
Primary sclerosing cholangitis
Clinical features and diagnosis
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease that is characterized by intra- and/or extrahepatic bile duct injury (1). The clinical presentation of PSC correlates with the sequence of inflammatory bile duct destruction and fibrosis, which results in bile duct stricturing, cholestasis, and eventually biliary cirrhosis with end-stage liver disease and hepatic dysfunction (2). PSC is increasingly diagnosed early in the stage of the disease course, and, as a result, the majority of patients do not have any clinical symptoms at the time of diagnosis (3). In the majority of cases, the diagnosis of PSC is prompted by the finding of cholestasis at the time of routine health evaluation or screening of high-risk patients such as those with inflammatory bowel disease (IBD). In patients who present with symptoms, abdominal pain is the most frequent symptom (20%) followed by pruritus (10%), jaundice (6%), and fatigue (6%) (3), but the presentation may differ widely among patients. Hepatomegaly and splenomegaly can be present in 44% and 39% of patients, respectively (4,5). Acute pruritus and/or cholangitis, presenting with jaundice, fever, and abdominal pain, may be a result of benign or malignant biliary tract obstruction. Indeed, worsening cholestatic signs and symptoms should raise concern about cholangiocarcinoma (CCA), the most feared and not uncommon complication of PSC. Presentation with variceal bleeding, ascites, or hepatic encephalopathy may occur once a patient has progression to end-stage liver disease. In patients with associated IBD, abdominal pain, diarrhea, and gastrointestinal bleeding may be the only presenting symptoms along with abnormal liver biochemistries.
Elevations in serum alkaline phosphatase (ALP) and gamma-glutamyl transferase values in a cholestatic pattern are the biochemical hallmark of PSC, though up to 30% to 40% of patients have normal ALP at diagnosis or during the course of their disease (6,7). Increases of serum aspartate and alanine aminotransferase levels are usually less pronounced and typically less than 5 times the upper limits of normal (ULN). The serum total bilirubin level is normal in the majority of cases at diagnosis. Elevation in transaminases might suggest a more inflammatory disease with features of autoimmune hepatitis (AIH), which is present in approximately 5–14% of cases (8). Elevation in bilirubin concentrations suggest presence of dominant stricture (DS) or advanced liver disease.
Currently, no autoimmune antibodies are sufficiently specific in the diagnosis of PSC. The most commonly positive autoantibodies are the perinuclear antineutrophilic autoantibodies (pANCA), which are found in approximately 80% of patients but lack diagnostic specificity (9-12). Other autoantibodies such as antinuclear antibodies and anti-smooth muscle antibodies have been reported in 20% to 50% of individuals, and their presence, especially along with elevations of total immunoglobulins, or subsets, may warrant evaluation for AIH and PSC with features of AIH. Antimitochondrial antibodies are seldom positive in PSC. Serum immunoglobulin 4 (IgG4) levels are observed in approximately 10% of patients with PSC (13,14) in the absence of IgG4-related disease (IgG4-RD) and may be associated with poorer outcomes. The specificity of IgG4 for IgG4-related disease increases when levels of more than 4 times the ULN or IgG4:IgG1 ratio is greater than 0.24 (15).
Cholangiographic evaluation in patients with PSC typically reveals a beaded appearance of the intrahepatic and/or extrahepatic bile ducts, caused by multifocal strictures with intervening segments of normal or dilated bile ducts. The diagnostic modality of choice for the diagnosis of PSC is magnetic resonance cholangiopancreatography (MRCP), which has been shown in a meta-analysis to have a sensitivity of 86% and specificity of 94% when compared to endoscopic retrograde cholangiopancreatography (ERCP) (16). ERCP was once the gold standard for diagnosis, but it is invasive, associated with complications, and less cost effective in comparison to MRCP (17), thus is currently reserved for therapeutic interventions or diagnostic assessments of bile duct strictures. Invasive cholangiography might be more sensitive in detecting early disease compared to MRCP (97% overall diagnostic accuracy versus 90, respectively) (18) and thus might be considered in cases in which MRCP (and potentially liver biopsy) is negative but a high degree of clinical suspicion remain.
The histological hallmark of PSC is the finding of concentric periductal fibrosis, also known as “onion-skin fibrosis”, but it is only detected in less than 15% of liver biopsies of patients with PSC. Furthermore, it is not specific, having been described occasionally in bile duct obstruction, primary biliary cholangitis, ductopenic rejection post-liver transplant and intra-arterial chemotherapy (19). Liver biopsy is not necessary to diagnose PSC (20), unless there is suspicion for small duct PSC or PSC with features of AIH. In patients with clinical and biochemical features of PSC but with normal cholangiography, a liver biopsy should be considered for evaluation of small duct PSC (21). In those with a disproportionate elevation in aminotransferases, biopsy is also recommended to exclude overlap syndrome (22). Liver biopsy might be of value to assess the degree of interface hepatitis consistent with PSC-AIH overlap syndrome and might help decide if immunosuppressive therapy is indicated. Though liver biopsy has been historically performed for staging purposes, prognostic models and non-invasive fibrosis markers have obviated the need for liver biopsy for staging of the disease.
The diagnosis of PSC is typically established in patients with chronic cholestasis when cholangiographic studies (MRCP, ERCP, or percutaneous transhepatic cholangiogram) show characteristic features of PSC, and secondary forms of sclerosing cholangitis (23), as summarized in Table 1, are excluded (22).

Full table
IgG4-associated cholangitis (IAC), the biliary manifestation of IgG4-RD, is a distinct entity which might mimic PSC. This condition is responsive to steroids and is not a pre-malignant condition, which makes it clinically significant to distinguish from PSC. Guidelines recommend measuring IgG4 in all patients with PSC to exclude IAC. In particular, IAC is frequently found to be associated with pancreatic involvement in IgG4-RD and is termed AIP-SC. However, IAC can also be seen without concurrent pancreatitis and can be difficult to distinguish between PSC using cholangiography alone. Diagnosis is usually established based on two or more main manifestations: elevated serum IgG4, suggestive pancreatic imaging findings, other organ involvement and bile duct/ampullary biopsy (with >10 IgG4 positive cells/high power field) along with a significant response to steroid therapy (24).
Small duct PSC
Though the large bile ducts are affected in the majority of patients PSC, up to 9% of cases have disease that affects bile ducts that are too small to be identified by cholangiogram, referred to as small duct PSC (25). Small duct PSC is characterized by consistent liver biopsy findings and cholangiographically normal bile ducts (26,27) and appears to have a less aggressive course and to be less likely to lead to CCA compared to large duct PSC (28,29). Approximately 5–15% of patients with small duct PSC ultimately progress to large duct disease over the course of the disease (28,30), but the frequency of progression and its predictors are poorly defined.
