
A comparison of magnetic resonance elastography (MRE) to biomarker testing for staging fibrosis in non-alcoholic fatty liver disease (NAFLD)
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the world’s most prevalent form of chronic liver disease. It affects up to 30% of the US population and is associated with increasing morbidity and mortality (1,2). Obesity, dyslipidemia and diabetes mellitus are known predisposing risk factors for the development and progression of this condition (3). Their rising incidence is believed by many to be of epidemic proportions (4). This has led to predictions that NAFLD will soon become the leading indication for liver transplantation (5,6). NAFLD is a slowly progressive disease with a calculated fibrosis progression rate of 7.7 years per each stage of fibrosis (7). Fibrosis can be divided into different stages to show progression of liver disease from no fibrosis to cirrhosis. Staging is based on histological scoring models such as the Knodell, Metavir, and Ishak systems (8-10).
NAFLD can lead to progressively worsening liver fibrosis and in some individuals eventually results in cirrhosis. This puts these individuals at risk for known complications of advanced liver disease such as hepatocellular carcinoma (HCC) and esophageal varices. The strongest association between NAFLD and HCC is the development of non-alcoholic steatohepatitis (NASH) and NASH associated cirrhosis (11-13). HCC is a serious consequence of NASH cirrhosis and as a result screening and surveillance are crucial (14). The incidence of HCC in patients with cirrhosis in the setting of NAFLD has been noted to range from 2.4% to 12.8% over a 3.2- to 7.2-year period (15). When it comes to treating NAFLD, lifestyle modifications and treatment of underlying metabolic syndromes such as obesity, hyperlipidemia and diabetes are believed to be the main beneficial options (16). There are no FDA approved medications specifically for NAFLD or NASH with or without cirrhosis, however this continues to be a field where advancements are actively being made (17,18). As a result, it is imperative that accurate NAFLD fibrosis staging be obtained in order to predict patient outcomes and ensure proper screening for conditions such as HCC and esophageal varices is performed (19).
Currently, there are several methods used for NAFLD fibrosis staging (20,21). Liver biopsy has historically been considered the gold standard despite being random, invasive, costly and timely (22). Reliability is compromised given biopsy is associated with great tissue sampling variability (23). Fibrosis staging, as determined by pathologists, has been shown to vary by as much as 20% in one study, while another reported a paired biopsy discordance of one or more fibrosis stage in 41% of cases (24,25). In addition, biopsy requires expertise and harbors risk with some patients being either unable or unwilling to undergo this invasive procedure (26). Other than biopsy, ultrasound based testing such as transient elastography (TE), radiographic testing such as magnetic resonance elastography (MRE) and biomarker testing using calculations such as Fibrosis-4 (FIB-4), NAFLD Fibrosis Score (NFS) and AST to Platelet Ratio Index (APRI) are additional clinical staging options.
TE has been proven effective, especially when diagnosing advanced fibrosis. As fibrosis increases, the sensitivity, specificity and NPV of TE improves in NAFLD patients (27). However, it is a test that only evaluates a limited portion of the liver and underlying medical conditions such as obesity, congestive hepatopathy, cholestasis and elevated liver enzymes are known confounders that result in testing inaccuracy (28). MRE, as compared to TE, has been shown to offer a higher degree of accuracy. This is of significant clinical importance given the ability to accurately predict adverse pathologic events related to increased liver stiffness (29-36). As a result, this new test has gained recognition as an important screening tool and is being used more frequently. Biomarker testing via calculations such as FIB-4, NFS and APRI is another important staging option (37). Although FIB-4 was originally created for use in viral hepatitis, it has seen been proven to accurately evaluate NAFLD as well (38,39). The FIB-4 calculator uses age, platelet count, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). AST and ALT are liver enzymes that are known to be elevated in the setting of liver damage. NFS uses the same values as FIB-4, in addition to Albumin, BMI and impaired fasting glucose/diabetes. These two tests are known to have good predictive value, most prominently when staging those with advanced fibrosis or cirrhosis (10,40,41). In addition, FIB-4 and NFS have been proven to correlate most accurately with other scoring algorithms when dealing with advanced fibrosis staging as well (42). APRI, however, has been shown to perform poorly as compared to NFS when discriminating between different stages of fibrosis (43,44).
