Reduced port laparoscopic gastrectomy for gastric cancer
Introduction
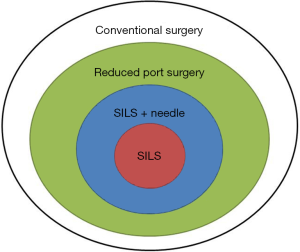
The use of reduced port laparoscopic surgery (RPS) has been increasing recently (1). RPS involves fewer ports than standard laparoscopic surgery and can allow for needlescopic surgery through narrower ports (2) by involving single-incision laparoscopic surgery (SILS). SILS, which is performed from a single incision at the umbilicus, can be considered the ultimate reduced-port technique. Needlescopic surgery and SILS were introduced almost simultaneously in the 1990s (3,4). Although they were originally used for surgical treatment of benign diseases, with the advances in techniques and devices, their applications have expanded to include malignant diseases such as colorectal and gastric cancers (5-8). Now, the concepts of SILS, RPS, and needlescopic surgery have been combined and are difficult to distinguish from each other (Figure 1). The term RPS integrates these concepts and is considered to be derived from the various efforts aimed at minimally invasive surgery.
The history of RPS use for malignant diseases such as gastric cancer is short, and its usefulness has not yet been fully established. This review describes the present concept, situations, and challenges of RPS for gastric resection of gastric cancer, and these issues are presented in light of the existing literature.
Concept and history of reduced-port laparoscopic gastrectomy for gastric cancer
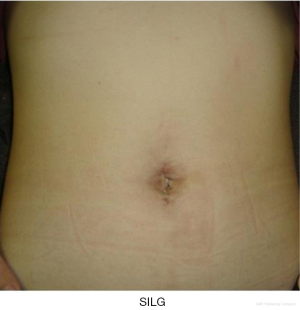
Laparoscopic surgery is a minimally invasive treatment, and its widely known advantages include reduced bleeding and its contribution to minute lymph node dissection (9,10). In the reduced-port laparoscopic gastrectomy (RPLG) concept, when laparoscopic surgery is performed for gastric cancer, the resected specimen and retrieved lymph nodes are usually extracted in a plastic bag through the umbilicus or another incision. In general, the incision must be at least around 3 cm. Therefore, gastric resection performed through an incision no larger than 3 cm can be the ultimate single-incision laparoscopic gastrectomy (SILG).
The application of RPS to gastric cancer was first reported by Omori et al. (7). Since then, many RPLG techniques have been reported (11-18). Reports have described the feasibility of RPLG and the techniques in detail. The minimal invasiveness, associated postoperative pain, and cosmetic outcomes of RPLG are reported to be similar to those of conventional laparoscopic gastrectomy (CLG). Interestingly, Kawamura et al. (16) reported that the amount of oral intake during the early postoperative period in RPLG exceeds that in CLG.
Many reports have indicated that the grade of lymph node dissection in RPLG also does not differ from that in CLG. Although the prospect for radical cure is thought to be not inferior, no long-term studies have confirmed this prediction. Lee et al. (18) verified the non-inferiority of RPLG to CLG in an animal study; the incidences of inflammatory reactions and complications were similar to those in CLG. We anticipate that such results will soon be verified in clinical studies.
The application of RPLG to gastric cancer remains controversial, and critical comments have been made regarding this (19). The fourth edition of the Japanese gastric cancer guidelines (20) states that laparoscopic surgery for gastric cancer is an option for patients with stage I disease, although the long-term results of the JCOG0912 phase III and KLASS-01 trials are awaited (21-23). In such a situation, consensus is difficult to reach on the usefulness of RPS, which is technically more challenging. Moreover, the benefit and effect of RPLG in comparison with those of CLG and open surgery should be discussed. Whether performing RPS requires special training and can be established as a standard operation remains to be elucidated.
The educational issue should also be considered for junior surgeons. Naturally, such an operation is technically difficult to perform from a single incision. Laparoscopic surgery requires the use of forceps, which restricts the view on the two-dimensional monitor and thus requires its own particular skill set. The degree of restriction is increased in RPS. Therefore, skilled surgeons who can perform laparoscopic surgery are needed (24). Generally, it is desirable that surgeons with adequate experience in ordinary laparoscopic gastric resection perform RPS as the next step.
