
Impact of baseline abnormal liver enzymes in the outcome of COVID-19 infection
Introduction
Abnormal liver function tests (LFT) were reported in 14–53% of the patients with SARS-CoV2 infection (1,2), particularly aspartate aminotransferase (AST) and alanine aminotransferase (ALT) up to two-fold the upper limit of normal (ULN) (3-5). Several reports have attributed LFT changes to drug-induced liver injury, systemic inflammatory response associated with viral infection or exacerbation of previous chronic liver disease (CLD) (1,2). The expression of the angiotensin-converting enzyme 2 entry receptor for the virus on host cells, mainly in cholangiocytes but not in hepatocytes, may explain why acute hepatitis due to SARS-CoV2 has never been reported despite the abnormal values of liver enzymes (6,7). Until now, the pathogenesis associated with the rare cases of severe liver injury or ischemic cholangiopathy were much more closely related to organ dysfunction seen in critically-ill patients due to sepsis or acute respiratory distress syndrome (8,9). Likewise, the majority of reports (1,2,8) have correlated LFT derangements with leukocyte markers of inflammation, SARS-CoV-2 cytokine storm, impaired immune responses and uncontrolled inflammation, which are related to organ dysfunction and increased risk of death (10). Most studies have associated the occurrence and magnitude of AST and/or AST increase in COVID-19 patients with disease severity (3-5,11,12) and mortality (5,12,13), particularly in Western reports when compared to Asian (2). AST and ALT levels were also shown to increase and peak during hospitalization (4,14,15), but there is controversy regarding whether LFT obtained at admission or during hospitalization could predict adverse outcomes (16). Little is known also about the impact and significance of baseline LFT abnormalities on prognosis of patients with COVID-19 admitted to the emergency department (ED) to evaluate whether they could be useful to help decision-making on hospitalization.
The purpose of the present study was to assess the frequency of AST and ALT abnormalities, their association with leukocyte markers of inflammation and outcomes in patients admitted to the ED due to SARS-CoV2 infection. We present the following article in accordance with the STROBE reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-41/rc).
Methods
Study design and setting
This is a retrospective cohort study using data from electronic medical charts of all patients admitted to the Emergency Department of the Portuguese Hospital of Salvador, Bahia, Brazil with clinical suspicion of SARS-CoV2 infection leading to determination of reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV2 infection. At the beginning of the COVID-19 pandemic in March 2020, a multidisciplinary COVID-19 committee comprising experts in Emergency Medicine, intensive care unit (ICU), infection control, healthcare management and information technology personnel was established for planning, implementing and monitoring the continuum of care of all subjects admitted to the hospital with respiratory symptoms. Practice recommendations were frequently updated for evidence-based decision-making. Patients with mild symptoms were discharged for outpatient management while the results of RT-PCR were pending and counseled to return to the ED in case of worsening of symptoms and/or health status. Patients with moderate to severe symptoms were admitted to the emergency ward or ICU and underwent laboratory evaluation including blood cell count, electrolytes, AST and ALT within two hours of admission. Arterial blood gases and lactate were determined at the physician’s discretion, as well as chest X rays or computed tomography (CT) scans.
Selection of participants
The institutional COVID-19 committee analyzed the database of admitted patients with respiratory symptoms on a weekly basis. The database was updated daily, according to RT-PCR results, admission and discharge from the ED, hospital ward or ICU, requirement of mechanical ventilation, renal replacement therapy or vasoactive drugs. In order to evaluate the impact of baseline LFT abnormalities on patient outcomes and the correlation with leukocyte ratios currently used as markers of systemic inflammation, AST, ALT and complete blood count (CBC) were collected at admission in the ED. Liver enzymes were categorized as within or above the normal range. When abnormal, their levels were further classified between 1.1–2 times, 2.1–5 times, or over 5 times the ULN. Neutrophil-lymphocyte (NLR) and monocyte-lymphocyte ratios (MLR) and systemic immune-inflammation index (SII) were calculated as previously described (17,18). Differential leucocyte counts were automatically generated for all white blood cell values using a Sysmex® XT-4000i hematology analyzer equipped with a flow cytometer.