Given the rising incidence of both NAFLD and its associated morbidity and mortality, it is important to establish protocols for both diagnostic purposes and fibrosis progression monitoring. There have been many studies evaluating the efficacy and accuracy of MRE and TE in NAFLD fibrosis staging (45,46). However, to date no studies have specifically assessed the relationship and correlation between MR Elastography and biomarker tests FIB-4 and NFS at different stages of liver fibrosis in patients with NAFLD. Most studies tend to assess their accuracy as compared to biopsy, not as compared to each other. Here, we evaluate how well biomarker staging corresponds with MRE staging in NAFLD. There are many cases where patients may be either unable or unwilling to undergo biopsy and we hope that our study can help physicians determine what the best and necessary next steps might be in these situations. Biomarker testing alone may be a sufficient means for staging, either in certain subgroups or as a whole. Of note, we present this article in accordance with the STARD reporting checklist (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-27/rc).
Methods
We conducted an IRB approved (Northwell Health Protocol Registration # 19-0465) retrospective chart review of all patients evaluated within our hospital system who were diagnosed with NAFLD with age ≥18 years old and no history of other concurrent liver disease. We looked at all patients who obtained MR Elastography for fibrosis staging between 2015 and 2020. Patients were excluded if they did not have labs drawn within 6 months of MRE. They were also excluded if they had a separate medical condition that could result in lab abnormalities and thus inaccurate biomarker test staging; for example thrombocytopenia from a non-liver etiology. We reviewed medical records containing patient demographics, medical conditions, medications, laboratory testing and radiologic imaging. Informed consent was not obtained in accordance with our IRB approved protocol for this retrospective chart review. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
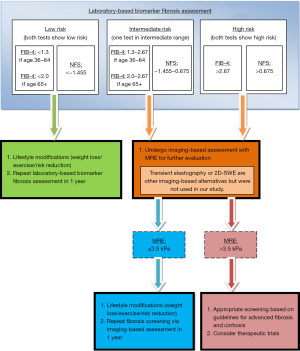
Biomarker testing was completed using FIB-4, which categorized patients as advanced fibrosis excluded (score of <1.3 if aged 36–64 and <2.0 if aged 65+), further investigation needed (1.3–2.67 if aged 36–64 and 2.0–2.67 if aged 65+), or advanced fibrosis likely (>2.67). Patients 35 years or younger were excluded from FIB-4 assessment. Biomarker testing using NFS categorized patients as F0-2 (score of <−1.455), Indeterminate (−1.455 to 0.675), or F3–F4 (>0.675).
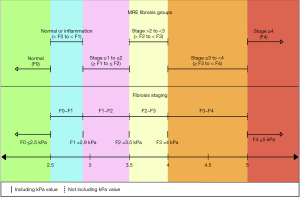
Patients were stratified into six different fibrosis groups based on MRE kPa values. The six groups are: Normal, Normal or Inflammation, Stage ≥1 to ≤2, Stage >2 to <3, Stage ≥3 to <4, Stage ≥4. These six groups are designed to differentiate NAFLD based on the five fibrosis stages: F0, F1, F2, F3 and F4. F4 is considered to be cirrhosis. The MRE kPa value cutoffs used for our study to divide the groups are 2.5, 2.9, 3.5, 4 and 5 (30). These kPa values are the standard cutoffs adopted by Radiologists throughout our hospital system. A value ≤2.5 kPa represents normal liver tissue while >2.5 kPa indicates the presence of inflammatory liver changes. Based on this, the ‘normal’ group as seen in Figure 1, which represents fibrosis stage F0, consists of all MRE kPa ≤2.5. At 2.9 kPa the liver stiffness is deemed to have stage 1 fibrosis while 3.5 kPa is the value used to signify that liver fibrosis has reached stage 2. As a result, the ‘normal or inflammation’ group, which represents fibrosis staging > F0 to < F1, contains MRE from kPa >2.5 to <2.9 kPa while ‘stage ≥1 to ≤2’, which represents fibrosis stage ≥F1 to ≤ F2, consists of MRE kPa from ≥2.9 kPa to ≤3.5 kPa. At 4 kPa the liver is considered to have reached stage 3 fibrosis and a value of 5 kPa is the final cutoff signifying progression to stage 4 fibrosis. Therefore, the ‘stage >2 to <3’ group, which represents fibrosis staging > F2 to < F3, contains MRE kPa >3.5 kPa to <4 kPa while ‘stage ≥3 to <4’, which represents fibrosis staging ≥ F3 to < F4, consists of MRE kPa values ≥4 kPa to <5 kPa. The final group, ‘stage ≥4’, represents fibrosis stage F4 and consists of all kPa ≥5 kPa.
