Clinical trials to reduce pancreatic fistula after pancreatic surgery—review of randomized controlled trials
Introduction
The morbidity rate after pancreatic surgery still remains high in the range of 15% to 65%, although mortality has decreased to less than 5% due to recent advances in surgical techniques and perioperative management (1-7). In particular, pancreatic fistula is one of the most severe postoperative complications after pancreatic surgery. Pancreatic fistula is reportedly associated with a higher incidence of life-threatening complications, such as intra-abdominal abscess, intra-abdominal hemorrhage, and sepsis (8-12). A strategy to decrease pancreatic fistula after pancreatic surgery is urgently required.
The various innovative techniques, including operative techniques, intensive care medicine and pharmacological agents have been utilized to prevent the incidence of pancreatic fistula after pancreatic surgery. This review summarizes the randomized controlled trials (RCTs) to prevent pancreatic fistula after pancreatic surgery.
Definition of pancreatic fistula
In 2005, an international study group of pancreatic surgeons (ISGPF) proposed a consensus definition and clinical grading of postoperative pancreatic fistula (13). Pancreatic fistula was defined by ISGPF guidelines as follows: amylase level in drainage fluid on POD 3 that was more than 3 times the serum amylase level. Pancreatic fistula was classified into three categories by ISGPF as follows: Grade A—“transient pancreatic fistula”, it has no clinical impact; Grade B—required a change in management or adjustment in the clinical pathway; Grade C—a major change in clinical management or deviation from the normal clinical pathway. Grade B and C were defined as “clinical pancreatic fistula”.
RCT regarding the operative technique to prevent pancreatic fistula after pancreaticoduodenectomy (PD)
Several clinical trials regarding operative technique were performed to prevent pancreatic fistula after PD as follows: (I) pancreaticojejunostomy (PJ) versus pancreaticogastrostomy (PG); and (II) pancreatic stent.
PJ versus PG
Both PJ and PG are established reconstructive procedures in PD for pancreatic or periampullary tumors. The meta-analysis of RCTs published in 2015 revealed a higher rate of pancreatic fistula after PD in PJ, when compared to PG (14). In this meta-analysis seven RCTs were reviewed, including 562 patients who underwent PG and 559 who underwent PJ. The pancreatic fistula incidence was significantly lower in the PG group than in the PJ group (11.2% vs. 18.7%, OR: 0.53, 95% CI: 0.38–0.75, P=0.0003). The overall mortality rate was 3.7% in the PG group and 3.9% in the PJ group (P=0.68). No significant differences regarding overall morbidity and mortality were found between PJ and PG. PG has been thought to be safer than PJ for the following reasons: (I) the gastric acid environment inhibits the activation of pancreatic enzymes; (II) the proximity of the stomach to the pancreatic remnant decreases tension on the anastomosis; (III) the rich gastric vascular supply reduces the tendency for ischemia of the anastomosis (15-17). However, there are some limitations in this meta-analysis as follows; (I) the type of intervention and the indications for surgery which are different among seven RCTs may lead to different results; (II) the definition of pancreatic fistula varied among these RCTs may cause the different decision of pancreatic fistula among each institution. There were nine RCTs to examine that PG reduces the incidence of pancreatic fistula comparing PJ (Table 1) (17-25). Afterward, a multicenter prospective randomized controlled trial comparing PG with PJ from Germany was published in 2015 (25). The impact of study was the currently largest (n=440) multicenter prospective randomized controlled trial comparing PG with PJ regarding postoperative complications including pancreatic fistula and long-term pancreatic function. The incidence of grade B/C pancreatic fistula after PJ was similar to that after PG (PJ: 22% vs. PG: 20%, P=0.617). On the other hand, this study reported that PG was associated with a significantly increased rate of postpancreatectomy hemorrhage compared to PJ (PJ: 12% vs. PG: 20%, P=0.023), although there was no significant difference regarding overall morbidity and mortality between PJ and PG.