No investigation of underlying liver disease or hepatitis B and C serology was systematically carried out in those patients with abnormal liver enzymes. Any further evaluation was left to the discretion of the attending physicians.
Outcome measurements
Hospital or ICU admission was decided at the attending physician’s discretion. Respiratory, renal and circulatory failures were defined as requirement of mechanical ventilation, renal replacement therapy and vasoactive drugs, respectively. Baseline AST and ALT levels were correlated with leukocyte ratios and indices and the following outcomes: hospital admission and ICU admission, organ failure (OF), length of stay (LOS) and death. Only RT-PCR proven SARS-CoV2 infections were included in the analysis. Subsequent admissions within 30 days were considered as readmissions and new infections were defined by RT-PCR proven infection only if occurring beyond 60 days after discharge. Patients were followed until death or hospital discharge.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Federal University of Bahia, School of Medicine Ethics Committee Board (No. CAAE 53069121.6.0000.5577). Informed consent was waived because the study was conducted using an anonymized database, that has already been collected, offering no risk of harm or patient identification or even procedures for which written consent is normally required.
Statistical analysis
Dichotomous variables were shown in text and tables as numbers and percentage and were compared using the chi-square test or Fisher’s test, when appropriate. Continuous variables were reported as mean and standard deviation, median and interquartile range, or range according to the shape of the data. Student’s t-test or the Mann-Whitney U test were used for comparisons when appropriate. Analysis was performed using the Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA), version 21.0 for Windows.
Results
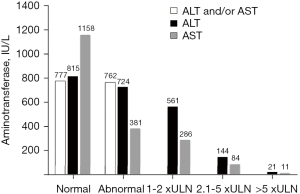
From March 2020 to March 2021, 6,495 patients were received in the ED with symptoms compatible with SARS-CoV-2 infection (Figure 1). All were tested for SARS-CoV-2 by RT-PCR, with diagnosis confirmed in 3,607 (56%) subjects; 1,458 (40%) of those RT-PCR positive patients were admitted to the emergency ward for further clinical, laboratory and/or imaging assessment. Eighty-one (4%) of the 2,149 patients initially discharged returned and were admitted to the emergency ward (Figure 1). The demographics, clinical and laboratory features, and the clinical outcomes of the remaining 1,539 cases are described in Table 1. Briefly, most were middle-aged women. The mean age of the entire cohort was 57±18 years. Hospital and subsequent ICU admissions were required in 57.5% and 30.9% of patients, respectively (Table 1); 273 (18%) subjects had at least one OF, particularly circulatory (17%), respiratory (13%), and renal (6%) failures; 194 patients died. Mortality was 12.6% among patients admitted to the emergency ward (n=1,539) and 5.4% among those COVID-19 positive subjects seeking care in the ED (n=3,607) (Figure 1). Abnormal AST and/or ALT were observed in 762 (50%) of patients. High AST appeared almost twice as frequently as ALT (47% versus 25%, P<0.0001). Most patients had AST abnormalities (77%) and ALT (75%) up to double the ULN. Few subjects had abnormal LFT over five times the ULN (Figure 2). Mean LFT, CBC and leukocyte ratios are shown in Table 1.