Statistical analysis
Patient demographics, biomarker testing classifications and MRE categories were analyzed using descriptive statistics. All variables were considered as categorical and were described using frequency and percentage. They were compared using a Chi-Square Test or Fisher’s Exact Test. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of biomarker testing (FIB-4 and NFS) as compared to MRE was computed two different ways. First, to rule out the presence of advanced fibrosis/cirrhosis, FIB-4 testing was dichotomized as Advanced Fibrosis Excluded vs. other (which includes the Further Investigation Needed and Advanced Fibrosis Likely groups) and NFS testing was dichotomized as F0-2 vs. other (which includes the Indeterminate and F3–F4 groups). These were compared to MRE, which was dichotomized as ‘stage ≥1 to ≤2’ fibrosis or lower vs. ‘stage >2 to <3’ fibrosis or higher. Second, to identify the presence of advanced fibrosis/cirrhosis, FIB-4 testing was dichotomized as Advanced Fibrosis Likely vs. other (which includes the Further Investigation Needed and Advanced Fibrosis Excluded groups) and NFS testing dichotomized as F3-4 vs. other (which includes the Indeterminate and F0-F2 groups). These were compared to MRE, which was dichotomized as ‘stage ≥3 to <4’ fibrosis or higher vs. ‘stage >2 to <3’ fibrosis or lower. Subsequently, the correlation between each dichotomized biomarker test was compared with the corresponding dichotomized MRE staging using Pearson Correlation Coefficients. For FIB-4 testing, correlations were additionally stratified by age category for those >35 years old. All statistical analysis was done using SAS (9.4) with P<0.05 considered statistically significant.
Results
Among 193 total patients, 22 (11.40%) did not receive a FIB-4 score as they were ≤35 years old. In terms of categorization, 100 (51.81%) were Advanced Fibrosis Excluded using FIB-4 while 39 (20.21%) were Further Investigation Needed and 32 (16.58%) were Advanced Fibrosis Likely. Among the same population, 98 (50.78%) patients were considered F0-2 using NFS, 63 (32.64%) were considered indeterminate and 32 (16.58%) were F3-4. There were 80 (41.45%) patients considered ‘Normal’ using MRE, 39 (20.21%) ‘Normal or Inflammation’, 23 (11.92%) ‘stage ≥1 to ≤2’, 9 (4.66%) ‘stage >2 to <3’ fibrosis, 21 (10.88%) ‘stage ≥3 to <4’ fibrosis, and 21 (10.88%) ‘stage ≥4’ fibrosis/cirrhosis. Demographic characteristics, FIB-4 and NFS scores were summarized for each MRE fibrosis stage (Table 1).
Table 1
| Demographics and biomarker scoring |
Fibrosis stage based on MRE | P value | |||||
|---|---|---|---|---|---|---|---|
| Normal | Normal or inflammation | Stage ≥1 to ≤2 |
Stage >2 to <3 |
Stage ≥3 to <4 |
Stage ≥4 | ||
| Age (years) | 0.0323 | ||||||
| ≤35 | 12 (15.00) | 5 (12.82) | 2 (8.70) | 0 (0.00) | 2 (9.52) | 1 (4.76) | |
| 36–64 | 55 (68.75) | 21 (53.85) | 13 (56.52) | 4 (44.44) | 9 (42.86) | 9 (42.86) | |
| ≥65 | 13 (16.25) | 13 (33.33) | 8 (34.78) | 5 (55.56) | 10 (47.62) | 11 (52.38) | |
| Sex | 0.7371 | ||||||
| Male | 34 (42.50) | 18 (46.15) | 13 (56.52) | 5 (55.56) | 12 (57.14) | 11 (52.38) | |
| Female | 46 (57.50) | 21 (53.85) | 10 (43.48) | 4 (44.44) | 9 (42.86) | 10 (47.62) | |
| FIB-4 score | <0.0001 | ||||||
| No score (≤35 years) | 12 (15.00) | 5 (12.82) | 2 (8.70) | 0 (0.00) | 2 (9.52) | 1 (4.76) | |
| Advanced fibrosis excluded | 56 (70.00) | 19 (48.72) | 9 (39.13) | 5 (55.56) | 9 (42.86) | 2 (9.52) | |
| Further investigation needed | 8 (10.00) | 12 (30.77) | 9 (39.13) | 2 (22.22) | 6 (28.57) | 2 (9.52) | |
| Advanced fibrosis likely | 4 (5.00) | 3 (7.69) | 3 (13.04) | 2 (22.22) | 4 (19.05) | 16 (76.19) | |
| NFS score | <0.0001 | ||||||
| F0-F2 | 61 (76.25) | 18 (46.15) | 8 (34.78) | 3 (33.33) | 7 (33.33) | 1 (4.76) | |
| Indeterminate | 16 (20.00) | 20 (51.28) | 11 (47.83) | 5 (55.56) | 7 (33.33) | 4 (19.05) | |
| F3-F4 | 3 (3.75) | 1 (2.56) | 4 (17.39) | 1 (11.11) | 7 (33.33) | 16 (76.19) | |
The numbers in parentheses represent the percentage with regards to the column within each subcategory. P value for sex using Chi-Square test, others using Fisher’s Exact test since cross tabulations had >25% of cells with counts <5. MRE, magnetic resonance elastography; FIB-4, Fibrosis-4; NFS, NAFLD Fibrosis Score; NAFLD, non-alcoholic fatty liver disease.