PSC with features of AIH or PSC-AIH overlap syndrome
A subset of patients with PSC have additional biochemical, serological, and histological features typical for AIH, including markedly elevated transaminases and IgG, characteristic autoantibodies and moderate to severe interface hepatitis. Though there is no consensus on the nomenclature, or diagnosis, of this presentation and whether it is a distinct entity, it is commonly referred to as PSC-AIH overlap syndrome or PSC with features of AIH. Adequate recognition of such features is clinically relevant as the component of AIH may be responsive to steroids and patients may benefit from therapy with immunosuppressants (31,32). In children, due to the high prevalence of such syndrome, MRCP is recommended for patients with a diagnosis of AIH, especially if there is failure to respond to first-line steroid therapy (33,34). In adults, the reported prevalence varies between 7–14% depending on the diagnostic criteria used (8), and the described long-term outcomes are worse than classic AIH but better than PSC (35).
Demographics and epidemiology
PSC is diagnosed more commonly in men (65–70%) between the ages of 30 and 40 years, though it can occur at any age.
The prevalence of PSC is highest among patients with underlying IBD. Indeed, up to 70% of patients have concurrent IBD (36), most often characterized as ulcerative colitis (UC). One study found that approximately 8% of patients with IBD who were screened with MRCP had cholangraphic features consistent with PSC (37). In general, IBD is diagnosed several years earlier than PSC (38), but it can be diagnosed at any time during the course of PSC, including after liver transplantation (LT). Likewise, PSC can occur at any time during the course of IBD (38), including many years after proctocolectomy for colitis (39). PSC is more common in those with pancolitis compared to those with isolated left-sided colitis or proctitis (40).
Though there is geographical variability, the prevalence of PSC is estimated at up to 16.2 per 100,000 population (41) and annual incidence up to 1 case per 100,000 population (42), being the highest in northern Europe and the United States and markedly lower in Asia. True population-based studies, however, are scarce and limit the understanding of the prevalence and incidence of PSC. A large population-based epidemiological study from the Netherlands reported an annual incidence of 0.5 per 100,000, with a point prevalence of 6 per 100,000 in 2008, with prevalence rates increasing significantly over time (25), as described in other studies (43,44). In the US, the incidence of PSC in a large, ethnically diverse cohort revealed lower incidence rates compared to studies including predominantly Caucasian populations (43,45). With estimated less than 200,000 cases in the US and less than 5 per 10,000 persons in Europe, PSC meets the criteria for a rare or “orphan” disease.
Pathogenesis
The pathogenesis of PSC is not fully understood but appears to be multifactorial and several mechanistic theories have been proposed. Cholangiocyte injury appears to result from environmental exposure and an abnormal cholangiocyte immune response leading to clinical disease in genetically susceptible individuals. Little is known about the role of the environmental risks including the influence of colonic toxins, gut microbiota, portal bacteria, or viral infections (46).
The role of genetic factors in the etiology of PSC is underscored by the finding that first-degree relatives of patients with PSC have an increased risk of PSC (up to 11-fold) (47). Genetic susceptibility factors for PSC may overlap with UC as first-degree relatives of patients with PSC without IBD are also at an increased risk of UC (8-fold) (47). Through the application of genome-wide association studies, greater than 20 susceptibility genes for PSC have been established, with the human leukocyte antigen (HLA) complex on chromosome six representing the strongest finding by several orders of magnitude (48). The overall genetic architecture of PSC appears to share features with both autoimmune diseases and IBD. Strong HLA gene associations, along with several susceptibility genes that are critically involved in T-cell function, support the involvement of adaptive immune responses in development of disease, and support the long-standing notion of PSC as an autoimmune disease (49). Nonetheless, genetic findings in PSC so far explain less than 10% of disease liability, and environmental risk factors are estimated to account for greater than 50% of the unexplained fraction (48).
Several factors support the involvement of the gut microbiota in the pathogenesis of PSC, including the long described association of PSC with IBD, genome-wide association study data identifying genetic variants in PSC that are associated with UC (such as those encoding GPR35) or influence biliary bacterial composition (such as those encoding FUT2) (50), the presence of bacterial products in the liver explants of patients with PSC (51), growth of bacteria and fungi from bile cultures acquired at time of first ERCP and increased T-cell response to microbial agents (52). Conversely, in vitro data have shown that biliary epithelial cells isolated from patients with PSC have aberrant TLR-nuclear factor-κB (NF-κB) immune responses to intestinal endotoxins with increased production of pro-inflammatory cytokines, such as IL-8 and TNF-α (53), suggesting that pro-inflammatory cytokines and endotoxins induce inappropriate innate immune responses in activated cholangiocytes in patients with PSC. Additionally, an overall reduction in bacterial diversity and altered abundance of certain bacteria in gut microbiota is observed in patients with PSC compared with the healthy state (54), but the mechanisms by which this alteration in gut microbial community results in disease remain unclear.
The strong HLA associations found in genetic studies suggest that adaptive immune responses are involved (54). The HLA class I and class II molecules present potentially antigenic peptides, derived from intracellular and extracellular sources, respectively, to the T cell receptor on CD8 and CD4 positive T cells. Gut derived antigens presented by PSC-associated HLA variants to the T cell receptor are potential triggers of these responses, and activated T cells may migrate to both the liver and gut following clonal expansion because of the overlapping expression in the gut and the liver of relevant lymphocyte homing components including mucosal vascular address in cell adhesion molecule 1 (MadCAM-1) and vascular cell adhesion molecule 1 (VCAM-1), α4β7 integrin, along with Chemokine C-C motif ligand 25 (CCL25) secretion, and contribute to the pathogenesis of PSC (55). In the liver, these recruited lymphocytes have been implicated in biliary inflammation leading to apoptosis and necrosis of cholangiocytes, and eventually fibrosis (56).
Inflammation and fibrosis lead to cholestasis and parenchymal injury. A distinct bile acid profile has been noted in IBD-PSC patients with a direct toxic effect of bile acids on cholangiocytes believed to contribute to disease progression (57,58). Primary or secondary disturbances in bile homeostasis as part of disease processes in the bile ducts or the colon (59-61), or deficiencies in protective or compensatory mechanisms, such as the so-called “bicarbonate umbrella” (62) have been implicated in the pathogenesis of PSC. The cholangiocytes show an activated phenotype in PSC, which may further trigger an immune response through interactions with hepatic stellate cells and/or portal myofibroblasts, promoting development of peribiliary fibrosis and eventually cirrhosis (63,64).
Natural history and prognosis
PSC is a progressive disease, with evolution to biliary cirrhosis and malignancy in the majority of patients (65). The estimated 10-year survival for patients with PSC is approximately 65% (43). In a large population-based study, in which 92% of patients were treated with UDCA, the estimated survival time from diagnosis until PSC-related death or LT was 21 years, compared to median 13 years transplant-free survival in a cohort from three liver transplant centers (37). Though patients who are asymptomatic have a better prognosis than those with symptoms at diagnosis, symptoms often develop over time (3). However, there is significant variation among individuals and between different subtypes. For example, patients with small duct PSC disease generally have better outcomes than those with classic disease and do not seem to develop CCA, unless the disease has progressed to large-duct PSC (21). Other favorable prognostic factors include younger age at diagnosis, and female sex (36). On the other hand, poor prognostic factors include extensive intrahepatic or extrahepatic biliary strictures (66), DSs (67), recurrent cholangitis (68), UC [compared to Crohn’s disease (CD) or no IBD] (36), evidence of liver synthetic dysfunction and cirrhosis with portal hypertension.