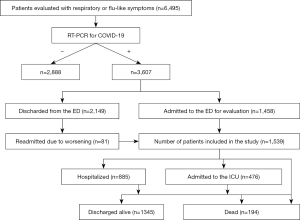
Table 1
| Characteristics | Values |
|---|---|
| Age (years) | 57±18 |
| Female gender, n (%) | 799 (51.9) |
| AST (IU/L)1 | 38 [29–55] (0–5,330) |
| ALT (IU/L)1 | 32 [20–52] (4–1,405) |
| Leukocytes ×109/L1 | 6.5 [4.9–8.8] (0–143.3) |
| Neutrophils ×109/L1 | 4.4 [2.9–6.7] (0–34.7) |
| Monocytes ×109/L1 | 0.5 [0.3–0.7] (0–7.2) |
| Platelets ×109/L1 | 217 [173–270] (0–788) |
| NLR1 | 3.8 [1.9–7.7] (0–119.2) |
| MLR1 | 0.4 [0.28–0.69] (0–2.36) |
| SII1 | 0.8 [0.4–1.7] (0–66.1) |
| Hospital admission, n (%) | 885 (57.5) |
| Admission to the ICU, n (%) | 476 (30.9) |
| Organ failure (number), n (%) | |
| 0 | 1,266 (82.3) |
| 1 | 72 (4.7) |
| 2 | 134 (8.7) |
| 3 | 67 (4.4) |
| Respiratory failure, n (%) | 193 (12.5) |
| Renal failure, n (%) | 85 (5.5) |
| Circulatory failure, n (%) | 263 (17.1) |
| Mortality, n (%) | 194 (12.6) |
1, data expressed by median, IQR in square brackets and minimum and maximum in parentheses. COVID-19, corona virus disease 2019; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; SII, systemic immune-inflammation index; ICU; intensive care unit; IQR, interquartile range.
The frequency and magnitude of AST and ALT abnormalities were significantly correlated with age, female sex, white blood cell count, neutrophil and monocyte numbers, NLR and SII (Tables 2,3). Likewise, LFT were further associated with hospital and ICU admission, number of OFs, respiratory and renal failure and mortality. AST abnormalities, but not ALT, were further related to a significantly higher frequency of OF occurrence, circulatory failure and a longer LOS (Tables 2,3).
Table 2
| Demographics and laboratory features | Normal | 1.1–2× ULN | 2.1–5× ULN | >5× ULN | P values |
|---|---|---|---|---|---|
| Age (years) | 53±19 | 61±17 | 61±16 | 57±20 | <0.0001 |
| Gender, n (%) | <0.0001 | ||||
| Male | 322 (40.5) | 322 (55.6) | 89 (61.8) | 9 (42.9) | |
| Female | 473 (59.5) | 249 (44.4) | 55 (38.2) | 12 (57.1) | |
| White blood cell count ×109/L¹ | 6.4 (0–70.2) | 6.7 (1.5–143.3) | 6.8 (1.5–27.6) | 7.6 (1.1–38.7) | 0.02 |
| Neutrophils ×109/L¹ | 4 (0–34.7) | 4.8 (0.025–34.2) | 5.3 (0.2–25.3) | 5.6 (0.8–20.1) | <0.0001 |
| Lymphocytes ×109/L¹ | 1.3 (0–59.2) | 1 (0–54.9) | 1 (0–22) | 1.1 (0–3.6) | 0.53 |
| Monocytes ×109/L¹ | 0.5 (0–7.2) | 0.5 (0–3.3) | 0.5 (0–1.9) | 0.5 (0–4.6) | 0.009 |
| Platelets ×109/L¹ | 223 (0–650) | 214 (45–788) | 205 (65–581) | 216 (78.5–549) | 0.3 |
| NLR¹ | 2.9 (0–97) | 4.9 (0.02–119) | 5.2 (0.26–48.3) | 5.57 (1.1–31.3) | <0.0001 |
| MLR¹ | 0.40 (0–6.65) | 0.48 (0.01–2.66) | 0.45 (0–3.17) | 0.52 (0.22–2.28) | 0.07 |
| SII¹ | 0.6 (0–49.6) | 1.1 (0.005-66.1) | 1.1 (0.058–12.4) | 1.7 (0.088–5.2) | <0.001 |
| Outcomes | |||||
| Hospital admission, n (%) | 342 (42.2) | 410 (73.1) | 113 (78.5) | 19 (90.5) | <0.0001 |
| Admission to the ICU, n (%) | 148 (18.2) | 240 (42.8) | 73 (50.7) | 15 (71.4) | <0.0001 |
| Organ failure, n (%) | 72 (9.2) | 141 (25.1) | 50 (34.7) | 9 (42.9) | <0.0001 |
| Number of organ failure, n (%) | <0.0001 | ||||
| 0 | 723 (90.9) | 420 (74.9) | 94 (65.3) | 12 (57.1) | |