The sensitivity, specificity, PPV and NPV for each dichotomized measure is presented in Tables 2,3 while the correlations between the dichotomized biomarker and MRE results are presented in Table 4. Table 2 shows the data for ruling out the presence of advanced fibrosis/cirrhosis while the data for identifying the presence of advanced fibrosis/cirrhosis is seen in Table 3. The raw data for these calculations can be seen in Tables 5-8. Most notably, for ruling out advanced fibrosis/cirrhosis, the NPV for FIB-4 (Advanced Fibrosis Excluded vs. other) when compared to MRE fibrosis staging was found to be 0.84 (95% CI: 0.77, 0.91) and the NPV for NFS (F0-2 vs. other) was found to be 0.89 (95% CI: 0.83, 0.95). For identifying the presence of advanced fibrosis/cirrhosis, the PPV for FIB-4 (Advanced Fibrosis Likely vs. other) was found to be 0.63 (95% CI: 0.46, 0.79), and the PPV for NFS (F3-4 vs. other) was found to be 0.72 (95% CI: 0.56, 0.87).
Table 2
| Biomarker test and classification | MRE fibrosis stage (‘stage ≥1 to ≤2’ fibrosis or lower vs. ‘stage >2 to <3’ fibrosis or higher) |
|||
|---|---|---|---|---|
| SE | SP | PPV | NPV | |
| FIB-4 (advanced fibrosis excluded vs. other) | 0.67 (0.53, 0.80) | 0.68 (0.60, 0.77) | 0.45 (0.34, 0.57) | 0.84 (0.77, 0.91) |
| NFS (F0-2 vs. other) | 0.78 (0.67, 0.90) | 0.61 (0.53, 0.69) | 0.42 (0.32, 0.52) | 0.89 (0.83, 0.95) |
Confidence intervals are listed in parentheses. SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; FIB-4, Fibrosis-4; NFS, NAFLD Fibrosis Score; NAFLD, non-alcoholic fatty liver disease.
Table 3
| Biomarker test and classification | MRE fibrosis stage (‘stage ≥3 to <4’ fibrosis or higher vs. ‘stage >2 to <3’ fibrosis or lower) |
|||
|---|---|---|---|---|
| SE | SP | PPV | NPV | |
| FIB-4 (advanced fibrosis likely vs. other) | 0.51 (0.36, 0.67) | 0.91 (0.86, 0.96) | 0.63 (0.46, 0.79) | 0.86 (0.81, 0.92) |
| NFS (F3-4 vs. other) | 0.55 (0.40, 0.70) | 0.94 (0.90, 0.98) | 0.72 (0.56, 0.87) | 0.88 (0.83, 0.93) |
Confidence intervals are listed in parentheses. SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; FIB-4, Fibrosis-4; NFS, NAFLD Fibrosis Score; NAFLD, non-alcoholic fatty liver disease.
Table 4
| Biomarker test classification | MRE fibrosis stage | |
|---|---|---|
| ‘Stage ≥1 to ≤2’ fibrosis or lower vs. ‘Stage >2 to <3’ fibrosis or higher |
‘Stage ≥3 to <4’ fibrosis or higher vs. ‘Stage >2 to <3’ fibrosis or lower |
|
| FIB-4 (ages 36+, N=171) | ||
| Advanced fibrosis excluded vs. other | 0.32 | – |
| Advanced fibrosis likely vs. other | – | 0.45 |
| FIB-4 (ages 36–64, N=111) | ||
| Advanced fibrosis excluded vs. other | 0.43 | – |
| Advanced fibrosis likely vs. other | – | 0.52 |
| FIB-4 (ages 65+, N=60) | ||
| Advanced fibrosis excluded vs. other | 0.11 | – |
| Advanced fibrosis likely vs. other | – | 0.33 |
| NFS (overall, N=193) | ||
| F0-2 vs. other | 0.35 | – |
| F3-4 vs. other | – | 0.54 |
Pearson correlation coefficients presented. FIB-4, Fibrosis-4; NFS, NAFLD Fibrosis Score; MRE, magnetic resonance elastography; NAFLD, non-alcoholic fatty liver disease.