Biomarkers to predict the pace of progression of any form of PSC have been described. One or 2 years after diagnosis, a serum ALP level of less than 1.5 times the ULN has been associated with better outcomes, regardless of treatment (6,7). Worsening cholestasis predicts poorer outcomes, including increased risk of LT, hepatobiliary cancer, and death (69,70). However, ALP has a naturally unpredictable fluctuating nature in PSC which limits the value of single measurements at any point in time for follow-up or clinical trials. The Enhanced Liver Fibrosis (ELF) Panel, a panel of three serum markers of fibrosis (hyaluronic acid, procollagen III aminoterminal peptide, and tissue inhibitor of metalloproteinase 1) has been shown to predict transplant-free survival in PSC (71). Interestingly, ELF can also be elevated when there is acute inflammation and biliary obstruction. Parenchymal changes on MRI (72), relative enhancement with hepatocyte-specific agents (73) and arterial peribiliary hyperenhancement (74,75) have been shown to correlate with clinical outcomes in several small studies. A recent retrospective study including an internal cohort and an external validation cohort of 238 patients showed that two MR risk scores, referred to as Anali with and without gadolinium, which combine cholangiographic changes and parenchymal features such as dysmorphy, portal hypertension and enhancement heterogeneity, were associated with the occurrence of clinically significant outcomes (cirrhosis decompensation and transplant-free survival) and may be useful to predict radiographic progression in patients with PSC (76). Liver stiffness as measured by transient elastography or by magnetic resonance elastography (MRE), both at baseline or its variation over time, has been shown to predict the clinical outcome of patients with PSC (77,78). Spleen size has also been shown to predict outcomes in those with PSC (79), probably as a reflection of progression of portal hypertension. The prognostic value of histological scores such as the Ludwig and Nakanuma staging systems have been confirmed to be independent predictors of long-term outcome is PSC (80). However, the routine applicability of liver biopsy in PSC is limited due to its invasive nature, high rate of sampling error, and slow rates of histological progression, particularly fibrosis.
Multiple prognostic scoring systems that incorporate various clinical factors have been proposed. The most commonly used scoring system is the Mayo risk score (MRS), which is calculated based on measurements of serum bilirubin, AST, and albumin; the age of the patient and the presence of variceal bleeding (81). It was developed to predict short-term mortality in PSC, but has not been validated to predict long-term outcomes or other clinically relevant events such as LT. The Amsterdam Cholangiographic Scoring System (66), in which cholangiographic classification of intra- and extrahepatic biliary lesions using ERCP estimate medium- and long-term prognosis in PSC, has been validated as a prognostic model in PSC. The need for an invasive procedure to evaluate biliary changes reduces applicability in clinical practice. Other prognostic scores have been recently proposed, including the Amsterdam-Oxford model (AOM) (82), the UK-PSC risk score (83) and the Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) (78). The AOM, which predicts PSC-related death or LT, was developed and internationally validated with recent work highlighting an incremental improvement in the model’s performance over time (such as at 5 years after diagnosis) (84). Nonetheless, the C statistic of 0.68 at the time of diagnosis was relatively modest, and its overall predictive ability was inferior compared with the MRS (C statistic 0.75). The UK-PSC risk score, which predicts LT and all-cause mortality, was derived from approximately 1,000 patients enrolled in the UK-PSC research cohort and independently validated. It includes a short-term (2-year) and a long-term (10-year) risk score, both of which outperformed the MRS (C statistics 0.81 and 0.80, respectively). The PREsTo is one of the newest prognostic models to accurately predict liver decompensation patients with PSC at 5 years. The PREsTo was derived from approximately 500 patients from a referral center and validated in about 300 patients in an international cohort, using a machine-based learning technique, and consists of nine variables: bilirubin, albumin, serum ALP times the ULN, platelets, aspartate aminotransferase (AST), hemoglobin, sodium, patient age, and number of years since PSC was diagnosed. It significantly outperforms current prediction scoring systems with a C statistic of at least 0.90.
Although they can be of benefit in research settings, the role of prognostic models in clinical practice and their use for individual patients is still evolving.
Medical management
No medical therapy has been shown to alter the course of PSC. Current treatments focus on symptom management (such as fatigue and pruritus), therapy of complications such as bacterial cholangitis and DSs, and management of coexisting conditions such as IBD, coexisting autoimmune diseases, and metabolic bone disease.
Ursodeoxycholic acid (UDCA) is the most extensively studied and most commonly used medication for PSC, but studies have not corroborated its effectiveness. In one randomized double blind controlled trial, UDCA at doses of 13–15 mg/kg/day was associated with a reduction in liver enzymes compared to placebo, but it failed to demonstrate any significant difference in clinically significant endpoints such as time to LT, progression to cirrhosis or mortality (85). A study evaluating moderate doses of UDCA (17–23 mg/kg/day) revealed reduction in ALP, but not clinically relevant endpoints of symptoms, need for LT or death (86). A large multi-center study comparing high dose UDCA (28–30 mg/kg/day) with placebo unexpectedly found the risk of primary endpoint of development of cirrhosis, CCA, liver transplant or death was 2.3 times higher in the treatment group, despite improvement in serum ALP levels, leading to early termination of the trial (87). Meta-analyses of published data report no benefit from UDCA in the treatment of PSC (88,89). Although guidelines advise against the use of high doses of UDCA, the use of lower and moderate doses (13–20 mg/kg/day) remains controversial, in part due to post-hoc analyses from clinical trial data that reveal normalization of the ALP level is associated with a better long-term prognosis (6,7). Current guidelines differ in terms of recommendation regarding the use of low to moderate doses of UDCA: the American Association for the Study of Liver Diseases recommends against its use for PSC (22), whereas the American College of Gastroenterology and the European Association for the Study of the Liver do not make any specific recommendations (90,91). Many centers prescribe low to moderate doses of UDCA for 6 months, to be continued only if normalization (or near normalization) of serum ALP levels is observed (92).
