Intraperitoneal free cancer cells in gastric cancer: pathology of peritoneal carcinomatosis and rationale for intraperitoneal chemotherapy/hyperthermic intraperitoneal chemotherapy in gastric cancer
Peritoneal carcinomatosis (PC) occurs synchronous with the primary tumor in about 14–43% of patients with gastric cancer (GC) and accounts for 35% of all synchronous metastasis (1). Moreover, metachronous PC known as peritoneal recurrence occurs in 10–46% of patients after a curative surgery for GC and accounts for 36–45% of all recurrences (1,2). The peritoneum is the first/sole site of tumor recurrence after D2 gastrectomy in 12–40% of patients (3). The prognosis of PC from GC is worse than other forms of metastases, with a median survival of only about 6 months and a 5-year survival of 0% (1,2,4).
Intraperitoneal free cancer cells (IFCCs), first exfoliating from primary tumor mass to the abdominal cavity, then attaching to peritoneal surface, and finally invading into the subperitoneal tissue to form proliferating nodules, are the most fundamental pathophysiology for both synchronous and metachronous PC (5,6).
The understanding that PC is a locoregional rather than a systemic disease has led to a resurgence of interest in regional therapies like cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC)/hyperthermic intraperitoneal chemotherapy (HIPEC) (7). Given the etiology of GC PC, it seems logic and promising to apply IPC/HIPEC as an essential component of the integrated treatment strategy.
This review focuses on the origin of IFCCs, the mechanism of PC formatting, the rationale of IPC/HIPEC, and the current clinical trials on IPC/HIPEC to treat advanced gastric cancer patients.
Origin of IFCCs
PC usually originates from IFCCs which in turn can occur from two different sources (7-9): (I) spontaneous exfoliation of cancer cells from the primary tumor as a result of the natural course of cancer invasion; and (II) iatrogenic dissemination of cancer cells due to ineffective tumor-free technique during surgical resection.
The IFCCs positive rate in abdominal cytology increases as the tumor invades deeply from mucosa to serosa. IFCCs can be seen in up to 24% patients with stage I and 40% patients with stage II or III GC (10). Moreover, Lee et al. (11) found that the IFCCs positive rate is significantly correlated with T3/T4 stage and positive lymph nodes. Surgery also plays a part in dissemination of tumor cells into the peritoneal cavity. During radical surgery for GC, cancer cells are released from transected lymphatic channels, tissue at the narrow margins of resection, and tumor-contaminated blood lost in the surgical field from the cancer specimen (7,12,13).
Mechanism of PC formation
Two different processes are proposed in the formation of peritoneal dissemination, designated as “transmesothelial” and “translymphatic” metastases by Yonemura et al. (14). Both processes start with the dissemination and survival of IFCCs.
Transmesothelial metastasis originates from the direct attachment of IFCCs on the mesothelium. Most IFCCs attach to the mesothelial cells die off because of poor nutrient environment as the result of strong attachment to each other of the peritoneal mesothelial cells and peritoneal-blood barrier. However, once IFCCs loosely attach to the mesothelial cells with adhesion molecules like CD44, cytokines are released to contract mesothelial cells by the phosphorylation of their cell skeleton (15). As a result, IFCCs migrate directly into the submesothelial space through the cleaved space between mesothelial cells and strongly attach to the exposed basement membrane by the expression of integrin molecules (16).
After the first step, IFCCs express motility factors and matrix proteinases to degrade the peritoneal-blood barrier and invade deeper into the subperitoneal tissue (17,18). When IFCCs invade near the subperitoneal capillary, they proliferate via autocrine or paracrine loop by the production of growth factors from cancer cells or stromal cells. Furthermore, angiogenic factors like VEGF-A and VEGF-C secreted from IFCCs induce neovascularization in the subperitoneal tissue (19). As a result, the integrity of the peritoneal-blood barrier is broken to create a ready soil for establishing PC.