Table 5
| MRE classification | Fibrosis category | |
|---|---|---|
| Advanced fibrosis likely | Further investigation needed and advanced fibrosis excluded | |
| ‘Stage ≥3 to <4’ fibrosis or higher | 20 | 19 |
| ‘Stage >2 to <3’ fibrosis or lower | 12 | 120 |
FIB-4, Fibrosis-4; MRE, magnetic resonance elastography.
Table 6
| MRE classification | Fibrosis category | |
|---|---|---|
| F3–F4 | Indeterminate and F0–F2 | |
| ‘Stage ≥3 to <4’ fibrosis or higher | 23 | 19 |
| ‘Stage >2 to <3’ fibrosis or lower | 9 | 142 |
NFS, NAFLD Fibrosis Score; NAFLD, non-alcoholic fatty liver disease; MRE, magnetic resonance elastography.
Table 7
| MRE classification | Fibrosis category | |
|---|---|---|
| Advanced fibrosis likely and further investigation needed | Advanced fibrosis excluded | |
| ‘Stage >2 to <3’ fibrosis or higher | 32 | 16 |
| ‘Stage ≥1 to ≤2’ fibrosis or lower | 39 | 84 |
FIB-4, Fibrosis-4; MRE, magnetic resonance elastography.
Table 8
| MRE classification | Fibrosis category | |
|---|---|---|
| F3–F4 and indeterminate | F0–F2 | |
| ‘Stage >2 to <3’ fibrosis or higher | 40 | 11 |
| ‘Stage ≥1 to ≤2’ fibrosis or lower | 55 | 87 |
NFS, NAFLD Fibrosis Score; NAFLD, non-alcoholic fatty liver disease; MRE, magnetic resonance elastography.
In Table 4 we see correlation values comparing the dichotomized biomarker results to MRE results. For ruling out the presence of advanced fibrosis/cirrhosis using FIB-4, the overall correlation between FIB-4 (Advanced Fibrosis Excluded vs. other) and MRE (‘stage ≥1 to ≤2’ fibrosis or lower vs. ‘stage >2 to <3’ fibrosis or higher) showed a low positive correlation (r=0.32). After stratifying by age it was even lower for those who are 65 and older (r=0.11), while slightly higher for those who are 36–64 (r=0.43). For ruling out the presence of advanced fibrosis/cirrhosis using NFS, there was also a low positive correlation (r=0.35) found between NFS (F0-2 vs. other) and MRE fibrosis stage (‘stage ≥1 to ≤2’ fibrosis or lower vs. ‘stage >2 to <3’ fibrosis or higher).
For identifying the presence of advanced fibrosis/cirrhosis using FIB-4, moderate positive correlation (r=0.45) was found between FIB-4 (Advanced Fibrosis Likely vs. other) and MRE fibrosis stage (‘stage ≥3 to <4’ fibrosis or higher vs. ‘stage >2 to <3’ fibrosis or lower). It was again higher for those 36–64 (r=0.52) and lower for those 65 and older (r=0.33). For identifying the presence of advanced fibrosis/cirrhosis using NFS, there was a moderate correlation (r=0.54) between NFS (F3-4 vs. other) and MRE fibrosis stage (‘stage ≥3 to <4’ fibrosis or higher vs. ‘stage >2 to <3’ fibrosis or lower).
Discussion
The main implication for NAFLD fibrosis staging is to assess for advanced liver disease. Staging stratifies patients with varying degrees of fibrosis to identify those who benefit from proper screening for complications such as HCC and esophageal varices. Noninvasive imaging techniques such as MRE have become widely used, however accessibility remains limited and cost effectiveness is unclear (47,48). It is important to note that liver biopsy remains the gold standard for fibrosis staging, however in patients who are either unable or unwilling to undergo biopsy, MRE and biomarker testing are well known and frequently used alternatives. The purpose of our study is not to imply that biopsy should be replaced by these other options but instead to determine whether or not patients who are planning to undergo MRE truly require it in all cases. Correlating NAFLD fibrosis staging via MRE to biomarker testing may prove MRE excessive in some situations (49). A study that were to show biomarker testing alone is sufficient for staging in certain cases would be of significant clinical importance, especially for those with financial constraints or those unable to undergo MRE. Our analysis suggested that some patients with NAFLD may only require biomarker testing to classify their fibrosis. We hope our results can help pave the way for cost-effective, standardized guidance to be used when staging fibrosis and recommending appropriate screening for NAFLD.