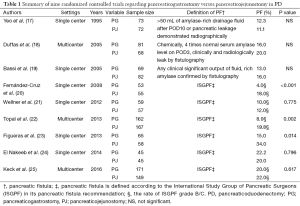
Full table
Regarding long-term pancreatic function between PJ and PG, two RCT have demonstrated that pancreatic exocrine insufficiency is more severe after PJ than PG (23,25). In contrast, one RCT has reported conflicting long-term outcomes regarding pancreatic function (24). However, pancreatic exocrine function in these RCTs was not measured directly. Alternatively, surrogate parameters including steatorrhea, body weight loss, and stool elastase level have represented pancreatic exocrine function indirectly. Moreover, surrogate parameters used for pancreatic exocrine function were different in each study. A furthermore large multicenter trial is required to evaluate long-term pancreatic function between PJ and PG.
Pancreatic duct stent in PJ
The impact of pancreatic duct stent to reduce pancreatic fistula after PD remains still controversial. There are three types for procedures of pancreatic duct stent as follows; lost stent, external stent and no stent. However, it remains unclear which is best procedure to reduce pancreatic fistula. There were five RCTs regarding pancreatic duct stent following PJ to prove the hypothesis that stent reduces the incidence of pancreatic fistula (Table 2) (10,26-29).

Full table
At first, three RCTs regarding external pancreatic duct stent versus no stent were reviewed. Poon et al. reported that pancreatic fistula occurred in 6.7% of patients with external drainage stent, and in 20% with no stent (P=0.032) in RCT which compared external drainage stent (n=60) with no stent (n=60) (10). However, this study included both soft and hard pancreatic parenchyma. Soft pancreas is well known to cause higher incidence of pancreatic fistula after PD than hard pancreas. Soft pancreas has been reported to be one of the risk factors for pancreatic fistula. In 2011, Pessaux et al. performed RCT to evaluate the impact of external duct stent among high-risk patients with soft pancreas or a non-dilated duct less than 3 mm (28). The study has reported that external pancreatic duct stent significantly reduced pancreatic fistula compared to no stent: 20 of 77 (26%) in external pancreatic duct stent group versus 34 of 81 (42%) in no stent group (P=0.03). Moreover, the stent group significantly reduced morbidity compared to no stent group (41.5% vs. 61.7%, P=0.01). Similarly, Motoi et al. also reported that among patients with a non-dilated duct, external pancreatic duct stent significantly reduced clinically relevant pancreatic fistula compared to no stent: two of 21 (10%) versus eight of 20 (40%) (P=0.033) (29). Pancreatic duct stent may protect PJ by diverting pancreatic juice away from the anastomosis, to improve long-term pancreatic duct patency, and to facilitate precise suture placement.
On the other hands, the impact of internal pancreatic duct stent remains still unclear. Winter et al. has reported that internal pancreatic duct stent did not reduce the incidence of pancreatic fistula, compared to no stent (11.3% in internal pancreatic duct stenting; n=115 versus 7.6% in no stent; n=119) (26). However, in this study, the technique of PJ anastomosis was not standardized as the use of duct-to-mucosa or invagination technique. The invagination technique is chosen in PJ for a small pancreatic duct which is more difficult for duct-to-mucosa. A bias of surgeons in selecting the anastomotic technique may influence outcomes in this study. Moreover, external stent may decrease the incidence of stent migration or offer a better diversion of pancreatic juice away from anastomosis compared to internal stent. However, Tani et al. has reported that no difference was found between external and internal stents regarding short-outcomes including the incidence of pancreatic fistula (27). It remains still controversial which is better external stent or internal stent. Meta-analysis has reported that pancreatic duct stent did not reduce the incidence of pancreatic fistula and other complications in PD compared with no stent (30). A large multicenter randomized controlled trial for standardized anastomotic techniques for PD is required to conclusively evaluate the benefits of using pancreatic duct stents.
RCT regarding the operative technique to prevent pancreatic fistula after distal pancreatectomy (DP)
DP is a procedure for treatment both benign and malignant diseases of the body and tail of the pancreas. In an effort to reduce the incidence of PF after DP, surgeons have attempted various surgical techniques to transect pancreatic parenchyma including a hand-sewn closure, stapler closure, scalpel, electrocautery or ultrasonic devices. However, appropriate procedure to transect the pancreas during DP remains still controversial. Table 3 summarizes RCTs regarding procedure to prevent pancreatic fistula after DP (31-36).