| 1 | 23 (2.9) | 37 (6.6) | 9 (6.3) | 3 (14.3) | |
| 2 | 31 (3.9) | 70 (12.5) | 30 (20.8) | 3 (14.3) | |
| 3 | 18 (2.3) | 34 (6.1) | 11 (7.6) | 3 (14.3) | |
| Respiratory failure, n (%) | 48 (5.9) | 98 (17.5) | 41 (28.5) | 6 (28.6) | <0.0001 |
| Renal failure, n (%) | 28 (3.4) | 42 (7.5) | 12 (8.3) | 3 (14.3) | <0.003 |
| Circulatory failure, n (%) | 66 (8.1) | 139 (24.8) | 49 (34) | 9 (42.9) | <0.0001 |
| Length of stay (days) | 5.6±16.1 | 9.6±15.6 | 14.7±21.0 | 18.0±29.4 | <0.0001 |
| Mortality, n (%) | 51 (6.3) | 96 (17.1) | 40 (27.8) | 7 (33.3) | <0.0001 |
1, data expressed by median and minimum and maximum in parentheses. COVID-19, corona virus disease 2019; AST, aspartate aminotransferase; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; SII, systemic immune-inflammation index, ICU, intensive care unit; ULN, upper limits of normal.
Table 3
| Demographics and laboratory features | Normal | 1.1–2× ULN | 2.1–5× ULN | >5× ULN | P values |
|---|---|---|---|---|---|
| Age (years) | 58±19 | 55±17 | 52±15 | 51±20 | 0.02 |
| Gender, n (%) | <0.0001 | ||||
| Male | 507 (43.8) | 177 (61.9) | 51 (60.7) | 5 (45.5) | |
| Female | 651 (56.2) | 109 (38.1) | 33 (39.3) | 6 (54.5) | |
| White blood cell count ×109/L¹ | 6.5 (0–143.3) | 6.8 (1.2–32) | 6.5 (2.3–17.1) | 12.4 (3.3–38.7) | <0.0001 |
| Neutrophils ×109/L¹ | 4.3 (0–34.7) | 5 (0–29) | 4.4 (1.3–14.5) | 8.8 (1.5–22.3) | <0.0001 |
| Lymphocytes ×109/L¹ | 1.1 (0–59.2) | 1 (0–21.5) | 1.2 (0–55) | 1.3 (0–3.6) | 0.38 |
| Monocytes ×109/L¹ | 0.5 (0–7.4) | 0.5 (0–1.6) | 0.5 (0–1.8) | 0.4 (0–4.6) | 0.005 |
| Platelets ×109/L¹ | 218 (0–788) | 215.5 (62–767) | 219 (123–461) | 183 (78.5–534) | 0.97 |
| NLR¹ | 3.50 (0–119.0) | 4.80 (0.25–93.5) | 3.90 (0.50–46.0) | 5.60 (1.13–62.8) | 0.006 |
| MLR¹ | 0.43 (0–6.0) | 0.47 (0.04–6.65) | 0.42 (0.07–2.20) | 0.54 (0.27–2.30) | 0.61 |
| SII¹ | 0.7 (0–66.1) | 1 (0.03–38.7) | 0.9 (0.097–12.3) | 1.2 (0.088–12.4) | 0.01 |
| Outcomes | |||||
| Hospital admission, n (%) | 620 (53.5) | 194 (67.8) | 61 (72.6) | 10 (90.9) | <0.0001 |
| Admission to the ICU, n (%) | 339 (29.3) | 101 (35.3) | 27 (32.1) | 9 (81.8) | 0.001 |
| Organ failure, n (%) | 205 (17.7) | 51 (17.8) | 12 (14.3) | 5 (45.5) | 0.09 |
| Number of organ failure, n (%) | |||||
| 0 | 953 (82.3) | 235 (82.2) | 72 (85.7) | 6 (54.5) | 0.01 |
| 1 | 59 (5.1) | 10 (3.5) | 3 (3.6) | 0 | |
| 2 | 100 (8.6) | 24 (8.4) | 8 (9.5) | 2 (18.2) | |
| 3 | 46 (4.0) | 17 (5.9) | 1 (1.2) | 3 (27.3) | |
| Respiratory failure, n (%) | 139 (12.0) | 40 (14.0) | 9 (10.7) | 5 (45.5) | 0.007 |
| Renal failure, n (%) | 63 (5.4) | 18 (6.3) | 1 (1.2) | 3 (27.3) | 0.004 |
| Circulatory failure, n (%) | 195 (16.8) | 51 (17.8) | 12 (14.3) | 5 (45.5) | 0.08 |
| Length of stay (days) | 7.7±17.4 | 9.2±15.4 | 7.2±10.4 | 19.9±34.7 | 0.06 |
| Mortality, n (%) | 141 (12.2) | 39 (13.6) | 7 (8.3) | 7 (63.6) | <0.0001 |
1, data expressed by median and minimum and maximum in parentheses. COVID-19, corona virus disease 2019; ALT, alanine aminotransferase; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; SII, systemic immune-inflammation index; ICU, intensive care unit; ULN, upper limits of normal.