Our results showed positive correlations for all of the subgroups analyzed in comparing MRE fibrosis staging to that of either FIB-4 or NFS. For ruling out advanced fibrosis, the lower correlation coefficients obtained for FIB-4 (r=0.32) and NFS (r=0.35) suggest that early stage NAFLD is certainly associated with some variability with regards to the exact stage of fibrosis determined via MRE as compared to FIB-4 or NFS. As NAFLD progresses to late stage disease, the higher correlation coefficients obtained for advanced fibrosis show that fibrosis staging as determined by FIB-4 (r=0.45) and NFS (r=0.54) corresponds more closely to the stage identified via MRE. These correlation coefficients suggest a higher probability of MRE characterizing liver fibrosis to be the same stage that either FIB-4 or NFS does in advanced disease. This supports the previously discussed and known general consensus that both MRE and the biomarker scoring methods tend to be more accurate in late stage disease (50-52).
The NPV and PPV values obtained suggest that, when grouping patients together as either early stage NAFLD (rule out advanced fibrosis) or late stage NAFLD (identify advanced fibrosis/cirrhosis), MRE corresponds well with FIB-4 and NFS in identifying late stage disease while having an even stronger association in those with early stage disease. For early stage disease, NPV values for FIB-4 (0.84) and NFS (0.89) in the ‘rule out advanced fibrosis’ category signify that 84% and 89% of respective biomarker scores (‘Advanced Fibrosis Excluded vs. other’ for FIB-4 and ‘F0-2 vs. other’ for NFS) correspond to MRE categories ‘stage ≥1 to ≤2’ fibrosis or lower. For late stage disease, PPV values for FIB-4 (0.63) and NFS (0.72) in the ‘identify advanced fibrosis/cirrhosis’ category signify that 63% and 72% of respective biomarker scores (‘Advanced Fibrosis Likely vs. other’ for FIB-4 and ‘F3-4 vs. other’ for NFS) correspond to MRE categories ‘stage ≥3 to <4’ fibrosis or higher. This has significant clinical implications as it suggests that those deemed to have early stage NAFLD using FIB-4 and NFS were also found to have early stage NAFLD using MRE 84% and 89% of the time respectively. MRE, NFS and FIB-4 are known to be accurate fibrosis staging options which are widely believed to perform better in late stage NAFLD. This enables them to effectively differentiate early from late stage disease. Early stage NAFLD may only require lifestyle modifications and risk reduction to prevent progression of disease, whereas late stage disease requires more regimented screening for serious complications. Our data suggested that patients with early stage disease on biomarker testing are very likely to show early stage disease on MRE as well. In our study, 100 individuals fell into the advanced fibrosis excluded category using FIB-4, suggesting early stage disease. Out of these, MRE provided contradictory results in 11 cases where MRE staging suggested advanced fibrosis/cirrhosis. With regards to NFS, 98 individuals were in the F0-2 range showing early stage disease. However, 8 of those 98 showed advanced fibrosis/cirrhosis on MRE. We were unable to determine whether biomarker testing or MRE was more accurate in these instances of contradictory results without liver biopsy.
There are several different components of MRE that must be performed with technical proficiency in order to ensure proper and accurate results (53). When done correctly, MRE is known to be very accurate with impressive interobserver agreement when head-to-head comparisons were performed to confirm appropriate fibrosis staging and reproducibility (54-57). One notable pitfall is that the MRE kPa cutoff values used in our study [2.5, 2.9, 3.5, 4, 5] were not universally used. The greatest uncertainty and variability involves the cutoff used for differentiating normal liver from inflammation in the setting of early fibrosis (58,59).
Based on our aforementioned results, MRE seemed to be excessive in a large majority of patients given it provided the same information obtained using FIB-4 or NFS. These findings pose a question as to whether or not patients with FIB-4 and NFS scores in the ‘rule out advanced fibrosis’ category truly benefit from MRE testing as well. Our study showed that FIB-4 and NFS scores indicating little to no fibrosis correspond quite well with respective ‘normal, normal or inflammation and stage ≥1 to ≤2’ MRE staging, while scores suggesting advanced fibrosis/cirrhosis do correspond with respective ‘stage ≥3 to <4 and stage ≥4’ MRE staging but less convincingly. Although the clinical implication of MRE as an alternative to liver biopsy has recently gained recognition, our study suggested that FIB-4 and NFS remain very effective and sufficient in assessing fibrosis for those with FIB-4 scores indicating Advanced Fibrosis Excluded and NFS scores indicating F0-2. A large majority of cases may fare well with monitoring simply via biomarker testing. MRE could be more useful when biomarker staging indicates progression of disease since MRE has proven most accurate when staging advanced fibrosis/cirrhosis (59). We provide a proposed fibrosis screening algorithm based upon this in Figure 2.
