HER2/neu as target in gastric adenocarcinoma
Gastric adenocarcinoma (GAC), a heterogeneous disease characterized by epidemiologic and histopathologic differences across countries, is a leading cause of cancer-related death. The modest activity and substantial toxicity of cytotoxic chemotherapy has raised the question, does palliative systemic therapy with available agents have clinical utility? In the setting of metastatic disease, many active chemotherapy agents can produce meaningful response alone or in combination with other agents, but the duration of response is often limited. Recent additions to the armamentarium include trastuzumab and ramucirumab, which have shown some survival advantage when added to cytotoxic(s). The HER2 proto-oncogene is the gene encoding the human EGF receptor 2. HER2 amplification or protein overexpression [found in 20% of gastric cancer (GC)] is clearly associated with accelerated cell growth and proliferation and the response to the monoclonal anti-HER2 antibody, trastuzumab. HER2 was more likely to be positive in patients with esophagogastric junction (EGJ) tumors than in more distal tumors (33% versus 20%); patients with diffuse GC were much less likely to have an HER2-positive (6%) tumor (1).
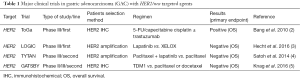
Table 1 summarizes representative important clinical trial results in HER2/neu-positive GAC. The preliminary results of the ToGA trial have been recently reported (2). Among 3,807 patients, 594 patients had HER2-positive GC. They were randomized to receive either 5-FU/cisplatin or capecitabine/cisplatin given every 3 weeks for six cycles or the same chemotherapy plus trastuzumab. The median OS was 13.8 months in the patients treated with trastuzumab plus chemotherapy and 11.1 months in the patients treated with chemotherapy. The most effective overall survival (OS) was seen in the immunohistochemical (IHC)-3+ group, with an HR of 0.66. In the IHC-2+ group, there was a trend toward benefit but it was not statistically significant, and treatment with trastuzumab was ineffective in the last group of patients, the 22% with IHC-0 or 1+. Trastuzumab has subsequently has been incorporated as a standard therapeutic options for patients with this disease. However, the benefit from trastuzumab diminished considerably when the results were reanalyzed after a longer follow-up by the U.S. Food and Drug Administration (the hazard ratio increased from 0.73 to 0.80, and the OS difference narrowed to a meager 1.4 months).
Full table
Lapatinib is the first dual inhibitor of HER1 (EGFR1) and HER2 (EGFR2). In contrast to the ToGA trial of the therapeutic antibody trastuzumab, results have been disappointing for HER2 directed kinase inhibitors. Two trials studied the reversible EGFR/ERBB2 inhibitor lapatinib in the second-line setting (4) and in the first-line setting (3), both yielding negative results. Lapatinib was evaluated in the phase III TYTAN study in the 2nd line setting for advanced GC. In Asian patients, 261 patients were randomly assigned to receive either weekly paclitaxel or paclitaxel + lapatinib and addition of lapatinib was not associated with an improvement in survival (P=0.104) (4). Benefit from the addition of lapatinib to first-line chemotherapy was also not shown in the TRIO013/LOGIC trial of capecitabine /oxaliplatin with or without lapatinib for first-line treatment in 545 patients with advanced gastroesophageal cancer (3). The primary end point of OS was 12.2 months compared with 10.5 months, with an HR of 0.9, but was not statistically significant (P=0.35). In prespecified subgroup analysis, Asian patients (median survival 16.5 versus 10.9 months, HR 0.68) and those under age 60 (median survival 12.9 versus 9 months, HR 0.69) seemed to benefit from lapatinib. Additionally, a phase III study of apatinib versus placebo was reported. This study randomized 267 patients who had progressed on at least two lines of therapy to apatinib (n=181 patients) or placebo (n=92). Patients assigned to apatinib experienced a longer median overall survival (6.5 versus 4.7 months, HR 0.709, P=0.0149) (6). Until further information becomes available, we suggest not using lapatinib as the initial therapy for the treatment of advanced esophagogastric cancer.