Many other medications are being assessed in ongoing clinical trials, as summarized in Table 2. The majority of the clinical trials focus on cholestatic and fibrotic targets [e.g., 24-nor-UDCA, obeticholic acid (OCA) and other farnesoid X receptor (FXR) agonists, anti- lysyl oxidase-like 2 (LOXL2)]. There is an ongoing phase 3 study of nor-UDCA, a synthetic bile acid that produces a bile acid-dependent bicarbonate-rich choleresis, based on promising results of a phase 2 randomized study including 161 patients that demonstrated a significant dose-dependent improvement in ALP in those receiving 500–1,500 mg nor-UDCA compared to placebo (93). OCA, a semisynthetic analogue of chenodeoxycholic acid and potent FXR agonist, was recently evaluated in a phase 2 randomized trial, which showed a significant reduction in the serum ALP at week 24 with OCA 5–10 mg compared to placebo, but not with OCA 1–3 mg (94). As seen in studies in primary biliary cholangitis, the most common adverse event was dose-dependent mild to moderate dose-related pruritus (94). Similarly, a phase 2 study including 52 patients demonstrated that treatment with cilofexor, a non-steroidal FXR agonist, resulted in a dose-dependent reduction in ALP and markers of cholestasis after 12 weeks of treatment, with lower observed rates of moderate to severe pruritus compared to placebo (95). A phase 3 study of cilofexor in patients with non-cirrhotic PSC is underway. Results of a small study of oral vancomycin, which may target the gut microbiota and may act as an immunomodulator, by increasing regulatory T-cells, appear promising, with reduction in ALP and bilirubin (96) and further randomized trials are ongoing.
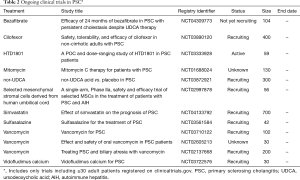
Full table
Notable recent negative studies include a phase 3 study of simtuzumab, a monoclonal antibody against LOXL2 with anti-fibrotic properties, which did not provide any clinical or histological benefit over placebo after 96 weeks in a randomized controlled trial including 234 patients with compensated PSC (97). A phase 2 study of NGM282, a non-tumorigenic FGF19 analogue, showed that after 12 weeks of treatment there were no significant changes in serum ALP levels from baseline between the NGM282 and placebo groups, despite significant reduction in markers of cholestasis and fibrosis in the treatment group (98). A retrospective analysis of 102 patients with PSC from a large international cohort who as part of their treatment for IBD received vedolizumab, which blocks the integrin α4β7 and thus targets T lymphocyte homing, reported a small increase in liver biochemistries including bilirubin at the end of the study; only one-fifth of patients had a significant reduction in level of serum ALP (99).
Clinical trial design and execution in PSC are hampered by multiple factors, including rarity of the disease, heterogeneity of presentation, protracted natural history course, variable rates of disease progression, competing risk events, and relatively low event rate of clinically relevant endpoints that are hard to predict. Regulatory agencies recognize that to promote drug development in serious and life-threatening rare diseases lacking effective medical therapy, accelerated pathways for approval are needed. Surrogate endpoints that are reasonably likely to predict clinical benefit or a clinical endpoint that can be measured earlier than irreversible morbidity (that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit) may be used as a basis for accelerated approval. So far, no biomarkers have been validated for use as surrogate endpoints in clinical trials for PSC, but many potential candidate surrogate biomarkers have been proposed (100,101) and are listed in Table 3 (further described in the “Natural history and prognosis” section). Combinations of biomarkers and/or clinical outcomes, such serum ALP levels and progression of fibrosis (using histology or noninvasive methods such as elastography), may increase content validity and be acceptable surrogate endpoints, recognizing that different endpoints may need to be individualized to patient phenotypes, drug mechanisms, and aspects of the disease targeted (i.e., inflammation, biliary stricturing, parenchymal fibrosis). Clinical trials that target symptoms associated with PSC may benefit from the use of a recently developed and preliminarily validated PSC-specific patient-reported outcome instrument (PSC PRO) as a surrogate endpoint (102).
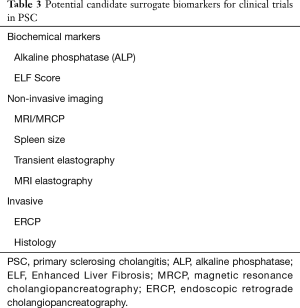
Full table
Management of complications and associated conditions
Bacterial cholangitis
As a result of biliary strictures and bile stasis, patients with PSC are at increased risk for bacterial cholangitis, which may occur spontaneously, particularly in the setting of a DS, or after biliary tract instrumentation. Due to increased risk of post-ERCP cholangitis (103), prophylactic antibiotics should be administered prior to and following biliary interventions (91). Bacterial cholangitis may contribute to disease progression (104). Empirical antibiotics are typically effective. Urgent endoscopic biliary decompression is recommended for patients who have acute cholangitis in the setting of a dominant biliary stricture.
Dominant stricture
DSs, defined as stenoses with a diameter of up to 1.5 mm in the common bile duct (CBD) or up to 1 mm in the hepatic ducts on ERCP (105), are a frequent finding in patients with PSC and occur in 45% to 65% of patients during follow-up. Patients can present with an increase in serum bilirubin, worsening symptoms of jaundice and pruritus, cholangitis, or progressive bile duct dilation on imaging. Though the majority of these strictures prove to be benign (105,106), a DS might harbor malignancy. One retrospective study including 128 patients showed one-fourth of patients with DS developed CCA during 10-year follow-up (67). DS portend a poor prognosis with markedly reduced survival rates (18-year survival of 25% in those with DSs compared to 75% in those without) (68), predominantly due to strong association with CCA. These patients should be evaluated by ERCP; brushings for cytology or biopsy for pathology to exclude CCA should be performed concomitantly with endoscopic treatment (22). A meta-analysis including 11 studies with 747 patients revealed that even though the specificity of endoscopic brushings for cytologic analysis in detecting CCA is very high (97%), their sensitivity is low (43%), and thus bile duct brushing is reliable in establishing a diagnosis CCA but not in excluding malignant strictures (107). Fluorescence in situ hybridization (FISH) increases sensitivity for detecting neoplasia to 68% (specificity 70%) from one cytology specimen, with more modest improvement if polysomy is considered diagnostic (sensitivity 51% and sensitivity 93%) (107). In patients with equivocal cytology results, the sensitivity increases to 74% but specificity is only 57% (107). The presence of FISH polysomy on two sequential specimens may denote a higher risk of development of CCA compared to those in patients in whom initial polysomy reverts to non-polysomy on subsequent specimen (108). Cholangioscopy allows for direct visualization of the bile duct with targeted biopsies of suspicious lesions, which has been reported to increase sensitivity to 65% and specificity to 97%, with a diagnostic accuracy of 96% (109). However, one of the disadvantages of this technique is that the passage of the cholangioscope may be difficult in the narrow, strictured bile ducts in PSC. Interestingly, a recent study suggests that while cholangioscopy-guided and transpapillary biopsies improve the sensitivity for the detection of CCA in combination with other ERCP-based techniques compared to brush cytology alone, these modalities in addition to FISH did not significantly improve the sensitivity for the detection of malignancy in patients with PSC. Further investigation is needed to determine the added benefit of endoscopic ultrasound including intraductal ultrasound and confocal endomicroscopy in the diagnosis of CCA in patients with PSC.
