Sorafenib in adjuvant setting: call for precise and personalized therapy
We read with great interest of the negative result of STORM (1). In the study, 1,114 patients were randomly assigned to sorafenib group and placebo group. After a median duration of treatment of 12.5 months, no difference in median recurrence-free survival (RFS) between the two groups was shown [33.3 months in the sorafenib group vs. 33.7 months in the placebo group; hazard ratio (HR) 0.940; 95% CI: 0.780–1.134; one-sided P=0.26].
The success of sorafenib in advanced hepatocellular carcinoma (HCC) has evoked much passion about its application in adjuvant setting (2). With the success of sorafenib in advance HCC, the expanding use of sorafenib in adjuvant setting in early stage BCLC A and intermediate stage BCLC B has been hotspots. However, the role of sorafenib when used as an adjuvant to transarterial chemoembolisation in a population with intermediate-stage HCC seems to be pessimistic. Recently released results of SPACE in HCC of intermediate stage are negative (3), which add to the negative evidence of sorafenib in adjuvant setting after TACE (4). And the recently publish STORM study implies that deeper thought of the mechanism of sorafenib in HCC is necessary to further improve the only effective drug in HCC systemic therapy.
There may be no target for sorafenib to have/exert its effect
Sorafenib is a multi-target small molecular drug with antiangiogenic, antitumor and off-target effect. The anti-tumor activity against established or advanced tumors is not necessarily associated with efficacy in the adjuvant setting against micrometastatic disease, since molecular target therapy need a “target”, and take the 75% recurrence rate in HCC in 5 years after operation into account, there will be 25% patients who have no recurrent disease thus probably don’t have a target available for sorafenib. Furthermore, the anti-vascular effect of sorafenib may also in vain, because tumor vasculation happened only if tumor cells grow to a mass larger than 1 centimeter in diameter, and before the tumor growing to certain size, the antiangiogenic effect of sorafenib as a multi-target drug will disappear. Still, tumor cells harbor the inherent resistant to molecular target therapy and even existence of micrometastatic disease may not respond to sorafenib treatment.
The clinical effect of molecular target therapy is different in the adjuvant setting and the advanced stage is distinct. For example, the clinical benefit of bevacizumab with respect to progression-free and overall survival was greater in patients with residual macrometastases in patients with surgically resected ovarian cancer in ICON7 study (5). In the STORM study (1), patients with 146 (32%) patients in sorafenib group and 147 (33%) patients has microscopic vascular invasion, and tumor satellite nodules existed in only 42 (9%) and 39 (9%) patients, which indicated that many patients who are not high-risk for recurrence were included in the study. As patients with high-risk for recurrence are more likely to have micro-metastatic disease, sorafenib probably will not find its target in these patients.
In previous retrospective cohort study, adjuvant use of sorafenib is safe and decreases risk of HCC recurrence in high-risk LT recipients (6). Another two retrospective cohort studies have also shown that sorafenib prevented recurrence and prolonged recurrence free survival in high-risk patients after resection (7,8) (Table 1). If stratification of patients with intermediate and high risk is performed, there may be more clues, just like the story in STORM (9).
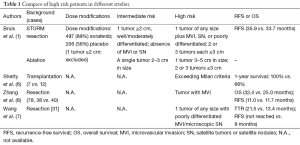
Full table
The dose of sorafenib matters
In STORM, the median duration of treatment was only 12.5 months for the sorafenib group versus 22.2 months for the placebo group, and 24% of patients discontinued sorafenib due to toxic effects compared with only 7% in the placebo group. Almost 90% of patients required dose modification in the sorafenib group compared with less than 40% for the placebo group, resulting in a much lower mean daily dose of sorafenib than placebo (577.7 vs. 777.9 mg). Compared with other studies, in the SHARP trial, 76% of patients received more than 80% of the planned daily dose of sorafenib (2). While in the ORIENTAL trial (10), 46 of 149 (30.9%) with sorafenib and 2 of 75 (2.7%) in placebo need dose reductions (Table 2). In the STORM trial, the rate of discontinuation of sorafenib (50% at 1 year) was higher than anticipated, and even much higher than that in SHARP study (38% vs. 37%) and ORIENTAL study (19.5% vs. 13.3%).
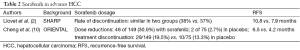
Full table
To our knowledge, the optimal dose of sorafenib is largely empirical, and pre-clinical study has implied that either high dose (11) or low dose sorafenib (12) will cause trouble by promoting the metastasis of tumor or increase the metastatic potential of tumor. For instance, treatment with anti-VEGFR antibodies or VEGFR tyrosine kinase inhibitors (VEGFR TKIs) in genetically engineered murine models of pancreatic islet-cell tumors, as well as in orthotopically transplanted murine GBM models, resulted in tumor adaptation and progression to increased invasion and malignant potential (13). Another study showed that, following short-term treatment of mice with various VEGFR TKIs prior to intravenous inoculation of human tumor cells accelerated metastases leading to shorter survival (11). Our previous study have shown that low dose sorafenib may promote the metastatic potential of HCC cells by down-regulating the anti-oncogene HTATIP2, and combination with aspirin (14) or metformin (15) are ways to increase the effect of sorafenib by upregulating HTATIP2. Furthermore, dose escalation may be one way to solve this problem (16).
On the other side, other preclinical data have not reported a prometastatic effect of VEGF-blockade, and support the strategy of continuing anti-angiogenic treatment.