The current use of molecular diagnostics, specifically gene expression arrays, is leading to an explosion of subcategories, so that by the second decade of the new millennium, several hundred distinct neoplastic disease entities are likely to be recognized, each following its own, reasonably predictable clinical course and exhibiting its own responsiveness to specific forms therapy. With the passage of time, cancer diagnoses will increasingly be made using bioinformatics rather than the trained eyes of a pathologist. Based upon observations from the molecular data, the The Cancer Genome Atlas (TCGA) team proposed a classification system where GAC is divided into four subtypes: EBV-positive, microsatellite-unstable, genomically stable and chromosomal instability (CIN) (7). Mesenchymal-like GCs had the worst prognosis, followed by TP53-inactive, TP53-active and finally microsatellite-instability tumors. Cell cycle-related genes such as CCND1, CCNE1 and CDK6 are commonly amplified in GC. For instance, CCNE1 is frequently co-amplified with HER2 (8) and GC patients with CCNE1/HER2 co-amplification typically developed resistance to lapatinib, a small molecular HER2 inhibitor (9).
The level of HER2 gene amplification significantly predicts sensitivity to therapy and overall survival in advanced GC treated with trastuzumab-based chemotherapy. A mean HER2/CEP17 ratio of 4.7 was identified as the optimal cutoff value discriminating sensitive and refractory patients (P=0.005). Similarly, the optimal cutoff for predicting survival longer than 12 months was 4.45 (P=0.005), and for survival longer than 16 months was 5.15 (P=0.004) (10). High levels of HER2 amplification should be considered as a predictive biomarker in LOGIC patients. These data not yet available but it should be dealt with in a separate article in the future.
In terms of guidelines, patients with HER2-positive metastatic disease would be considered eligible if they were IHC 3+ or 2+ fluorescence in situ hybridization (FISH) positive or 2+ IHC with in situ hybridization positivity. The criteria for HER2 positivity on surgical and biopsy specimens are of some measure of complication and differ slightly based on GC as opposed to breast cancer, particularly because the expression in GC is somewhat spottier and less diffuse as opposed to breast cancer. The potential adequacy of a single small biopsy to accurately assess HER2 status is questionable as opposed to a surgical specimen (11-13). How many unique specimens should be tested also remains unresolved (14-16). Similarly, the relationship between the level of HER2 amplification and the outcome of HER2-positive GC treated with anti HER2/neu agents remains unclear (17). In addition, whether there is concordance in HER2/neu results if metastatic cancer or primary cancer is tested, remains generally unresolved (18-20). Today, the association between HER2 expression/amplification and prognosis in esophagogastric cancer remains unknown.
So despite the failure of lapatinib, other approaches for HER2-positive disease are under way, again based on the benefits of various approaches in breast cancer. Trastuzumab emtansine (T-DM1) is a novel antibody-drug conjugate. T-DM1 consists of the cytotoxic agent DM1 (derivative of maytansine) linked to trastuzumab. The EMILIA trial for patients with HER2/neu-positive breast cancer was positive, resulting in a survival benefit of 5.8 months; however, a T-DM1 trial for HER2/neu-positive GAC in the second-line setting was a disappointment (5). Pertuzumab is that monoclonal antibody that blocks the dimerization of HER2 to other ERBB family components and therefore has been shown to be effective when added to trastuzumab in HER2-positive breast cancer. One question is: is there a benefit of adding pertuzumab to trastuzumab in HER2-positive GC? The JACOB study included patients with previously untreated HER2-positive gastric and GE-junction adenocarcinoma who were randomized to standard fluoropyrimidine, cisplatin, trastuzumab treatment, plus or minus pertuzumab. It is being evaluated in an ongoing clinical trial that is expected to conclude by the end of 2016. Ultimately, the biggest challenge of drug development now and in the future is to demonstrate long-term efficacy: does a drug being tested have significant effects on extending the life expectancy of cancer patients, doing so with acceptable levels of side-effect toxicities? And do we dare to hope that it can achieve durable responses, including cures?