One of the technical solutions for the oncological and educational problems described earlier is the use of thinner forceps such as needle forceps, which are generally 3 mm or less in diameter (25,26). These needle forceps are useful for establishing the working angle in each procedure, which contributes to maintaining the quality of lymphadenectomy and reconstruction. They also keep the assistant motivated to participate in the operation by holding the needle forceps, which can contribute to the education of junior surgeons. Moreover, the cosmetic outcome and degree of invasiveness could be maintained even if these needle forceps are added.
Authors have also ranked RPS from standpoints other than the cosmetic outcome and degree of invasiveness. In physically small patients, the area of the abdominal cavity that receives the ports is small. If ports of conventional size and number are used, forceps interfere with each other; and as a result, the target angle needed to reach an organ is difficult to achieve.
In this case, if forceps are centralized in a single incision at the umbilicus, we have to resolve the problem of interference, but the target angle is easy to achieve. We overcome the problem by inserting a total of three needle forceps, one held in the operator’s left hand, and two held in the assistant’s two hands, from incisions other than those at the umbilicus. We have adopted the concept of needle-assisted surgery. The mobilization of internal organs and the procedure of lymph node dissection and reconstruction in gastric resection are performed as in CLG. Thus, the introduction of RPS seems to be relatively easy for the surgeon who is used to CLG.
Indications
The indications for RPLG include early gastric cancer in patients who are slim and have little visceral fat. Patients with a belly wall area are also in this category. Specifically, the distance from the umbilicus to the xiphoid process of 15 cm or less and a BMI of 20 kg/m2 or less are preferable. RPLG is also particularly appropriate for young women because of its cosmetic benefit (27). Some institutions consider advanced gastric cancer as an indication or do not take sex or physical condition into consideration when deciding whether RPLG should be indicated (28).
Variation of the reduced-port laparoscopic gastrectomy (RPLG) procedure
RPLG includes SILG and various RPLG procedures, as it requires fewer ports or introducing a smaller port incision than that in CLG. The SILG and RPLG procedures that were introduced in the authors’ institute are described herein by referring to published articles.
Single-incision laparoscopic gastrectomy
SILG in this chapter refers to the procedure where only one small incision is made without any additional port, which we call “pure SILG.” Reports on pure SILG (7,12-15,17,28-34) described its feasibility, cosmetic results, and minimal invasiveness. The feature of SILG is based on the concept of standardizing SILG in each institute.
Set-up
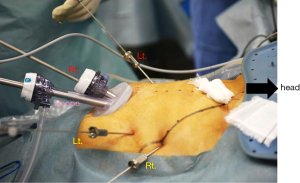
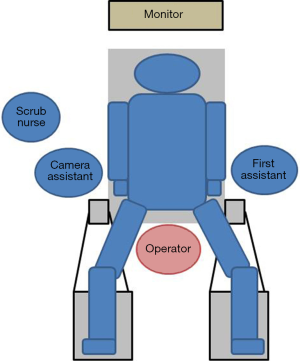
The patient is placed supine in the dorsosacral position. The surgeon stands between the patient’s legs, the camera assistant stands at the patient’s right side, and the first assistant stands at the patient’s left side. A monitor is placed above the patient’s head (Figure 2). A 10-mm endoscope with a high-definition camera is preferred. A flexible endoscope is preferable than a rigid endoscope because its flexibility can prevent conflict between the forceps.
Access device
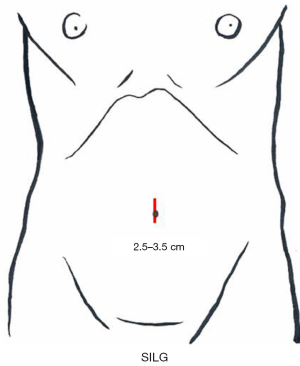
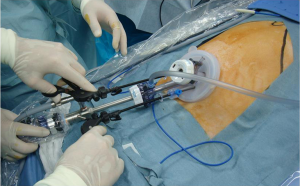
A 2.5- to 3.5-cm incision is made in the umbilicus (Figure 3). Different kinds of access device have been developed and commercialized, and are chosen according to the discretion of the surgeons’ in each institute. The present authors use Lap-Protector (Hakko Co., Ltd., Nagano, Japan) for wound protection and EZ Access (Hakko Co., Ltd., Nagano, Japan), which can be assembled as an umbilical access device. Two 12- and 5-mm trocars each are inserted through the EZ Access device. By using a 10-mm, 30-degree endoscope, both the surgeon’s and assistant’s forceps are inserted through the respective trocars. The energy device, stapler, and gauze are inserted and removed through the 12-mm trocar (Figure 4). Four trocars are also generally inserted in previous reports.