Endoscopic treatment of dominant stenoses relieves obstruction and improves cholestasis in the short-term (110). Radiographic improvement is often noted on follow-up cholangiography. Long-term benefits are less clear—model-based comparisons suggest endoscopic therapy prolongs survival in comparison to predicted survival (105,111,112). In an intriguing large retrospective study including 268 patients with DS who were offered either scheduled ERCP and dilatation, or procedures based on clinical symptoms, transplant-free survival at 5 years was significantly higher in those with scheduled procedures compared to those in the clinically indicated ERCP group (51% vs. 29%) (113), but these findings have not yet been confirmed and surveillance ERCP is not recommended in the management of PSC. Balloon dilatation and plastic stents are the main modalities for endoscopic therapy of DS. Due to an apparent increased risk of procedure-related cholangitis with placement of biliary stents, short-term stenting is likely preferable to long-term (110). While both balloon dilatation and stents have been shown to be effective, a recent randomized trial was halted early due to significantly higher occurrence of treatment-related adverse events, particularly pancreatitis and cholangitis, in patients who were treated with short-term stent placement (114), suggesting balloon dilatation should be the initial treatment of choice for DS in patients with PSC and stenting should be reserved for strictures that are refractory to dilation. Antibiotic prophylaxis is recommended to reduce cholangitis for all patients with PSC undergoing ERCP (115).
For patients with severe or recurrent obstruction, surgical management might be an option. In highly selected patients without cirrhosis and with predominantly extrahepatic biliary strictures, resection of the extrahepatic biliary tree may improve jaundice and delay LT (116), and long-term survival rates may be comparable between noncirrhotic patients with PSC status post resection compared to that of cirrhotic patients undergoing LT (117).
Cholangiocarcinoma
CCA is the most common malignancy in PSC. Compared to the general population, patients with PSC have an almost 400-fold increased risk of developing CCA, with the annual incidence estimated at 0.6% per year and a cumulative lifetime risk of 10–20% (25). Patients with DSs are at higher risk of CCA, whereas patients with small-duct PSC are at low risk (2). Duration of PSC does not appear to be a risk factor for development of CCA, and up to half of the patients are diagnosed with CCA within a year of diagnosis of PSC (118). Although symptoms such as weight loss, fever, jaundice and elevation in ALP and bilirubin may suggest superimposed CCA, in early stages CCA can be asymptomatic thus early diagnosis can be quite challenging despite the availability of a variety of diagnostic modalities. CCA accounts for a large proportion of mortality in patients with PSC, with reported mortality rates as high as 80% at median of 1 year of follow-up (25). Timely identification of malignancy may have a significant impact on management, including therapeutic options and long-term outcomes. Though guidelines do not universally recommend surveillance for CCA (22,91,115), a retrospective study including 79 patients with PSC who developed hepatobiliary cancer revealed that patients who underwent surveillance had a significantly improved 5-year survival compared to the no-surveillance group (68% vs. 20%) (119). MRCP, combined with contrast-enhanced MRI of the liver, is the imaging modality most widely used for annual assessment, often combined with annual CA19-9 (91), yet the optimal method for surveillance for CCA is unknown. Though meta-analysis suggests elevated CA19-9 might be a useful tumor marker for the diagnosis of CCA, with an overall sensitivity and specificity of 72% and 84%, respectively (120), CA19-9 levels are influenced by complicated cholangitis and biliary strictures, and should not be used alone for screening or diagnosis of CCA. The diagnosis of CCA in PSC typically is established based on a combination of tumor markers (CA19-9), multiple imaging modalities, and ERCP-based brush cytology, biopsy and FISH, as discussed in the previous section.
Early or locally-advanced stage CCA may be associated with a better prognosis and a higher chance of survival (121). Surgery with complete resection or LT are the only treatments with curative intent for CCA. In carefully selected patients with unresectable, perihilar early-stage CCA, LT following neoadjuvant therapy, including external beam radiotherapy combined with radio-sensitizing chemotherapy, endoluminal brachytherapy and maintenance chemotherapy can be considered (121). However, in the majority patients CCA is diagnosed at an advanced stage when surgery and LT are not appropriate options (122). For those who are ineligible for surgery or LT, systemic chemotherapy is recommended as the mainstay palliative treatment modality; a combination of gemcitabine with cisplatin is typically used as first-line treatment (123). Other palliative treatment strategies include endoscopic stenting and photodynamic therapy. For unresectable CCA, the median survival time is only 5–12 months after diagnosis with or without chemotherapy (124).
Gallbladder polyps and adenocarcinoma
Gallbladder lesions or polyps are observed in 4% to 6% of patients with PSC (125,126), many of which are malignant. Studies looking at these lesions have shown that greater than 50% are adenocarcinoma (126,127). At minimum, annual ultrasound examinations are recommended for screening (22,91). Though small lesions may harbor neoplasia, a cut-off of 0.8 cm for cholecystectomy has been proposed (128,129) given the low prevalence of malignancy in small polyps and high early post-operative morbidity associated with cholecystectomy in patients with PSC, particularly when advanced liver disease is present (128).
IBD and colorectal malignancy
The estimated prevalence of IBD in patients with PSC is approximately 60–80% (22). The majority of these patients have UC, while a minority have CD with colonic involvement and many patients may have “indeterminate colitis” (not easily classified as having UC or colonic CD). Interestingly, the IBD pattern in patients with PSC appears to differ from that of patients without PSC. The disease presents earlier in life, is often quiescent and may be asymptomatic. Involvement of the entire colon is very common (either UC or CD) (41), with a right-side predominance, and features such as backwash ileitis and rectal sparing are more common in this population compared with patients with IBD without PSC (40). Patients may be more likely to develop pouchitis after ileo-anal anastomosis. Pancolectomy with ileostomy is associated with a risk of peristomal varices. Patients with IBD-PSC also tend to have a more quiescent course compared with regular IBD patients (130). In most cases, diagnosis of IBD precedes PSC; however, IBD can also present at any point after diagnosis of PSC (40), even after LT. Nonetheless, treatment of IBD in patients with PSC is based on general IBD guidelines.
The risk of colorectal dysplasia and cancer in patients with IBD-PSC compared to patients with IBD alone is approximately 4- to 5-fold higher (131), and patients with PSC-IBD should undergo annual surveillance colonoscopy with segmental mucosal biopsies (91,115). The role of UDCA as chemoprophylaxis against colorectal cancer in PSC-IBD remains unclear (132).
In newly diagnosed patients without known diagnosis of IBD, evaluation for concurrent IBD with colonoscopy with biopsies is recommended, regardless of the presence of symptoms (91,115). The frequency of surveillance colonoscopy is still unclear for patients who are not diagnosed with IBD on initial colonoscopy (22,54), but many clinicians repeat colonoscopy with biopsies in 3–5 years.
Cirrhosis and LT
Once patients with PSC develop cirrhosis as a result of the natural history of the disease, the management and surveillance strategies used are similar to those used for other causes of cirrhosis and are beyond the scope of this review.
Because of the progressive nature of PSC, approximately 40% of patients with this disease will ultimately require LT (4). The main indication for LT in PSC is decompensated cirrhosis caused by the disease, but those with refractory cholangitis may benefit and LT is also recommended for highly selected patients with unresectable early-stage hilar CCA (133). PSC has become the dominant autoimmune indication for LT in the US (134), accounting for 5% of LTs performed in the US from 1988 through 2015 (5).