One notable limitation in our study is the possibility of confounding medical conditions that may have unexpectedly affected biochemical results leading to inaccurate biomarker testing scores. We excluded patients from our study that had obvious conditions that could impact our results, however undiagnosed diseases could always be playing a role. With regards to MRE, patients are told to fast at least 4 hours prior to imaging to ensure the most accurate results. Unknown patient adherence was another aspect that we could not control and could have impacted our results. In addition, our limited sample size poses another limitation while the retrospective nature of the study also contributes to further constraints. Future prospective large series studies are warranted to further investigate the relationship between MRE and biomarker testing with regards to NAFLD fibrosis staging. As MRE continues to be utilized more often and data supporting its accuracy as compared to biopsy becomes more readily available, it is possible that it may become a noninvasive gold standard for NAFLD fibrosis staging and monitoring (60-62). However, there is a need for further universal guidance to standardize the kPa cutoff values of MRE for NAFLD, most notably in differentiating normal liver from mild fibrosis. We have noted cases where biomarker testing and MRE give conflicting results and it would be beneficial to further investigate these instances in an attempt to uncover the reasons for discrepancy in such cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-27/rc
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-27/dss
Peer Review File: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-22-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by our ethics committee (Northwell Health Protocol Registration # 19-0465). Informed consent was not obtained in accordance with our IRB approved protocol for this retrospective chart review. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11-20. [Crossref] [PubMed]
- Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531-44. [Crossref] [PubMed]
- Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med 2020;17:e1003100. [Crossref] [PubMed]
- Jennings J, Faselis C, Yao MD. NAFLD-NASH: An Under-Recognized Epidemic. Curr Vasc Pharmacol 2018;16:209-13. [Crossref] [PubMed]
- Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci 2016;17:774. [Crossref] [PubMed]
- Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017;152:1090-1099.e1. [Crossref] [PubMed]
- Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643-54.e1-9; quiz e39-40.
- Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431-5. [Crossref] [PubMed]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15-20. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018;155:1828-1837.e2. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019;156:1264-1281.e4. [Crossref] [PubMed]
- Reig M, Gambato M, Man NK, et al. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation 2019;103:39-44. [Crossref] [PubMed]
- Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 2016;36:317-24. [Crossref] [PubMed]
- Leoni S, Tovoli F, Napoli L, et al. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol 2018;24:3361-73. [Crossref] [PubMed]
- Dajani A, AbuHammour A. Treatment of nonalcoholic fatty liver disease: Where do we stand? an overview. Saudi J Gastroenterol 2016;22:91-105. [PubMed]
- Loomba R, Neuschwander-Tetri BA, Sanyal A, et al. Multicenter Validation of Association Between Decline in MRI-PDFF and Histologic Response in NASH. Hepatology 2020;72:1219-29. [Crossref] [PubMed]
- Galizzi HO, Couto CA, Taranto DOL, et al. Accuracy of non-invasive methods/models for predicting esophageal varices in patients with compensated advanced chronic liver disease secondary to nonalcoholic fatty liver disease. Ann Hepatol 2021;20:100229. [Crossref] [PubMed]
- Karanjia RN, Crossey MM, Cox IJ, et al. Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol 2016;22:9880-97. [Crossref] [PubMed]
- Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep 2020;2:100067. [Crossref] [PubMed]
- Berger D, Desai V, Janardhan S. Con: Liver Biopsy Remains the Gold Standard to Evaluate Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Liver Dis (Hoboken) 2019;13:114-6. [Crossref] [PubMed]
- Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med 2017;377:756-68. [Crossref] [PubMed]
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495-500. [Crossref] [PubMed]
- Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898-906. [Crossref] [PubMed]
- Heyens LJM, Busschots D, Koek GH, et al. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne) 2021;8:615978. [Crossref] [PubMed]
- Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Caspian J Intern Med 2016;7:242-52. [PubMed]
- Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26:1431-40. [Crossref] [PubMed]