Liver retraction
Retraction of the left lobe of the liver is important for gastrectomy. It is simply performed with percutaneous stitching nylon thread or by using a penlose drain. The present authors use a 2-0 nylon thread and a medium-sized Silicon-disc (Hakko Co., Ltd., Nagano, Japan).
Single-incision laparoscopic gastrectomy (SILG) procedure
Almost all procedures are performed in the same manner as those in CLG. The unique tips of the SILG are as follows: (I) the operating table is more tilted into the head-up position to gain the benefit of counter traction by gravity; (II) a curved instrument is frequently used to prevent the conflict between forceps; (III) an innovation that uses small clips, which are hanged with percutaneous thread and then retracted. Instead of organ traction by using forceps, each clip is used to grasp the tissue of the organ and create the working field. Thus, the technical quality of SILG is maintained so as not to be inferior to RPLG or CLG. Reconstruction is also performed through a single incision. In this situation, stapling should be carefully executed. A 10-mm stapler is rather large for the access device, and assist forceps should be appropriately inserted. The present authors perform Roux-en-Y reconstruction after gastrectomy with the use of a linear stapler. The Y limb is created extracorporeally to shorten the execution time. Gastrojejunostomy is performed intracorporeally. We always choose side-to-side anastomosis by using a 60-mm linear stapler. The stapler entry hole is sutured intracorporeally. The 15-cm 3-0 V-Loc 180 (Covidien, Mansfield, MA, USA), a barbed suture material, is used for the suturing. This material prevents line slack during the suturing and does not require knotting, which facilitates laparoscopic suturing especially for SILG. Petersen’s defect and the mesenteric space around the Y limb are also closed with a continuous barbed suture. Finally, the single umbilical opening is cosmetically closed with an appropriate buried suture, and the scar is generally invisible after 3 months (Figure 5).
Reduced-port laparoscopic gastrectomy
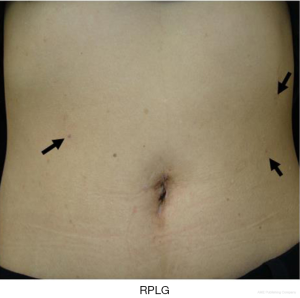
The term RPLG can be applied to various procedures with various efforts to achieve minimal invasiveness. Many literatures indicated that RPLG procedures are comparable with CLG procedures (11,35-41). The present authors’ concept of RPLG was derived from SILG. Sometimes, we encounter instrument conflicts at SILG and cannot maintain an adequate working angle. Additional ports should be inserted if necessary, but in a minimally invasive manner. We prefer to use the 2.1-mm-diameter BJ Needle forceps (NITI-ON Co., Ltd., Chiba, Japan), not through an additional port, but via a dedicated puncture port (Figure 6). The tiny wound made by the BJ needle does not cause postoperative pain or an unsatisfactory cosmetic outcome (Figure 7). Even one additional BJ Needle in the surgeon’s left hand is useful for obtaining the proper manipulation angle in the laparoscopic view. According to the authors’ experience, a maximum of three additional BJ Needles, one in the surgeon’s left hand and one each in the assistant’s right and left hands, will create almost the same tissue traction as in conventional laparoscopic surgery. The authors named this standardized procedure “needle device-assisted single-incision laparoscopic gastrectomy (NA-SILG).”
Set-up
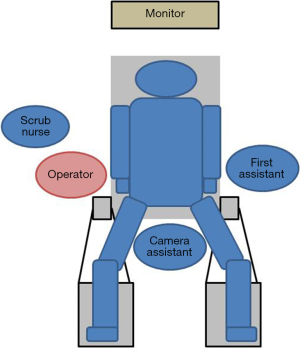
The set-up for NA-SILG is identical to that for SILG. The surgeon stands at the patient’s right side, the camera assistant stands between the patient’s legs, and the first assistant stands at the patient’s left side (Figure 8). The other equipment is placed as it is for SILG.