Outcomes after LT are excellent compared to other indications, yet it is estimated that approximately 20% of patients have recurrent PSC after LT (91), which is associated with significant morbidity and mortality, often requiring retransplantation (135). Though no single risk factor has consistently been reported to impact the risk of recurrent PSC, several studies investigating post-transplant outcomes have found that active IBD after LT is a risk factor for recurrent PSC, whereas colectomy appears to be protective (135-138).
Conclusions
PSC remains a poorly understood disease for which medical therapy is lacking, despite numerous scientific advances over the recent years. Combination medical treatment targeting various aspects of pathogenesis in parallel might offer new opportunities. Clinical trial design to study drugs to improve prognosis is hampered by rarity of the disease, heterogeneity of presentation and relatively low event rate of clinically relevant endpoints that are hard to predict. To address this, biomarkers are necessary to serve as surrogate endpoints in clinical trials, but none have been established for use in clinical trials. Research is needed in many areas, including pathogenesis, medical and endoscopic treatment, comorbidities, risk of malignancy, complications after liver transplant. A collaborative approach is crucial for undertaking large studies to meaningfully advance the knowledge in the field of PSC and benefit the individuals afflicted by it.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luca Fabris and Mario Strazzabosco) for the series “Recent Advances in Rare Liver Diseases” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh-20-266). The series “Recent Advances in Rare Liver Diseases” was commissioned by the editorial office without any funding or sponsorship. Dr. MGS reports grants from Norvartis, grants from Gilead, grants from Cymabay, grants from Target-Pharmasolutions, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med 1995;332:924-33. [Crossref] [PubMed]
- Dyson JK, Beuers U, Jones DEJ, et al. Primary sclerosing cholangitis. Lancet 2018;391:2547-59. [Crossref] [PubMed]
- Kaplan GG, Laupland KB, Butzner D, et al. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 2007;102:1042-9. [Crossref] [PubMed]
- Tischendorf JJ, Hecker H, Kruger M, et al. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol 2007;102:107-14. [Crossref] [PubMed]
- Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161-70. [Crossref] [PubMed]
- Stanich PP, Bjornsson E, Gossard AA, et al. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis 2011;43:309-13. [Crossref] [PubMed]
- de Vries EM, Wang J, Leeflang MM, et al. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int 2016;36:1867-75. [Crossref] [PubMed]
- Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54:374-85. [Crossref] [PubMed]
- Chapman RW, Cottone M, Selby WS, et al. Serum autoantibodies, ulcerative colitis and primary sclerosing cholangitis. Gut 1986;27:86-91. [Crossref] [PubMed]
- Mulder AH, Horst G, Haagsma EB, et al. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology 1993;17:411-7. [Crossref] [PubMed]
- Bansi D, Chapman R, Fleming K. Antineutrophil cytoplasmic antibodies in chronic liver diseases: prevalence, titre, specificity and IgG subclass. J Hepatol 1996;24:581-6. [Crossref] [PubMed]
- Chapman RW. The enigma of anti-neutrophil antibodies in ulcerative colitis primary sclerosing cholangitis: important genetic marker or epiphenomenon? Hepatology 1995;21:1473-4. [Crossref] [PubMed]
- Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 2006;101:2070-5. [Crossref] [PubMed]
- Berntsen NL, Klingenberg O, Juran BD, et al. Association between HLA haplotypes and increased serum levels of IgG4 in patients with primary sclerosing cholangitis. Gastroenterology 2015;148:924-7.e2. [Crossref] [PubMed]
- Boonstra K, Culver EL, de Buy Wenniger LM, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 2014;59:1954-63. [Crossref] [PubMed]
- Dave M, Elmunzer BJ, Dwamena BA, et al. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology 2010;256:387-96. [Crossref] [PubMed]
- Talwalkar JA, Angulo P, Johnson CD, et al. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology 2004;40:39-45. [Crossref] [PubMed]
- Angulo P, Pearce DH, Johnson CD, et al. Magnetic resonance cholangiography in patients with biliary disease: its role in primary sclerosing cholangitis. J Hepatol 2000;33:520-7. [Crossref] [PubMed]
- Scheuer PJ. Ludwig Symposium on biliary disorders--part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc 1998;73:179-83. [Crossref] [PubMed]
- Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol 2003;98:1155-8. [Crossref] [PubMed]
- Björnsson E, Olsson R, Bergquist A, et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology 2008;134:975-80. [Crossref] [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [Crossref] [PubMed]
- Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology 2006;44:1063-74. [Crossref] [PubMed]
- Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 2008;134:706-15. [Crossref] [PubMed]
- Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58:2045-55. [Crossref] [PubMed]
- Björnsson E, Chapman RW. Sclerosing cholangitis. Curr Opin Gastroenterol 2003;19:270-5. [Crossref] [PubMed]
- Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol 2000;95:1130-8. [Crossref] [PubMed]
- Björnsson E, Boberg KM, Cullen S, et al. Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut 2002;51:731-5. [Crossref] [PubMed]
- Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology 2002;35:1494-500. [Crossref] [PubMed]
- Boberg KM, Schrumpf E, Fausa O, et al. Hepatobiliary disease in ulcerative colitis. An analysis of 18 patients with hepatobiliary lesions classified as small-duct primary sclerosing cholangitis. Scand J Gastroenterol 1994;29:744-52. [Crossref] [PubMed]
- Lüth S, Kanzler S, Frenzel C, et al. Characteristics and long-term prognosis of the autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. J Clin Gastroenterol 2009;43:75-80. [Crossref] [PubMed]
- Zenouzi R, Lohse AW. Long-term outcome in PSC/AIH "overlap syndrome": does immunosuppression also treat the PSC component? J Hepatol 2014;61:1189-91. [Crossref] [PubMed]
- Gregorio GV, Portmann B, Karani J, et al. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology 2001;33:544-53. [Crossref] [PubMed]
- Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193-213. [Crossref] [PubMed]
- Al-Chalabi T, Portmann BC, Bernal W, et al. Autoimmune hepatitis overlap syndromes: an evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol Ther 2008;28:209-20. [Crossref] [PubMed]
- Weismuller TJ, Trivedi PJ, Bergquist A, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 2017;152:1975-84.e8. [Crossref] [PubMed]
- Lunder AK, Hov JR, Borthne A, et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology 2016;151:660-9.e4. [Crossref] [PubMed]
- Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis 1991;11:31-9. [Crossref] [PubMed]
- Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis 2006;26:31-41. [Crossref] [PubMed]
- Loftus EV Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 2005;54:91-6. [Crossref] [PubMed]
- Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012;56:1181-8. [Crossref] [PubMed]
- Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology 2011;53:1590-9. [Crossref] [PubMed]
- Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003;125:1364-9. [Crossref] [PubMed]
- Lindkvist B, Benito de Valle M, Gullberg B, et al. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in Sweden. Hepatology 2010;52:571-7. [Crossref] [PubMed]
- Toy E, Balasubramanian S, Selmi C, et al. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol 2011;11:83. [Crossref] [PubMed]
- Yokoda RT, Carey EJ. Primary biliary cholangitis and primary sclerosing cholangitis. Am J Gastroenterol 2019;114:1593-605. [Crossref] [PubMed]
- Bergquist A, Montgomery SM, Bahmanyar S, et al. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2008;6:939-43. [Crossref] [PubMed]
- Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol 2017;14:279-95. [Crossref] [PubMed]
- Farh KK, Marson A, Zhu J, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015;518:337-43. [Crossref] [PubMed]
- Fabris L, Fiorotto R, Spirli C, et al. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol 2019;16:497-511. [Crossref] [PubMed]
- Olsson R, Bjornsson E, Backman L, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol 1998;28:426-32. [Crossref] [PubMed]
- Katt J, Schwinge D, Schoknecht T, et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013;58:1084-93. [Crossref] [PubMed]
- Mueller T, Beutler C, Pico AH, et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int 2011;31:1574-88. [Crossref] [PubMed]
- Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017;67:1298-323. [Crossref] [PubMed]
- Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med 2004;200:1511-7. [Crossref] [PubMed]
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 2006;6:244-51. [Crossref] [PubMed]
- Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med 1998;339:1217-27. [Crossref] [PubMed]
- Banales JM, Huebert RC, Karlsen T, et al. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019;16:269-81. [Crossref] [PubMed]
- Sinakos E, Marschall HU, Kowdley KV, et al. Bile acid changes after high-dose ursodeoxycholic acid treatment in primary sclerosing cholangitis: relation to disease progression. Hepatology 2010;52:197-203. [Crossref] [PubMed]
- Bell LN, Wulff J, Comerford M, et al. Serum metabolic signatures of primary biliary cirrhosis and primary sclerosing cholangitis. Liver Int 2015;35:263-74. [Crossref] [PubMed]
- Milkiewicz M, Klak M, Kempinska-Podhorodecka A, et al. Impaired hepatic adaptation to chronic cholestasis induced by primary sclerosing cholangitis. Sci Rep 2016;6:39573. [Crossref] [PubMed]
- Hohenester S, Wenniger LM, Paulusma CC, et al. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 2012;55:173-83. [Crossref] [PubMed]
- Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:2823. [Crossref] [PubMed]
- Lemoinne S, Cadoret A, Rautou PE, et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015;61:1041-55. [Crossref] [PubMed]
- Bergquist A, Said K, Broome U. Changes over a 20-year period in the clinical presentation of primary sclerosing cholangitis in Sweden. Scand J Gastroenterol 2007;42:88-93. [Crossref] [PubMed]
- Ponsioen CY, Reitsma JB, Boberg KM, et al. Validation of a cholangiographic prognostic model in primary sclerosing cholangitis. Endoscopy 2010;42:742-7. [Crossref] [PubMed]
- Chapman MH, Webster GJ, Bannoo S, et al. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol 2012;24:1051-8. [Crossref] [PubMed]
- Rudolph G, Gotthardt D, Kloters-Plachky P, et al. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol 2009;51:149-55. [Crossref] [PubMed]
- Lindström L, Hultcrantz R, Boberg KM, et al. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2013;11:841-6. [Crossref] [PubMed]
- Hilscher M, Enders FB, Carey EJ, et al. Alkaline phosphatase normalization is a biomarker of improved survival in primary sclerosing cholangitis. Ann Hepatol 2016;15:246-53. [PubMed]
- Vesterhus M, Hov JR, Holm A, et al. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology 2015;62:188-97. [Crossref] [PubMed]
- Kitzing YX, Whitley SA, Upponi SS, et al. Association between progressive hepatic morphology changes on serial MR imaging and clinical outcome in primary sclerosing cholangitis. J Med Imaging Radiat Oncol 2017;61:636-42. [Crossref] [PubMed]
- Schulze J, Lenzen H, Hinrichs JB, et al. An imaging biomarker for assessing hepatic function in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2019;17:192-9.e3. [Crossref] [PubMed]
- Ni Mhuircheartaigh JM, Lee KS, Curry MP, et al. Early Peribiliary hyperenhancement on MRI in patients with primary sclerosing cholangitis: significance and association with the Mayo Risk Score. Abdom Radiol (NY) 2017;42:152-8. [Crossref] [PubMed]
- Petrovic BD, Nikolaidis P, Hammond NA, et al. Correlation between findings on MRCP and gadolinium-enhanced MR of the liver and a survival model for primary sclerosing cholangitis. Dig Dis Sci 2007;52:3499-506. [Crossref] [PubMed]
- Cazzagon N, Chazouilleres O, Corpechot C, et al. Predictive criteria of response to endoscopic treatment for severe strictures in primary sclerosing cholangitis. Clin Res Hepatol Gastroenterol 2019;43:387-94. [Crossref] [PubMed]
- Ruiz A, Lemoinne S, Carrat F, et al. Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology 2014;59:242-50. [Crossref] [PubMed]
- Eaton JE, Vesterhus M, McCauley BM, et al. Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) predicts outcomes of the disease: a derivation and validation study using machine learning. Hepatology 2020;71:214-24. [Crossref] [PubMed]
- Ehlken H, Wroblewski R, Corpechot C, et al. Spleen size for the prediction of clinical outcome in patients with primary sclerosing cholangitis. Gut 2016;65:1230-2. [Crossref] [PubMed]
- de Vries EM, de Krijger M, Farkkila M, et al. Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: an international cohort study. Hepatology 2017;65:907-19. [Crossref] [PubMed]
- Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 2000;75:688-94. [Crossref] [PubMed]
- de Vries EM, Wang J, Williamson KD, et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut 2018;67:1864-9. [Crossref] [PubMed]
- Goode EC, Clark AB, Mells GF, et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system. Hepatology 2019;69:2120-35. [Crossref] [PubMed]
- Goettel JA, Kotlarz D, Emani R, et al. Low-dose interleukin-2 ameliorates colitis in a preclinical humanized mouse model. Cell Mol Gastroenterol Hepatol 2019;8:193-5. [Crossref] [PubMed]
- Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med 1997;336:691-5. [Crossref] [PubMed]
- Olsson R, Boberg KM, de Muckadell OS, et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology 2005;129:1464-72. [Crossref] [PubMed]
- Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50:808-14. [Crossref] [PubMed]
- Shi J, Li Z, Zeng X, et al. Ursodeoxycholic acid in primary sclerosing cholangitis: meta-analysis of randomized controlled trials. Hepatol Res 2009;39:865-73. [Crossref] [PubMed]
- Triantos CK, Koukias NM, Nikolopoulou VN, et al. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther 2011;34:901-10. [Crossref] [PubMed]