Summary and recommendations
GC remains an important problem worldwide. The majority of patients with esophageal or GC will require palliative treatment at some point in the course of their disease. In recent years, a better understanding of the biological properties of tumors and the development and application of molecular targeted drugs have created hope for the individualized treatment of advanced GC. At the present time, trastuzumab remains the only drug of this type that has demonstrated efficacy, in combination with cytotoxic chemotherapy, in GC. The National Comprehensive Cancer Network (NCCN) suggests the addition of trastuzumab to chemotherapy in patients with HER2-positive tumors (as defined by 3+ IHC staining or FISH positivity), as long as they do not have a contraindication to trastuzumab. The future prospects are excellent for defining biomarker based subsets of patients and the application of specific therapeutics.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Rulin Miao, MD [Key laboratory of Carcinogenesis and Translational Research (Ministry of education/Beijing), Gastrointestinal Tumor Center, Peking University Cancer Hospital & Institute, Beijing, China].
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bang Y, Chung H, Sawaki A, et al. HER2-positivity rates in advanced gastric cancer (GC): results from a large international phase III trial. J Clin Oncol 2008;26: abstr 4526.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol 2016;34:443-51. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Knag YK, Shah MA, Ohtsu A, et al. A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine (T-DM1) versus a taxane (TAX) in patients (pts) with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma (LA/MGC/GEJC). J Clin Oncol 2016;34: abstr 5.
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012;61:673-84. [Crossref] [PubMed]
- Kim J, Fox C, Peng S, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest 2014;124:5145-58. [Crossref] [PubMed]
- Gomez-Martin C, Plaza JC, Pazo-Cid R, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol 2013;31:4445-52. [Crossref] [PubMed]
- Yoshida H, Yamamoto N, Taniguchi H, et al. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch 2014;465:145-54. [Crossref] [PubMed]
- Wang T, Hsieh ET, Henry P, et al. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol 2014;45:970-5. [Crossref] [PubMed]
- van Hagen P, Biermann K, Boers JE, et al. Human epidermal growth factor receptor 2 overexpression and amplification in endoscopic biopsies and resection specimens in esophageal and junctional adenocarcinoma. Dis Esophagus 2015;28:380-5. [Crossref] [PubMed]
- Grillo F, Fassan M, Ceccaroli C, et al. The Reliability of Endoscopic Biopsies in Assessing HER2 Status in Gastric and Gastroesophageal Junction Cancer: A Study Comparing Biopsies with Surgical Samples. Transl Oncol 2013;6:10-6. [Crossref] [PubMed]
- Ge X, Wang H, Zeng H, et al. Clinical significance of assessing Her2/neu expression in gastric cancer with dual tumor tissue paraffin blocks. Hum Pathol 2015;46:850-7. [Crossref] [PubMed]
- Qiu Z, Sun W, Zhou C, et al. HER2 expression variability between primary gastric cancers and corresponding lymph node metastases. Hepatogastroenterology 2015;62:231-3. [PubMed]
- Kumarasinghe MP, de Boer WB, Khor TS, et al. HER2 status in gastric/gastro-oesophageal junctional cancers: should determination of gene amplification by SISH use HER2 copy number or HER2: CEP17 ratio? Pathology 2014;46:184-7. [Crossref] [PubMed]
- Kochi M, Fujii M, Masuda S, et al. Differing deregulation of HER2 in primary gastric cancer and synchronous related metastatic lymph nodes. Diagn Pathol 2013;8:191. [Crossref] [PubMed]
- Cho EY, Park K, Do I, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol 2013;26:677-84. [Crossref] [PubMed]
- Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 2011;104:1372-6. [Crossref] [PubMed]
Cite this article as: Cetin B, Ozet A. HER2/neu as target in gastric adenocarcinoma. Transl Gastroenterol Hepatol 2016;1:59.