Translymphatic metastasis via lymphatic orifices opening on the peritoneal surface, requires fewer steps and forms PC earlier than transmesothelial metastasis. IFCCs migrate into the lymphatic orifices and proliferate in the submesothelial lymphatic space beneath the lymphatic stomatas. The distribution of lymphatic orifices on the peritoneum is uneven. There are many lymphatic orifices on the greater omentum, appendices, epiploicae of the colon, inferior surface of the diaphragm, falciform ligament, Douglas’ pouch, and small bowel mesentery. Accordingly, PC occurs earlier in these places (14). In contrast, there are much fewer lymphatic stomatas on the liver capsule, the peritoneum covering the abdominal wall, or the serosal surface of small bowel and splenic capsule. These peritoneal parts are not affected until late stages of peritoneal dissemination.
Rationale for IPC/HIPEC
The presence of plasm-peritoneal barrier makes the peritoneal cavity a relatively closed space lacking blood vessels, accounting for the poor effect of intravenous chemotherapy (IVC). IPC/HIPEC is designed according to the function of plasm-peritoneal barrier, to achieve a high intraperitoneal concentration of chemotherapeutics with a low plasma concentration. Morgan et al. (20) found that the median peak peritoneal concentration of Gemcitabine was 1,116 folds (range, 456–1,886 folds) higher than the peak plasma level. This positive gradient of chemotherapy in the peritoneum could intensifying its direct antitumor effect and effectively eradicate IFCCs, micrometastases, and tumor nodules with less systemic adverse effects. Moreover, the drugs administered into the peritoneal cavity are ultimately absorbed through the portal vein into the liver and may have anti-tumor effect on liver micrometastasis as well (21).
Hyperthermia itself has direct detrimental effects on IFCCs, and also enhances the effects of IPC (22). The tolerance of normal tissue and cancer tissue to hyperthermia is different. Temperature over 43 °C has direct cytostatic effect on human gastric carcinoma SGC-7901 cells (23,24), colorectal carcinoma HT-29 cells (25), and ovarian SKOV-3 cells (26). Several mechanisms account for the multiple adverse effects of hyperthermia on IFCCs. First, hyperthermia causes tumor microvessel embolism at the tissue level, resulting in ischemic necrosis of tumor tissue. Second, hyperthermia disturbs cancer cell homeostasis and energy metabolism, activates the lysosomes, and destroys the cytoplasm and nucleus, directly killing cancer cells in S and M phases of the cell cycle. Third, hyperthermia also disrupts cancer cell membrane proteins at the molecular level, and interferes with the synthesis of DNA, RNA and protein. Hyperthermia enhances the effects of IPC in two ways. Firstly, hyperthermia increases the cytotoxic activity of the chemotherapy by a synergistic effect. Secondly, hyperthermia increases the penetration of chemotherapy drugs into the tumor nodule, increases the drug uptake in the tumor cells and increases the chemosensibility of neoplastic cells (27-29).
Clinical trials of IPC/HIPEC to treat gastric cancer
IPC/HIPEC has been increasingly applied in gastric cancer patients and can be categorized into three types according to the different purposes and timing of administration: neoadjuvant IPC (Table 1) (30-36), prophylactic IPC/HIPEC for gastric cancer patients with serosa invasion (Table 2) (37-53), and therapeutic IPC/HIPEC for gastric cancer patients with macroscopic PC (Table 3) (42,47,54-67).
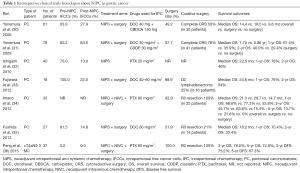
Full table
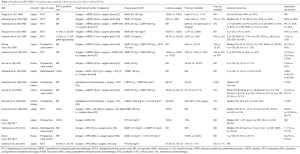
Full table

Full table
Assessment methods for IPC/HIPEC
In the past, the clinical outcome was the only method to evaluate the efficacy of IPC/HIPEC. This method is the most authoritative, but it takes a very long time, which may limit the development of IPC/HIPEC. IFCCs are the recognized root pathologic cause for PC and the technique of detecting IFCCs has been developed in recent years. Ji et al. (5) tried to assess the efficacy of HIPEC by detecting pre- and post-HIPEC IFCCs viability using traditional cytological method and RT-PCR method. This new method makes it possible to rapidly evaluate the efficacy of IPC/HIPEC, and it can be used in routine clinical setting to evaluate patients’ response to IPC/HIPEC and predict the prognosis, and in preclinical research of evaluate new chemotherapeutic agents or new treatment strategies.