- Gidener T, Ahmed OT, Larson JJ, et al. Liver Stiffness by Magnetic Resonance Elastography Predicts Future Cirrhosis, Decompensation, and Death in NAFLD. Clin Gastroenterol Hepatol 2021;19:1915-1924.e6. [Crossref] [PubMed]
- Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 2019;17:630-637.e8. [Crossref] [PubMed]
- Imajo K, Honda Y, Kobayashi T, et al. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2022;20:908-917.e11. [Crossref] [PubMed]
- Glaser KJ, Manduca A, Ehman RL. Review of MR elastography applications and recent developments. J Magn Reson Imaging 2012;36:757-74. [Crossref] [PubMed]
- Chen J, Yin M, Glaser KJ, et al. MR Elastography of Liver Disease: State of the Art. Appl Radiol 2013;42:5-12. [Crossref] [PubMed]
- Yin M, Glaser KJ, Talwalkar JA, et al. Hepatic MR Elastography: Clinical Performance in a Series of 1377 Consecutive Examinations. Radiology 2016;278:114-24. [Crossref] [PubMed]
- Yin Z, Murphy MC, Li J, et al. Prediction of nonalcoholic fatty liver disease (NAFLD) activity score (NAS) with multiparametric hepatic magnetic resonance imaging and elastography. Eur Radiol 2019;29:5823-31. [Crossref] [PubMed]
- Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver Int 2020;40:2242-51. [Crossref] [PubMed]
- Lee J, Vali Y, Boursier J, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int 2021;41:261-70. [Crossref] [PubMed]
- Younossi ZM, Noureddin M, Bernstein D, et al. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol 2021;116:254-62. [Crossref] [PubMed]
- Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin Gastroenterol Hepatol 2019;17:1877-1885.e5. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [Crossref] [PubMed]
- Hernandez Roman J, Siddiqui MS. The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Endocrinol Diabetes Metab 2020;3:e00127. [Crossref] [PubMed]
- Kim D, Kim WR, Talwalkar JA, et al. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology 2013;268:411-9. [Crossref] [PubMed]
- Peleg N, Issachar A, Sneh-Arbib O, et al. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis 2017;49:1133-8. [Crossref] [PubMed]
- Udelsman BV, Corey K, Hutter MM, et al. Use of noninvasive scores for advanced liver fibrosis can guide the need for hepatic biopsy during bariatric procedures. Surg Obes Relat Dis 2021;17:292-8. [Crossref] [PubMed]
- Venkatesh SK. Magnetic Resonance Elastography: An Update. Top Magn Reson Imaging 2018;27:303. [Crossref] [PubMed]
- Furlan A, Tublin ME, Yu L, et al. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol 2020;214:W20-6. [Crossref] [PubMed]
- Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 2018;15:274-82. [Crossref] [PubMed]
- Ryan JD, Tsochatzis EA. MRE in NAFLD: Promising but Further Validation is Required. Ann Hepatol 2017;16:331-2. [Crossref] [PubMed]
- Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther 2015;41:1271-80. [Crossref] [PubMed]
- Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617-49. [Crossref] [PubMed]
- Alkhouri N, McCullough AJ. Noninvasive Diagnosis of NASH and Liver Fibrosis Within the Spectrum of NAFLD. Gastroenterol Hepatol (N Y) 2012;8:661-8. [PubMed]
- Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920-8. [Crossref] [PubMed]
- Guglielmo FF, Venkatesh SK, Mitchell DG, Liver MR. Elastography Technique and Image Interpretation: Pearls and Pitfalls. Radiographics 2019;39:1983-2002. [Crossref] [PubMed]
- Rustogi R, Horowitz J, Harmath C, et al. Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging 2012;35:1356-64. [Crossref] [PubMed]
- Liang Y, Li D. Magnetic resonance elastography in staging liver fibrosis in non-alcoholic fatty liver disease: a pooled analysis of the diagnostic accuracy. BMC Gastroenterol 2020;20:89. [Crossref] [PubMed]
- Runge JH, Bohte AE, Verheij J, et al. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging 2014;39:283-90. [Crossref] [PubMed]
- Hines CD, Bley TA, Lindstrom MJ, et al. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging 2010;31:725-31. [Crossref] [PubMed]
- Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: clinical applications. J Comput Assist Tomogr 2013;37:887-96. [Crossref] [PubMed]
- Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells 2020;9:1005. [Crossref] [PubMed]
- Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2021;19:2274-2283.e5. [Crossref] [PubMed]
- Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab 2021;50:101167. [Crossref] [PubMed]
- Allen AM, Shah VH, Therneau TM, et al. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery. Hepatology 2020;71:510-21. [Crossref] [PubMed]
Cite this article as: Kaplan JM, Alexis J, Grimaldi G, Islam M, Izard SM, Lee TP. A comparison of magnetic resonance elastography (MRE) to biomarker testing for staging fibrosis in non-alcoholic fatty liver disease (NAFLD). Transl Gastroenterol Hepatol 2023;8:7.