Full table
Stapler closure has recently become a standard technique for pancreatic stump closure. The meta-analyses on hand-sewn suture and stapler closure reported by Knaebel et al. showed that stapler closure (22.8%) had reduced pancreatic fistula more than hand-sewn suture (34.9%) (37) and those reported by Zhou et al. showed that stapler closure (22.1%) had reduced pancreatic fistula more than hand-sewn suture (31.2%) (38). These two reports of meta-analyses demonstrated that stapler closure in DP tended to reduce pancreatic fistula as compared to manual suturing, but could not prove that stapler closure was statistically useful. In 2011, the results of RCT of hand-sewn suture and stapler closure were published (31). However, the multicenter randomized DISPACT trial found that stapler closure did not significantly reduce the incidence of pancreatic fistula after DP in comparison to hand-sewn closure. In this study, 352 patients were randomized both treatment groups, 177 patients were stapler group, 175 patients were another group. The incidence of pancreatic fistula did not differ between both groups (stapler closure; 32% vs. hand-sewn; 28%, OR: 0.84, 95% CI: 0.53–1.33, P=0.56). Afterward, there are RCTs regarding absorbable material (32,34,35) or seromuscular patch (33) to reinforce the staple line. In 2012, it has been reported that the resection with a stapler having reinforcing absorbable materials significantly reduced clinically relevant pancreatic fistula (32). However, in two RCTs, an absorbable fibrin sealant patch (TachoSil) to stapling technique did not reduce the incidence of pancreatic fistula. Montorsi et al. have reported the incidence of pancreatic fistula was not significantly different between groups (with TachoSil group; 62% vs. without TachoSil group; 68%, P=0.267) in a multicenter, randomized, controlled trial (34). Park et al. also examined a similar prospective, multicenter, randomized controlled study (35). In this RCT, the incidence of clinically relevant postoperative complications (grade B and C, ISGPF) (with TachoSil group; 22.9% vs. without TachoSil group; 28.3%, P=0.536). These two studies demonstrated that the TachoSil patch did not reduce the incidence of pancreatic fistula after DP. TachoSil had no significant effect on the incidence of pancreatic fistula. On the other hand, a RCT has reported that covering the stapled pancreatic remnants with seromuscular patch significantly decreased the overall rate of pancreatic-related complications, although the rates of clinically relevant postoperative complications (grade B and C, ISGPF) were comparable between two groups (33).
A multicenter randomized controlled trial has evaluated whether PJ of pancreatic stump decreases the incidence of pancreatic fistula after DP compared with stapler technique in a multicenter randomized controlled trial (36). This RCT demonstrated that PJ of the pancreatic stump during DP does not reduce pancreatic fistula compared with stapler closure. However, this study has reported that PJ of pancreatic stump in thickness of pancreas greater than 12 mm tended to reduce the incidence of clinically relevant pancreatic fistula compared to stapler closure (22.2% of the stapler closure group vs. 6.2% of the PJ group; P=0.080).
Efficacy the use of somatostatin or its analogues after pancreatic surgery
Somatostatin and somatostatin analogues, including octreotide and vapreotide, have well-recognized inhibitory effects on pancreatic exocrine secretion. Therefore, somatostatin or octreotide have been used as prophylactic agents to prevent pancreatic fistula after pancreas resection. Table 4 summarizes RCTs regarding the administration of somatostatin and somatostatin analogues after pancreatic surgery. Two RCTs reported that prophylactic somatostatin or octreotide significantly reduced the incidence of pancreatic fistula after PPPD (39,40). On the other hand, four recent RCTs reported that the use of somatostatin analogues including octreotide and vapreotide, did not reduce pancreatic fistula after pancreas surgery (41-45). A meta-analysis regarding the benefit of somatostatin and its analogues reported that these agents reduced overall morbidity (P=0.003) and pancreas-specific complications (P=0.002), but did not reduce the incidence of clinically relevant pancreatic fistula after pancreatic surgery (47). In contrast, another meta-analysis report concluded that these agents didn’t have advantages of utility for mortality, re-operation rate, and hospital stay, and the incidence of clinical pancreatic fistula after pancreatic surgery (48). Recently, one RCT reported that pasireotide which is another long-acting somatostatin analogue significantly reduced the incidence of pancreatic fistula after pancreatic surgery (46). As the reason to reduce pancreatic fistula, the report discussed that pasireotide has a long half-life and a strong affinity to some SSTR-subtypes compared to other somatostatin analogues. The impact of somatostatin and its analogues to reduce pancreatic fistula after pancreatic surgery remains controversial, as study design is heterogeneity by each study. Furthermore large multicenter RCTs are required to clarify the benefits of somatostatin and its analogues after pancreatic surgery.