Access device
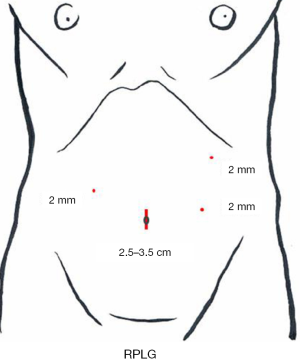
A 3-cm incision (a little smaller than the incision for SILG) is made at the umbilicus, and the Lap-Protector is used, with only two 12-mm trocars inserted through the EZ Access device. A 10-mm 30-degree endoscope and the surgeon’s right forceps are inserted through the respective trocars. The energy device, stapler, and gauze are inserted and removed through the 12-mm trocar. As additional punctures, a puncture for the 2.1-diameter BJ Needle forceps is made at the right lateral side of the abdomen for the operator’s left hand and two punctures are made at the left side for the assistant’s both hands (Figure 9).
Liver retraction
The liver retraction procedure is performed in the same manner as in SILG.
Needle device-assisted single-incision laparoscopic gastrectomy (NA-SILG) procedure
Dissection and reconstruction are performed in the same manner as in SILG. The difference is that the forceps in the operator’s left hand are inserted via the patient’s right abdomen, and the two forceps in the assistant’s hands are inserted via the left abdomen. The same operative field attained in CLG is thereby attained in NA-SILG. The distance between the operator’s two hands becomes comfortably wide, and a comfortable operating angle is obtained. The assistant performs the same procedure as in CLG. Thus, in NA-SILG, operators can easily master their roles, which can solve the educational problem in RPLG. The postoperative incisional pain and the incision required are minimal. The authors believe that the needle instruments are useful not only in single-incision surgery but also in facilitating RPS.
Results in reduced-port laparoscopic gastrectomy (RPLG)
Feasibility
The feasibility of RPLG seems to be almost established in previous reports (7,11,14,17,25-43). The median operative time in previous reports was 241.9 minutes (range, 186–302 minutes). The complication rate ranged from 0% to 20.8%. Furthermore, the procedure was not associated with any mortality and conversion to open surgery. However, the operator of the procedure is limited to an elected surgeon and institute where CLG has already been established and standardized. Almost all patients who underwent RPS are also elected according to the surgeon’s decision. That is, the possibility of selection bias should be taken into consideration in the interpretation of the results.
Oncological validity
So far, no report has described the long-term results concerning oncological validity. Predictive parameter may be the number of retrieved lymph nodes. In addition, that of previous reports was 36.5 (range, 24–66), which seemed to be comparable with that of CLG (7,11,14,17,25-43). The long-term results can be expected.
Training and education
The technical demand in RPLG is high (42). The preferable indications are early-stage cancer and slim patients, as described previously. However, surgeons in training would want to perform CLG for patients under the same indication as in the surgical training. That is, junior surgeons may lose their chance to assuming the role of operator. One solution is to standardize RPLG for many patients, including fat or advanced-stage cancer patients. In fact, introduction of thinner forceps as the needle device facilitates RPS, and junior surgeons can participate and assist during RPLG in the same way as they can in CLG.
Discussion
As described earlier, the institutions that have reported on RPLG so far, including our facility, are those that have standardized CLG. Therefore, we can argue that RPLG cannot be introduced as easily as CLG. Moreover, because RPLG is regarded mainly for its cosmetic benefit, it will be interesting to determine how effective and ontologically valid the procedure is. Whether RPLG can be taught and mastered as well as CLG should also be investigated.
For future prospect, standardizing the RPLG technique, shortening the learning curve, and reducing the difficulty of the technique are important issues to be addressed. Developing a new device is warranted. Novel devices such as flexible forceps, forceps with flexible tips, and an oval access port (43) have been reported. Single-port devices for robot-assisted surgery have also been developed, and these devices are expected to improve the ease with which SILG is performed. However, the size and cost of the apparatus are still problematic. Certain robot-supporting technology will be indispensable for the breakthrough in RPS (44-48).
Conclusions
RPLG for gastric cancer has been developed through advances in technology and devices, and the technical issues have been overcome in the same way. Problems in feasibility, oncological validity, training, and education will be solved with various efforts. The short-term results reported in literatures are acceptable. Long-term results that verify positive results or radical cure even for cases of cancer have not yet been published; thus, patients indicated for RPLG should be selected carefully. Prospective multicenter studies should also be conducted to establish RPS as a truly evidence-based practice that addresses not only cosmesis but also the proper balance between minimal invasiveness and radical cure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cuschieri A. Single-incision laparoscopic surgery. J Minim Access Surg 2011;7:3-5. [PubMed]
- Naito T. Chapter 3. Terminology. In: Mori T, Dapri G. editors. Reduced port laparoscopic surgery. Tokyo: Springer Japan, 2014:23-6.