Molecular biomarker are promising to predict the effect of sorafenib and its side effects as well
Molecular therapies targeting signaling cascades involved in hepatocarcinogenesis have been explored in phase III clinical trials, but none of the drugs tested showed positive results in first-(brivanib, sunitinib, erlotinib, and linifanib) or second-line therapy (brivanib and everolimus) after progression on sorafenib (17).
Sorafenib is only moderately effective and a substantial proportion of patients have to discontinue the medication either due to intolerable side effects or drug resistance. Hence the ability to predict response and prevent unnecessary adverse effects is an important area of active research and a number of biomarkers predictive of response to sorafenib have been identified. An analysis of 77 patients enrolled prospectively in three sorafenib trials who had pretreatment tumor biopsy available showed that elevated tissue expression of pERK and VEGFR-2 were predictive of poor outcome in advanced HCC treated with sorafenib (18). With further validation of these biomarkers it should eventually be possible to predict the response to sorafenib and patients who are nonresponders can be offered other systemic therapies, once available, thus avoiding unnecessary side effects.
Emerging preclinical evidence suggests that VEGF-targeted treatment, despite being effective in reducing primary tumor growth initially, may subsequently elicit an adaptive-evasive response, resulting in a more invasive phenotype and in some cases increasing metastasis (11,13). Hence, the benefits from VEGF inhibition monotherapy may, in certain settings, be offset by increased invasiveness and metastatic potential. Our previous studies have shown that sorafenib promoted metastatic potential by down-regulating HTATIP2 (12).
Thus, there is an urgent need to identify molecular subclasses of HCC driven by specific genetic aberrations that can be effectively targeted, just like the success of crizotininb in ALK-rearranged lung cancer (19) or Gleevec in gastrointestinal tumor (GIST) expressing CD117. Recent studies have indicated that focal amplifications in FGF3/4 and VEGF-A can predict response to sorafenib (20). Once these results are more extensively validated, these biomarkers can potentially be used for patient selection. Biomarkers predicting adverse reactions to sorafenib have not been clearly identified as yet. Early onset of skin toxicity has been shown to herald a good response to sorafenib (21), and we have found that the presence of adverse effects predicted improved survival in sorafenib treated advanced HCC (22). There are also efforts underway to sensitize HCCs to sorafenib and a recent study has suggested that silencing Mapk14 sensitizes HCCs to sorafenib. Combination of sorafenib with Mapk14 block¬ade therefore has the potential to overcome sorafenib resistance in human HCC (23).
In conclusion, the benefit of patients to sorafenib treatment is largely empirical and precise treatment according to specific activated pathway is urgently needed to select subclasses of patients who will benefit sorafenib treatment. In the adjuvant setting, both patients with residual disease and specific molecular marker of tumor should be identified to ensure personalized treatment. Furthermore, the dose of sorafenib may be decreased through drug combination to improve patients’ quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or Placebo plus TACE with Doxorubicin-Eluting Beads for Intermediate-Stage HCC: Phase II, Randomized, Double-Blind SPACE Trial. J Hepatol 2016. [Crossref]
- Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. [Crossref] [PubMed]
- Mountzios G, Pentheroudakis G, Carmeliet P. Bevacizumab and micrometastases: revisiting the preclinical and clinical rollercoaster. Pharmacol Ther 2014;141:117-24. [Crossref] [PubMed]
- Shetty K, Dash C, Laurin J. Use of adjuvant sorafenib in liver transplant recipients with high-risk hepatocellular carcinoma. J Transplant 2014;2014:913634.
- Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: A pilot study. Hepatol Res 2014;44:523-31. [Crossref] [PubMed]
- Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends 2014;8:333-8. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009;15:232-9. [Crossref] [PubMed]
- Zhang W, Sun HC, Wang WQ, et al. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology 2012;143:1641-1649.e5.
- Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009;15:220-31. [Crossref] [PubMed]
- Lu L, Sun HC, Zhang W, et al. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS One 2013;8:e65023. [Crossref] [PubMed]
- Guo Z, Cao M, You A, et al. Metformin inhibits the pro-metastatic effect of Sorafenib in hepatocellular carcinoma by upregulating the expression of TIP30. Cancer Sci 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Desar IM, Timmer-Bonte JN, Burger DM, et al. A phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer. Br J Cancer 2010;103:1637-43. [Crossref] [PubMed]
- Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res 2014;20:2072-9. [Crossref] [PubMed]
- Negri FV, Dal Bello B, Porta C, et al. Expression of pERK and VEGFR-2 in advanced hepatocellular carcinoma and resistance to sorafenib treatment. Liver Int 2015;35:2001-8. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Horwitz E, Stein I, Andreozzi M, et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov 2014;4:730-43. [Crossref] [PubMed]
- Brose MS, Frenette CT, Keefe SM, et al. Management of sorafenib-related adverse events: a clinician's perspective. Semin Oncol 2014;41 Suppl 2:S1-S16. [Crossref] [PubMed]
- Song T, Zhang W, Wu Q, et al. A single center experience of sorafenib in advanced hepatocellular carcinoma patients: evaluation of prognostic factors. Eur J Gastroenterol Hepatol 2011;23:1233-8. [Crossref] [PubMed]
- Avila M, Berasain C. Making sorafenib irresistible: In vivo screening for mechanisms of therapy resistance in hepatocellular carcinoma hits on Mapk14. Hepatology 2015;61:1755-7. [Crossref] [PubMed]
Cite this article as: Zhang W. Sorafenib in adjuvant setting: call for precise and personalized therapy. Transl Gastroenterol Hepatol 2016;1:13.