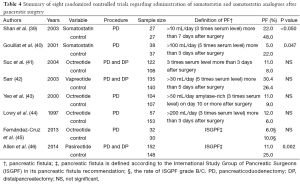
Full table
Conclusions
Consensus on the best way to prevent pancreatic fistula after pancreatic surgery remains still controversial. However, several RCTs steadily clarify a useful procedure to reduce the incidence of pancreatic fistula after pancreatic surgery. Therefore, further RCTs to study innovative approaches remain a high priority for pancreatic surgeons to prevent pancreatic fistula after pancreatic surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60. [PubMed]
- Büchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 2003;138:1310-4; discussion 1315. [PubMed]
- Balcom JH 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391-8. [PubMed]
- Tani M, Kawai M, Terasawa H, et al. Complications with reconstruction procedures in pylorus-preserving pancreaticoduodenectomy. World J Surg 2005;29:881-4. [PubMed]
- Ban D, Shimada K, Konishi M, et al. Stapler and nonstapler closure of the pancreatic remnant after distal pancreatectomy: multicenter retrospective analysis of 388 patients. World J Surg 2012;36:1866-73. [PubMed]
- Zhang H, Zhu F, Shen M, et al. Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br J Surg 2015;102:4-15. [PubMed]
- Sell NM, Pucci MJ, Gabale S, et al. The influence of transection site on the development of pancreatic fistula in patients undergoing distal pancreatectomy: A review of 294 consecutive cases. Surgery 2015;157:1080-7. [PubMed]
- van Berge Henegouwen MI, De Wit LT, Van Gulik TM, et al. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 1997;185:18-24. [PubMed]
- Wada K, Traverso LW. Pancreatic anastomotic leak after the Whipple procedure is reduced using the surgical microscope. Surgery 2006;139:735-42. [PubMed]
- Poon RT, Fan ST, Lo CM, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 2007;246:425-33; discussion 433-5. [PubMed]
- Adam U, Makowiec F, Riediger H, et al. Risk factors for complications after pancreatic head resection. Am J Surg 2004;187:201-8. [PubMed]
- Kawai M, Tani M, Terasawa H, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg 2006;244:1-7. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [PubMed]
- Menahem B, Guittet L, Mulliri A, et al. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg 2015;261:882-7. [PubMed]
- Shen Y, Jin W. Reconstruction by Pancreaticogastrostomy versus Pancreaticojejunostomy following Pancreaticoduodenectomy: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract 2012;2012:627095.
