastric cancer. Gut 1995;36:684-90. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2016. [Epub ahead of print].
- Sano T, Yamamoto S, Sasako M, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 2002;32:363-4. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006;93:559-63. [Crossref] [PubMed]
- Csendes A, Burdiles P, Rojas J, et al. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 2002;131:401-7. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 2008;207:e6-11. [Crossref] [PubMed]
- Liu DL, Xia S, Xu W, et al. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery 1996;119:27-33. [Crossref] [PubMed]
- Li P, Huang CM, Lin JX, et al. A preoperatively predictive difficulty scoring system for laparoscopic spleen-preserving splenic hilar lymph node dissection for gastric cancer: experience from a large-scale single center. Surg Endosc 2016;30:4092-101. [Crossref] [PubMed]
- Huang CM, Chen QY, Lin JX, et al. Laparoscopic spleen-preserving no. 10 lymph node dissection for advanced proximal gastric cancer using a left approach. Ann Surg Oncol 2014;21:2051. [Crossref] [PubMed]
- Kinoshita T, Shibasaki H, Enomoto N, et al. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc 2016;30:2613-9. [Crossref] [PubMed]
- Wang JB, Huang CM, Zheng CH, et al. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol 2014;20:4797-805. [Crossref] [PubMed]
- Son T, Lee JH, Kim YM, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606-15. [Crossref] [PubMed]
Cite this article as: Kinoshita T. Splenic hilar dissection in the treatment of proximal advanced gastric cancer: what is an adequate strategy? Transl Gastroenterol Hepatol 2016;1:72.