Future perspectives and conclusions
From perspectives of clinical oncology, there are literally three forms of cancer metastases, i.e., lymph-route metastasis, blood-route metastasis, and peritoneal metastasis. For lymph-route metastasis, universally adopted treatment guidelines and technical standards have long been established, and surgery undoubtedly plays a major role. For blood-route metastasis, such as liver metastasis from colorectal cancer, it has also been universally accepted that that surgery is the treatment of choice for selected patients with limited liver metastases and good function reserve (68). Compared with these two types of cancer metastasis, peritoneal metastasis is the last stronghold of cancer. Confronted with such a tremendous adverse, the oncology community in whole still remains pessimistic, with palliative approaches gaining no concrete benefits other than psychologic consoling to both patients and the doctors alike.
Thanks to the groundbreaking works of the early pioneers in the field of peritoneal metastasis, the landscape is changing, slowly but steadily for the better. The first generation pioneers such as Dr. Sugarbaker (69) from the United States, Dr. Elias (70) from France, Dr. Deraco (71) from Italy, and Dr. Yonemura (72) from Japan have paved the way for the right direction. Dr. Piso (73) from Germany, Dr. Glehen (74) from France, Dr. Morris (75) from Australia, Dr. Verwaal (76) from the Netherlands, Dr. Li (77) from China and many others have also contributed enormously to push the endeavor to a new height. Currently, specialized PC treatment centers for standardized HIPEC treatments have been established in many parts of the world. A broad united front against GC PC have been established and increasingly higher levels of evidence has been obtained. It has been unequivocally proved that IPC/HIPEC is the most effective treatment strategy for GC PC, and more future work is required to promote this comprehensive treatment.
Acknowledgements
Funding: This study was supported by the Key Discipline Development Fund of Beijing Shijitan Hospital affiliated to the Capital Medical University (2016fmzlwk), and the Special Fund for the Capital Characteristic Clinical Medicine Development Project (Z161100000516077) (both to Yan Li).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622-8. [Crossref] [PubMed]
- Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236-42. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol 2016;22:1114-30. [Crossref] [PubMed]
- Ji Z, Sun J, Wu H, et al. Assessment of Hyperthermic Intraperitoneal Chemotherapy to Eradicate Intraperitoneal Free Cancer Cells. Transl Oncol 2016;9:18-24. [Crossref] [PubMed]
- Yonemura Y, Endou Y, Fujimura T, et al. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg 2001;71:521-8. [Crossref] [PubMed]
- Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol 2003;21:233-48. [Crossref] [PubMed]
- Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol 1984;108:236-8. [Crossref] [PubMed]
- Iitsuka Y, Kaneshima S, Tanida O, et al. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer 1979;44:1476-80. [Crossref] [PubMed]
- Juhl H, Stritzel M, Wroblewski A, et al. Immunocytological detection of micrometastatic cells: comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal and pancreatic cancer patients. Int J Cancer 1994;57:330-5. [Crossref] [PubMed]
- Lee SD, Ryu KW, Eom BW, et al. Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg 2012;99:397-403. [Crossref] [PubMed]
- Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011;18:2818-25. [Crossref] [PubMed]
- Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res 2003;9:678-85. [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res 2007;169:11-23. [PubMed]
- Yonemura Y, Endou Y, Nojima M, et al. A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol 1997;11:349-58. [PubMed]
- Yonemura Y, Endou Y, Yamaguchi T, et al. Roles of VLA-2 and VLA-3 on the formation of peritoneal dissemination in gastric cancer. Int J Oncol 1996;8:925-31. [PubMed]
- Takaishi K, Sasaki T, Kato M, et al. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene 1994;9:273-9. [PubMed]
- Kaji M, Yonemura Y, Harada S, et al. Participation of c-met in the progression of human gastric cancers: anti-c-met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther 1996;3:393-404. [PubMed]
- Yonemura Y, Endo Y, Tabata K, et al. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol 2005;10:318-27. [Crossref] [PubMed]
- Morgan RJ Jr, Synold TW, Xi B, et al. Phase I trial of intraperitoneal gemcitabine in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Clin Cancer Res 2007;13:1232-7. [Crossref] [PubMed]
- Speyer JL, Sugarbaker PH, Collins JM, et al. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res 1981;41:1916-22. [PubMed]
- González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol 2010;2:68-75. [Crossref] [PubMed]
- Zhou NX, Jiang YY, Wen ZM, et al. Biologic effects of hyperthermia and radiation on gastric cancer cells (SGC-7901) in vitro. II. Ultrastructural changes of cells. Zhonghua Zhong Liu Za Zhi 1987;9:176-8, 12.