- European Association for the Study of the L. EASL Clinical Practice Guidelines. management of cholestatic liver diseases. J Hepatol 2009;51:237-67. [Crossref] [PubMed]
- Lindor KD, Kowdley KV, Harrison ME, et al. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol 2015;110:646-59. [Crossref] [PubMed]
- Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:2501-2. [Crossref] [PubMed]
- Fickert P, Hirschfield GM, Denk G, et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol 2017;67:549-58. [Crossref] [PubMed]
- Kowdley KV, Vuppalanchi R, Levy C, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol 2020;73:94-101. [Crossref] [PubMed]
- Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 2019;70:788-801. [Crossref] [PubMed]
- Tabibian JH, Weeding E, Jorgensen RA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther 2013;37:604-12. [Crossref] [PubMed]
- Muir AJ, Levy C, Janssen HLA, et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology 2019;69:684-98. [Crossref] [PubMed]
- Hirschfield GM, Chazouilleres O, Drenth JP, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol 2019;70:483-93. [Crossref] [PubMed]
- Lynch KD, Chapman RW, Keshav S, et al. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18:179-87.e6. [Crossref] [PubMed]
- Ponsioen CY, Lindor KD, Mehta R, et al. Design and endpoints for clinical trials in primary sclerosing cholangitis. Hepatology 2018;68:1174-88. [Crossref] [PubMed]
- Ponsioen CY, Chapman RW, Chazouilleres O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology 2016;63:1357-67. [Crossref] [PubMed]
- Younossi ZM, Afendy A, Stepanova M, et al. Development and validation of a primary sclerosing cholangitis-specific patient-reported outcomes instrument: the PSC PRO. Hepatology 2018;68:155-65. [Crossref] [PubMed]
- Bangarulingam SY, Gossard AA, Petersen BT, et al. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol 2009;104:855-60. [Crossref] [PubMed]
- Pohl J, Ring A, Stremmel W, et al. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol 2006;18:69-74. [Crossref] [PubMed]
- Stiehl A, Rudolph G, Kloters-Plachky P, et al. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol 2002;36:151-6. [Crossref] [PubMed]
- Lindberg B, Arnelo U, Bergquist A, et al. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy 2002;34:909-16. [Crossref] [PubMed]
- Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:783-9. [Crossref] [PubMed]
- Quinn KP, Tabibian JH, Lindor KD. Clinical implications of serial versus isolated biliary fluorescence in situ hybridization (FISH) polysomy in primary sclerosing cholangitis. Scand J Gastroenterol 2017;52:377-81. [Crossref] [PubMed]
- Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther 2016;44:1139-51. [Crossref] [PubMed]
- Ponsioen CY, Kuiper H, Ten Kate FJ, et al. Immunohistochemical analysis of inflammation in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol 1999;11:769-74. [Crossref] [PubMed]
- Stiehl A, Rost D. Endoscopic treatment of dominant stenoses in patients with primary sclerosing cholangitis. Clin Rev Allergy Immunol 2005;28:159-65. [Crossref] [PubMed]
- Stiehl A, Rudolph G, Sauer P, et al. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol 1997;26:560-6. [Crossref] [PubMed]
- Leo S, Lazarevic V, Gaia N, et al. The intestinal microbiota predisposes to traveler's diarrhea and to the carriage of multidrug-resistant Enterobacteriaceae after traveling to tropical regions. Gut Microbes 2019;10:631-41. [Crossref] [PubMed]
- Ponsioen CY, Arnelo U, Bergquist A, et al. No superiority of stents vs balloon dilatation for dominant strictures in patients with primary sclerosing cholangitis. Gastroenterology 2018;155:752-9.e5. [Crossref] [PubMed]
- European Society of Gastrointestinal E, European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol 2017;66:1265-81. [Crossref] [PubMed]
- Domajnko B, Ahrendt SA. Indications for non-transplant surgery in primary sclerosing cholangitis. HPB (Oxford) 2005;7:292-7. [Crossref] [PubMed]
- Pawlik TM, Olbrecht VA, Pitt HA, et al. Primary sclerosing cholangitis: role of extrahepatic biliary resection. J Am Coll Surg 2008;206:822-30; discussion 830-2. [Crossref] [PubMed]
- Fevery J, Verslype C, Lai G, et al. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci 2007;52:3123-35. [Crossref] [PubMed]
- Ali AH, Tabibian JH, Nasser-Ghodsi N, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology 2018;67:2338-51. [Crossref] [PubMed]
- Liang B, Zhong L, He Q, et al. Diagnostic accuracy of serum CA19-9 in patients with cholangiocarcinoma: a systematic review and meta-analysis. Med Sci Monit 2015;21:3555-63. [Crossref] [PubMed]
- Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int 2010;23:692-7. [Crossref] [PubMed]
- Rizvi S, Eaton JE, Gores GJ. Primary sclerosing cholangitis as a premalignant biliary tract disease: surveillance and management. Clin Gastroenterol Hepatol 2015;13:2152-65. [Crossref] [PubMed]
- Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. [Crossref] [PubMed]
- Song J, Li Y, Bowlus CL, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis (PSC): a comprehensive review. Clin Rev Allergy Immunol 2020;58:134-49. [Crossref] [PubMed]
- Brandt DJ, MacCarty RL, Charboneau JW, et al. Gallbladder disease in patients with primary sclerosing cholangitis. AJR Am J Roentgenol 1988;150:571-4. [Crossref] [PubMed]
- Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol 2008;48:598-605. [Crossref] [PubMed]
- Buckles DC, Lindor KD, Larusso NF, et al. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol 2002;97:1138-42. [Crossref] [PubMed]
- Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol 2012;107:431-9. [Crossref] [PubMed]
- Bowlus CL, Lim JK, Lindor KD. AGA clinical practice update on surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis: expert review. Clin Gastroenterol Hepatol 2019;17:2416-22. [Crossref] [PubMed]
- Lundqvist K, Broome U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum 1997;40:451-6. [Crossref] [PubMed]
- Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc 2002;56:48-54. [Crossref] [PubMed]
- Hansen JD, Kumar S, Lo WK, et al. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Dig Dis Sci 2013;58:3079-87. [Crossref] [PubMed]
- Sapisochín G, Fernández de Sevilla E, Echeverri J, et al. Liver transplantation for cholangiocarcinoma: current status and new insights. World J Hepatol 2015;7:2396-403. [Crossref] [PubMed]
- Webb GJ, Rana A, Hodson J, et al. Twenty-year comparative analysis of patients with autoimmune liver diseases on transplant waitlists. Clin Gastroenterol Hepatol 2018;16:278-87.e7. [Crossref] [PubMed]
- Ravikumar R, Tsochatzis E, Jose S, et al. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol 2015;63:1139-46. [Crossref] [PubMed]
- Cholongitas E, Shusang V, Papatheodoridis GV, et al. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl 2008;14:138-43. [Crossref] [PubMed]
- Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl 2009;15:330-40. [Crossref] [PubMed]
- Hildebrand T, Pannicke N, Dechene A, et al. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: a retrospective multicenter analysis. Liver Transpl 2016;22:42-52. [Crossref] [PubMed]
Cite this article as: Rabiee A, Silveira MG. Primary sclerosing cholangitis. Transl Gastroenterol Hepatol 2021;6:29.