Discussion
This is one of the largest single-center studies concerning the frequency and impact of abnormal baseline liver enzymes on outcomes from COVID-19. Almost half of patients had high AST and/or ALT levels. Similar frequencies of LFT abnormalities were described in patients from the United States and Europe (2,4,5,19-22). This is in contrast to the much lower rates of LFT elevations reported in China (2,3), supporting the observation that aminotransferase derangements seen in COVID-19 are much more common in Western subjects, when compared to those originating from the East (2,23). Interestingly, one previous study from Brazil has also reported much lower frequencies of AST and ALT elevations (24), but the authors only took those with levels of double the upper limit into account, which are less commonly seen in COVID-19. Indeed, most patients with COVID-19 in the present study, and several other reports had mild abnormalities of AST and/or ALT (2,4,5,11,14,19,25). The preponderance of abnormal levels of AST over ALT has been reported in several studies (4,5,19,22,25), particularly from the United States (14-16,20,26,27). Some studies have reported a parallel increase of ALT and AST suggesting that this concordance may reflect a pattern of liver injury (22), which could be induced by different mechanisms including drug-induced, aggravation of previous CLD or cholangiocyte and/or endothelial cell dysfunction due to a cytokine storm (1,2,28,29). Others have attributed the prominent AST fluctuations seen in COVID-19 to mitochondrial injury reflecting uncontrolled systemic inflammation and extrahepatic organ dysfunction (9,28).
Several xenobiotics (30) have been linked to drug-induced liver injury (DILI) in subjects with SARS-CoV-2 infection including lopinavir/ritonavir (26,31); remdesevir (26) and immunomodulatory drugs (29). In the present study, baseline LFT assessed at ED were evaluated prior to the use of most of the aforementioned drugs, but we may not exclude the other commonly prescribed drugs for outpatients with COVID-19, such as analgesics, antipyretics or antibiotics, could have influenced the LFT derangements seen in our patients. Worsening of subjacent CLD seems to be a very unlikely reason for those LFT abnormalities, since they were reported in less than 4% of those affected subjects worldwide (16,25,32) and are not so commonly found in the general Brazilian population (33). It is therefore plausible to hypothesize that uncontrolled inflammation could give rise to abnormal LFT seen in COVID-19. In agreement with several other reports, the frequency and magnitude of AST and ALT elevations in the present study correlated with leukocyte and neutrophil counts as well as with other leukocyte markers of inflammation (5,14,27,34). This suggests that aminotransferase elevations seen in COVID-12 may be the liver component of the cytokine storm seen in SARS-CoV-2 infections, that may lead to OF and increased mortality (35,36).