- Zhou NX, Jiang YY, Wen ZM. Biologic effects of hyperthermia and radiation on gastric cancer cells (SGC-7901) in vitro--I. Influence on cell growth curve, number of colonies and mitosis. Zhonghua Zhong Liu Za Zhi 1987;9:17-20. [PubMed]
- Vorotnikova E, Ivkov R, Foreman A, et al. The magnitude and time-dependence of the apoptotic response of normal and malignant cells subjected to ionizing radiation versus hyperthermia. Int J Radiat Biol 2006;82:549-59. [Crossref] [PubMed]
- Michalakis J, Georgatos SD, de Bree E, et al. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann Surg Oncol 2007;14:1220-8. [Crossref] [PubMed]
- Overgaard J. Effect of hyperthermia on malignant cells in vivo. A review and a hypothesis. Cancer 1977;39:2637-46. [Crossref] [PubMed]
- Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am 2003;12:689-701. [Crossref] [PubMed]
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 2004;5:219-28. [Crossref] [PubMed]
- Yonemura Y, Bandou E, Sawa T, et al. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol 2006;32:661-5. [Crossref] [PubMed]
- Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol 2009;100:311-6. [Crossref] [PubMed]
- Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol 2010;21:67-70. [Crossref] [PubMed]
- Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol 2012;105:38-42. [Crossref] [PubMed]
- Imano M, Yasuda A, Itoh T, et al. Phase II study of single intraperitoneal chemotherapy followed by systemic chemotherapy for gastric cancer with peritoneal metastasis. J Gastrointest Surg 2012;16:2190-6. [Crossref] [PubMed]
- Fushida S, Kinoshita J, Kaji M, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol 2013;71:1265-72. [Crossref] [PubMed]
- Peng YF, Imano M, Itoh T, et al. A phase II trial of perioperative chemotherapy involving a single intraperitoneal administration of paclitaxel followed by sequential S-1 plus intravenous paclitaxel for serosa-positive gastric cancer. J Surg Oncol 2015;111:1041-6. [Crossref] [PubMed]
- Koga S, Hamazoe R, Maeta M, et al. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 1988;61:232-7. [Crossref] [PubMed]
- Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994;73:2048-52. [Crossref] [PubMed]
- Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 1994;18:150-5. [Crossref] [PubMed]
- Ikeguchi M, Kondou A, Oka A, et al. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg 1995;161:581-6. [PubMed]
- Fujimoto S, Takahashi M, Mutou T, Kobayashi K, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999;85:529-34. [Crossref] [PubMed]
- Hirose K, Katayama K, Iida A, et al. Efficacy of continuous hyperthermic peritoneal perfusion for the prophylaxis and treatment of peritoneal metastasis of advanced gastric cancer: evaluation by multivariate regression analysis. Oncology 1999;57:106-14. [Crossref] [PubMed]
- Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 2001;48:1776-82. [PubMed]
- Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). Gastric Cancer 2001;4:27-33. [Crossref] [PubMed]
- Shimada S, Tanaka E, Marutsuka T, et al. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer 2002;5:168-72. [Crossref] [PubMed]
- Newman E, Potmesil M, Ryan T, et al. Neoadjuvant chemotherapy, surgery, and adjuvant intraperitoneal chemotherapy in patients with locally advanced gastric or gastroesophageal junction carcinoma: a phase II study. Semin Oncol 2005;32:S97-100. [Crossref] [PubMed]
- Zhu ZG, Tang R, Yan M, et al. Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion. A long-term follow-up study. Dig Surg 2006;23:93-102. [Crossref] [PubMed]
- Brenner B, Shah MA, Karpeh MS, et al. A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locally advanced gastric cancer. Ann Oncol 2006;17:1404-11. [Crossref] [PubMed]
- Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. [Crossref] [PubMed]
- Imano M, Imamoto H, Itoh T, et al. Impact of intraperitoneal chemotherapy after gastrectomy with positive cytological findings in peritoneal washings. Eur Surg Res 2011;47:254-9. [Crossref] [PubMed]
- Yarema RR, Ohorchak MA, Zubarev GP, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: results of a single-centre retrospective study. Int J Hyperthermia 2014;30:159-65. [Crossref] [PubMed]
- Kwon OK, Chung HY, Yu W. Early postoperative intraperitoneal chemotherapy for macroscopically serosa-invading gastric cancer patients. Cancer Res Treat 2014;46:270-9. [Crossref] [PubMed]
- Kodera Y, Takahashi N, Yoshikawa T, et al. Feasibility of weekly intraperitoneal versus intravenous paclitaxel therapy delivered from the day of radical surgery for gastric cancer: a preliminary safety analysis of the INPACT study, a randomized controlled trial. Gastric Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg 1991;15:530-5; discussion 535-6. [Crossref] [PubMed]
- Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996;119:437-44. [Crossref] [PubMed]
- Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997;79:884-91. [Crossref] [PubMed]
- Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004;139:20-6. [Crossref] [PubMed]
- Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454-63. [Crossref] [PubMed]
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370-5. [Crossref] [PubMed]
- Cheong JH, Shen JY, Song CS, et al. Early postoperative intraperitoneal chemotherapy following cytoreductive surgery in patients with very advanced gastric cancer. Ann Surg Oncol 2007;14:61-8. [Crossref] [PubMed]
- Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 2008;34:1246-52. [Crossref] [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [Crossref] [PubMed]
- Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol 2010;101:457-64. [Crossref] [PubMed]
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [Crossref] [PubMed]
- Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2014;21:1448-55. [Crossref] [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [Crossref] [PubMed]
- Wu HT, Peng KW, Ji ZH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur J Surg Oncol 2016;42:1024-34. [Crossref] [PubMed]
- Orcutt ST, Massarweh NN, Li LT, et al. Patterns of Care for Colorectal Liver Metastasis Within an Integrated Health System: Secular Trends and Outcomes. Ann Surg Oncol 2016. [Epub ahead of print].
- Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat Rev 2016;48:42-9. [Crossref] [PubMed]
- Elias D, Goéré D, Dumont F, et al. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer 2014;50:332-40. [Crossref] [PubMed]
- Kusamura S, Baratti D, Deraco M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 2012;255:348-56. [Crossref] [PubMed]
- Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 2016;42:1123-31. [Crossref] [PubMed]
- Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2009;7:5. [Crossref] [PubMed]
- Passot G, Vaudoyer D, Villeneuve L, et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experiece with 1,125 procedures. J Surg Oncol 2016;113:796-803. [Crossref] [PubMed]
- Alzahrani N, Ferguson JS, Valle SJ, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: long-term results at St George Hospital, Australia. ANZ J Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [Crossref] [PubMed]
- Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016;22:6906-16. [Crossref] [PubMed]
Cite this article as: Ji ZH, Peng KW, Li Y. Intraperitoneal free cancer cells in gastric cancer: pathology of peritoneal carcinomatosis and rationale for intraperitoneal chemotherapy/hyperthermic intraperitoneal chemotherapy in gastric cancer. Transl Gastroenterol Hepatol 2016;1:69.