- Tanaka J, Andoh H, Koyama K. Minimally invasive needlescopic cholecystectomy. Surg Today 1998;28:111-3. [Crossref] [PubMed]
- Inoue H, Takeshita K, Endo M. Single-port laparoscopy assisted appendectomy under local pneumoperitoneum condition. Surg Endosc 1994;8:714-6. [Crossref] [PubMed]
- Remzi FH, Kirat HT, Kaouk JH, et al. Single-port laparoscopy in colorectal surgery. Colorectal Dis 2008;10:823-6. [Crossref] [PubMed]
- Lee JH, Ryu KW, Kim YW, et al. Staging laparoscopy in gastric cancer: a single port method. J Surg Oncol 2003;84:50-2. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Ozdemir BA, Thomas RL, Soon Y. Single-port laparoscopic subtotal gastrectomy with DIα lymphadenectomy. Surg Innov 2011;18:NP1-4. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [Crossref] [PubMed]
- Usui S, Tashiro M, Haruki S, et al. Triple-incision laparoscopic distal gastrectomy for the resection of gastric cancer: comparison with conventional laparoscopy-assisted distal gastrectomy. Asian J Endosc Surg 2014;7:197-205. [Crossref] [PubMed]
- Omori T, Masuzawa T, Akamatsu H, et al. A simple and safe method for Billroth I reconstruction in single-incision laparoscopic gastrectomy using a novel intracorporeal triangular anastomotic technique. J Gastrointest Surg 2014;18:613-6. [Crossref] [PubMed]
- Ahn SH, Son SY, Lee CM, et al. Intracorporeal uncut Roux-en-Y gastrojejunostomy reconstruction in pure single-incision laparoscopic distal gastrectomy for early gastric cancer: unaided stapling closure. J Am Coll Surg 2014;218:e17-21. [Crossref] [PubMed]
- Ertem M, Ozveri E, Gok H, et al. 2013.
- Ahn SH. Single-incision laparoscopic total gastrectomy with D1+beta lymph node dissection for proximal early gastric cancer. Gastric Cancer 2014;17:392-6. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Kuji M, et al. The initial experience of dual port laparoscopy-assisted total gastrectomy as a reduced port surgery for total gastrectomy. Gastric Cancer 2013;16:602-8. [Crossref] [PubMed]
- Omori T, Tanaka K, Tori M, et al. Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc 2012;26:1490-4. [Crossref] [PubMed]
- Lee JH, Lee MS, Kim HH, et al. Comparison of single-incision laparoscopic distal gastrectomy and laparoscopic distal gastrectomy for gastric cancer in a porcine model. J Laparoendosc Adv Surg Tech A 2011;21:935-40. [Crossref] [PubMed]
- Kodera Y. Reduced port surgery for gastric cancer: another giant leap for mankind? Gastric Cancer 2013;16:457-9. [Crossref] [PubMed]
- Available online: http://www.jgca.jp/guideline/fourth/index.html
- Nakamura K, Katai H, Mizusawa J, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol 2013;43:324-7. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Kim HH, Han SU, Kim MC, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc 2013;84:123-30. [Crossref] [PubMed]
- Curcillo PG, Wu AS, Podolsky ER, et al. Reduced port surgery: Developing a safe pathway to single port access surgery. Chirurg 2011;82:391-7. [Crossref] [PubMed]
- Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg 2015;8:1-10. [Crossref] [PubMed]
- Shibao K, Matayoshi N, Sato N, et al. Reduced Port Distal Gastrectomy With a Multichannel Port Plus One Puncture (POP). Surg Technol Int 2015;26:92-9. [PubMed]
- Inaki N. Chapter 18. Total gastrectomy. In: Mori T, Dapri G. editors. Reduced port laparoscopic surgery. Tokyo: Springer Japan, 2014:197-220.
- Omori T, Nishida T. Chapter 17. Distal gastrectomy. In: Mori T, Dapri G. editors. Reduced port laparoscopic surgery. Tokyo: Springer Japan, 2014:183-96.