Several studies have associated abnormal baseline LFT either with adverse outcomes, such as ICU admission, or OF (3,4,11,16,22,31), or mortality (13,14,16,19,21,24,31,34) in patients with COVID-19, but others have failed to disclose an association either with severity (20,37) or mortality (4,37) or reported an association with severity (3) or mortality (14) restricted or more marked in subjects with abnormal AST. Other reports have associated adverse outcomes to peak, rather than baseline, LFT levels, obtained during hospitalization (5,25,27,38). In the present study, several adverse outcomes, such as need for hospital or ICU admission, number and type of OF, LOS and mortality were associated with baseline LFT abnormalities. All adverse outcomes were significantly correlated to AST and/or ALT derangements, with the exception of OF presence, circulatory failure and LOS which were more frequently observed only in the group of patients with high ALT. Our findings suggest that abnormal LFT could be viewed as red flags in COVID-19, in association with other clinical and laboratory parameters, to identify subjects with a higher risk for adverse outcomes requiring hospitalization or at least scheduled reevaluation after discharge.
It is important to highlight that our study has several limitations due to its retrospective design. It is debatable whether those subjects with LFT abnormalities could be biased for hospitalization, but this is very unlikely because current triage recommendations for hospital admission do not take LFT levels into consideration at present. Another important limitation is the lack of LFT evaluation in all patients seeking the ED, nor their longitudinal assessment after hospital admission.
AST and ALT abnormalities are frequently seen in patients with COVID-19 evaluated at the ED. Their levels reflect ongoing COVID-19 related inflammatory response and may predict disease severity and mortality. Liver enzyme levels could be useful as red flags to adverse outcomes and may help decision-making for hospital admission or scheduled outpatient reassessment. Most patients with liver enzyme derangements have gradual decline of their AST and/or AST levels over two to three weeks without requirement of changes in clinical management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-41/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-41/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Federal University of Bahia, School of Medicine Ethics Committee Board (No. CAAE 53069121.6.0000.5577). Informed consent was waived because the study was conducted using an anonymized database, that has already been collected, offering no risk of harm or patient identification or even procedures for which written consent is normally required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jothimani D, Venugopal R, Abedin MF, et al. COVID-19 and the liver. J Hepatol 2020;73:1231-40. [Crossref] [PubMed]
- Bertolini A, van de Peppel IP, Bodewes FAJA, et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology 2020;72:1864-72. [Crossref] [PubMed]
- Kovalic AJ, Huang G, Thuluvath PJ, et al. Elevated Liver Biochemistries in Hospitalized Chinese Patients With Severe COVID-19: Systematic Review and Meta-analysis. Hepatology 2021;73:1521-30. [Crossref] [PubMed]
- Hundt MA, Deng Y, Ciarleglio MM, et al. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology 2020;72:1169-76. [Crossref] [PubMed]
- Phipps MM, Barraza LH, LaSota ED, et al. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology 2020;72:807-17. [Crossref] [PubMed]
- Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int 2020;40:2038-40. [Crossref] [PubMed]
- Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135-40. [Crossref] [PubMed]
- Du M, Yang S, Liu M, et al. COVID-19 and liver dysfunction: Epidemiology, association and potential mechanisms. Clin Res Hepatol Gastroenterol 2022;46:101793. [Crossref] [PubMed]
- Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the Liver: Lessons Learnt from the EAST and the WEST, A Year Later. J Viral Hepat 2022;29:4-20. [Crossref] [PubMed]
- Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 2021;93:250-6. [Crossref] [PubMed]