- Takano S, Ito Y, Watanabe Y, et al. Pancreaticojejunostomy versus pancreaticogastrostomy in reconstruction following pancreaticoduodenectomy. Br J Surg 2000;87:423-7. [PubMed]
- Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 1995;222:580-8; discussion 588-92. [PubMed]
- Duffas JP, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg 2005;189:720-9. [PubMed]
- Bassi C, Falconi M, Molinari E, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg 2005;242:767-71, discussion 771-3. [PubMed]
- Fernández-Cruz L, Cosa R, Blanco L, et al. Pancreatogastrostomy with gastric partition after pylorus-preserving pancreatoduodenectomy versus conventional pancreatojejunostomy: a prospective randomized study. Ann Surg 2008;248:930-8. [PubMed]
- Wellner UF, Sick O, Olschewski M, et al. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg 2012;16:1686-95. [PubMed]
- Topal B, Fieuws S, Aerts R, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol 2013;14:655-62. [PubMed]
- Figueras J, Sabater L, Planellas P, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg 2013;100:1597-605. [PubMed]
- El Nakeeb A, Hamdy E, Sultan AM, et al. Isolated Roux loop pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: a prospective randomized study. HPB (Oxford) 2014;16:713-22. [PubMed]
- Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg 2016;263:440-9. [PubMed]
- Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2006;10:1280-90; discussion 1290. [PubMed]
- Tani M, Kawai M, Hirono S, et al. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. Am J Surg 2010;199:759-64. [PubMed]
- Pessaux P, Sauvanet A, Mariette C, et al. External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg 2011;253:879-85. [PubMed]
- Motoi F, Egawa S, Rikiyama T, et al. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg 2012;99:524-31. [PubMed]
- Xiong JJ, Altaf K, Mukherjee R, et al. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg 2012;99:1050-61. [PubMed]
- Diener MK, Seiler CM, Rossion I, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22. [PubMed]
- Hamilton NA, Porembka MR, Johnston FM, et al. Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy: a single-blinded, randomized controlled trial. Ann Surg 2012;255:1037-42. [PubMed]
- Oláh A, Issekutz A, Belágyi T, et al. Randomized clinical trial of techniques for closure of the pancreatic remnant following distal pancreatectomy. Br J Surg 2009;96:602-7. [PubMed]
- Montorsi M, Zerbi A, Bassi C, et al. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: a multicenter, randomized, controlled trial. Ann Surg 2012;256:853-9; discussion 859-60. [PubMed]
- Park JS, Lee DH, Jang JY, et al. Use of TachoSil(®) patches to prevent pancreatic leaks after distal pancreatectomy: a prospective, multicenter, randomized controlled study. J Hepatobiliary Pancreat Sci 2016;23:110-7. [PubMed]
- Kawai M, Hirono S, Okada KI, et al. Randomized Controlled Trial of Pancreaticojejunostomy versus Stapler Closure of the Pancreatic Stump During Distal Pancreatectomy to Reduce Pancreatic Fistula. Ann Surg 2015. [Epub ahead of print]. [PubMed]
- Knaebel HP, Diener MK, Wente MN, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 2005;92:539-46. [PubMed]
- Zhou W, Lv R, Wang X, et al. Stapler vs suture closure of pancreatic remnant after distal pancreatectomy: a meta-analysis. Am J Surg 2010;200:529-36. [PubMed]
- Shan YS, Sy ED, Lin PW. Role of somatostatin in the prevention of pancreatic stump-related morbidity following elective pancreaticoduodenectomy in high-risk patients and elimination of surgeon-related factors: prospective, randomized, controlled trial. World J Surg 2003;27:709-14. [PubMed]
- Gouillat C, Chipponi J, Baulieux J, et al. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br J Surg 2001;88:1456-62. [PubMed]
- Suc B, Msika S, Piccinini M, et al. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection: a prospective, multicenter randomized controlled trial. Arch Surg 2004;139:288-94; discussion 295. [PubMed]
- Sarr MG; Pancreatic Surgery Group. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg 2003;196:556-64; discussion 564-5; author reply 565. [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000;232:419-29. [PubMed]
- Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997;226:632-41. [PubMed]
- Fernández-Cruz L, Jiménez Chavarría E, Taurà P, et al. Prospective randomized trial of the effect of octreotide on pancreatic juice output after pancreaticoduodenectomy in relation to histological diagnosis, duct size and leakage. HPB (Oxford) 2013;15:392-9. [PubMed]
- Allen PJ, Gönen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med 2014;370:2014-22. [PubMed]
- Connor S, Alexakis N, Garden OJ, et al. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg 2005;92:1059-67. [PubMed]
- Koti RS, Gurusamy KS, Fusai G, et al. Meta-analysis of randomized controlled trials on the effectiveness of somatostatin analogues for pancreatic surgery: a Cochrane review. HPB (Oxford) 2010;12:155-65. [PubMed]
Cite this article as: Kitahata Y, Kawai M, Yamaue H. Clinical trials to reduce pancreatic fistula after pancreatic surgery—review of randomized controlled trials. Transl Gastroenterol Hepatol 2016;1:4.