- Kim SM, Lee SH, Ha MH, et al. Techniques of the Single-Port Totally Laparoscopic Distal Gastrectomy. Ann Surg Oncol 2015;22 Suppl 3:S341. [Crossref] [PubMed]
- Ahn SH, Son SY. Solo Intracorporeal Esophagojejunostomy Reconstruction Using a Laparoscopic Scope Holder in Single-Port Laparoscopic Total Gastrectomy for Early Gastric Cancer. J Gastric Cancer 2015;15:132-8. [Crossref] [PubMed]
- Suh YS, Park JH, Kim TH, et al. Unaided Stapling Technique for Pure Single-Incision Distal Gastrectomy in Early Gastric Cancer: Unaided Delta-Shaped Anastomosis and Uncut Roux-en-Y Anastomosis. J Gastric Cancer 2015;15:105-12. [Crossref] [PubMed]
- Jiang ZW, Zhang S, Wang G, et al. Single-incision laparoscopic distal gastrectomy for early gastric cancer through a homemade single port access device. Hepatogastroenterology 2015;62:518-23. [PubMed]
- Ahn SH. Pure single-incision laparoscopic D2 lymphadenectomy for gastric cancer: a novel approach to 11p lymph node dissection (midpancreas mobilization). Ann Surg Treat Res 2014;87:279-83. [Crossref] [PubMed]
- Ahn SH, Son SY. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 2014;219:933-43. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Shibuya K, et al. Comparison of the invasiveness between reduced-port laparoscopy-assisted distal gastrectomy and conventional laparoscopy-assisted distal gastrectomy. Int Surg 2013;98:247-53. [Crossref] [PubMed]
- Kim SM, Ha MH, Seo JE, et al. Comparison of single-port and reduced-port totally laparoscopic distal gastrectomy for patients with early gastric cancer. Surg Endosc 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Dauser B, Görgei A, Stopfer J, et al. Conventional laparoscopy vs. single port surgery from a patient's point of view: influence of demographics and body mass index. Wien Klin Wochenschr 2012;124:834-41. [Crossref] [PubMed]
- Kim SM, Ha MH, Seo JE, et al. Comparison of Reduced Port Totally Laparoscopic Distal Gastrectomy (Duet TLDG) and Conventional Laparoscopic-Assisted Distal Gastrectomy. Ann Surg Oncol 2015;22:2567-72. [Crossref] [PubMed]
- Kunisaki C, Ono HA, Oshima T, et al. Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg 2012;29:261-8. [Crossref] [PubMed]
- Kunisaki C, Makino H, Kimura J, et al. Application of reduced-port laparoscopic total gastrectomy in gastric cancer preserving the pancreas and spleen. Gastric Cancer 2015;18:868-75. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Monma E, et al. Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 2015;29:1321-6. [Crossref] [PubMed]
- Rehman H, Fitzgerald JE, Frantzias J, et al. The need for training frameworks and scientific evidence in developing scarless surgery: a national survey of surgeons' opinions on single port laparoscopic surgery. Int J Surg 2013;11:73-6. [Crossref] [PubMed]
- Shibao K, Sato N, Higure A, et al. A new oval multichannel port to facilitate reduced port distal gastrectomy. Minim Invasive Ther Allied Technol 2015;24:135-40. [Crossref] [PubMed]
- Kim HH, Ahn SH. The current status and future perspectives of laparoscopic surgery for gastric cancer. J Korean Surg Soc 2011;81:151-62. [Crossref] [PubMed]
- Ostrowitz MB, Eschete D, Zemon H, et al. Robotic-assisted single-incision right colectomy: early experience. Int J Med Robot 2009;5:465-70. [Crossref] [PubMed]
- Ragupathi M, Ramos-Valadez DI, Pedraza R, et al. Robotic-assisted single-incision laparoscopic partial cecectomy. Int J Med Robot 2010;6:362-7. [Crossref] [PubMed]
- Petroni G, Niccolini M, Caccavaro S, et al. A novel robotic system for single-port laparoscopic surgery: preliminary experience. Surg Endosc 2013;27:1932-7. [Crossref] [PubMed]
- Horise Y, Nishikawa A, Sekimoto M, et al. Development and evaluation of a master-slave robot system for single-incision laparoscopic surgery. Int J Comput Assist Radiol Surg 2012;7:289-96. [Crossref] [PubMed]
Cite this article as: Inaki N, Tsuji T, Doden K, Sakimura Y, Tawara H, Matsui R, Yamamoto D, Kitamura H, Bando H, Yamada T. Reduced port laparoscopic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol 2016;1:38.