- Youssef M, H, Hussein M, Attia AS, et al. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol 2020;92:1825-33. [Crossref] [PubMed]
- Yadav DK, Singh A, Zhang Q, et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut 2021;70:807-9. [Crossref] [PubMed]
- Ding ZY, Li GX, Chen L, et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol 2021;74:1295-302. [Crossref] [PubMed]
- Lei F, Liu YM, Zhou F, et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology 2020;72:389-98. [Crossref] [PubMed]
- Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol 2020;73:455-8. [Crossref] [PubMed]
- Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther 2020;52:584-99. [Crossref] [PubMed]
- Lowsby R, Gomes C, Jarman I, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J 2015;32:531-4. [Crossref] [PubMed]
- Bernsmeier C, Cavazza A, Fatourou EM, et al. Leucocyte ratios are biomarkers of mortality in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. Aliment Pharmacol Ther 2020;52:855-65. [Crossref] [PubMed]
- Bernstein D, Roth N, Kim A, et al. Presentation, patterns and predictive value of baseline liver tests on outcomes in COVID-19 patients without chronic liver disease. World J Gastroenterol 2021;27:7350-61. [Crossref] [PubMed]
- Leal T, Costa E, Arroja B, et al. Gastrointestinal manifestations of COVID-19: results from a European centre. Eur J Gastroenterol Hepatol 2021;33:691-4. [Crossref] [PubMed]
- Weber S, Hellmuth JC, Scherer C, et al. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut 2021;70:1925-32. [Crossref] [PubMed]
- Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology 2021;73:890-900. [Crossref] [PubMed]
- Sultan S, Altayar O, Siddique SM, et al. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020;159:320-334.e27. [Crossref] [PubMed]
- Pozzobon FM, Perazzo H, Bozza FA, et al. Liver injury predicts overall mortality in severe COVID-19: a prospective multicenter study in Brazil. Hepatol Int 2021;15:493-501. [Crossref] [PubMed]
- Siddiqui MA, Suresh S, Simmer S, et al. Increased Morbidity and Mortality in COVID-19 Patients with Liver Injury. Dig Dis Sci 2022;67:2577-83. [Crossref] [PubMed]
- Leo M, Galante A, Pagnamenta A, et al. Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points. Dig Liver Dis 2022;54:565-71. [Crossref] [PubMed]
- Sobotka LA, Esteban J, Volk ML, et al. Acute Liver Injury in Patients Hospitalized with COVID-19. Dig Dis Sci 2022;67:4204-14. [Crossref] [PubMed]
- Yu D, Du Q, Yan S, et al. Liver injury in COVID-19: clinical features and treatment management. Virol J 2021;18:121. [Crossref] [PubMed]
- McConnell MJ, Kawaguchi N, Kondo R, et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol 2021;75:647-58. [Crossref] [PubMed]
- Gabrielli M, Franza L, Esperide A, et al. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines (Basel) 2022;10:192. [Crossref] [PubMed]
- Cai Q, Huang D, Yu H, et al. COVID-19: Abnormal liver function tests. J Hepatol 2020;73:566-74. [Crossref] [PubMed]
- Sharma A, Jaiswal P, Kerakhan Y, et al. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol 2021;21:100273. [Crossref] [PubMed]
- Perazzo H, Pacheco AG, De Boni R, et al. Age-Standardized Mortality Rates Related to Cirrhosis in Brazil from 2000 to 2012: A nationwide Analysis. Ann Hepatol 2017;16:269-78. [Crossref] [PubMed]
- Chen LY, Chu HK, Bai T, et al. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis 2020;21:512-8. [Crossref] [PubMed]
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 2020;383:2255-73. [Crossref] [PubMed]
- Zhao W, Li H, Li J, et al. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J Med Virol 2022;94:1886-92. [Crossref] [PubMed]
- Shehab M, Alrashed F, Shuaibi S, et al. Gastroenterological and hepatic manifestations of patients with COVID-19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta-analysis. BMJ Open Gastroenterol 2021;8:e000571. [Crossref] [PubMed]
- Wagner J, Garcia-Rodriguez V, Yu A, et al. Elevated transaminases and hypoalbuminemia in Covid-19 are prognostic factors for disease severity. Sci Rep 2021;11:10308. [Crossref] [PubMed]
Cite this article as: Farias JP, Codes L, Vinhaes D, Amorim AP, D’Oliveira RC, Farias AQ, Bittencourt PL. Impact of baseline abnormal liver enzymes in the outcome of COVID-19 infection. Transl Gastroenterol Hepatol 2023;8:5.


