
Procalcitonin and C-reactive protein in the diagnosis of spontaneous bacterial peritonitis
Introduction
Spontaneous bacterial peritonitis (SBP) is one of the turning points in the natural history of progression of cirrhosis which heralds the onset of decompensated disease. It is associated with a high incidence of morbidity and mortality (up to 20–30%) as it puts cirrhotic patients at risk of developing hepatic encephalopathy, renal failure, acidosis and shock (1). Prompt initiation of antibiotics to treat SBP is the cornerstone of optimal therapy as it improves patient outcomes and survival (2). Primary prophylaxis with antibiotics should be instituted in high-risk patients (e.g., cirrhotics with low protein ascites, gastrointestinal hemorrhage) and secondary prophylaxis for those with a prior history of SBP (3,4).
Practice guidelines from various medical societies recommend doing a paracentesis in order to make a diagnosis of SBP based on ascitic fluid neutrophil count prior to initiation of antibiotics (5,6). Given that the process of paracentesis requires skill, expertise in specimen handling and time delay associated with obtaining ascitic fluid cell count, many physicians in busy office practices in the United States initiate antibiotics based on clinical suspicion alone which adds to the problem of growing antibiotic resistance from overuse of unwarranted antibiotics. Alternatively, the diagnosis of SBP can be entirely missed if clinical suspicion is low and paracentesis is overlooked. In high volume emergency rooms and busy outpatient clinics throughout the country, availability of a quick, reliable, non-invasive marker for SBP would help crucial decision-making regarding need for paracentesis and promote appropriate antibiotic use.
Procalcitonin (PCT), which is widely used as a marker of bacterial infection in various clinical conditions (e.g., pneumonia, meningitis, bacterial gastroenteritis, septic shock) has also been studied in the setting of SBP and has been found to accurately predict the presence of SBP when present at elevated levels (7-17) (Table 1). Procalcitonin has been studied alone or in combination with other inflammatory markers such as TNF-alpha, IL-6, lipocalin/NGAL, MIP-1 beta and has been shown to be a sensitive and specific marker for the diagnosis of SBP (7,13,18) (Table 1). Similarly, CRP which is an acute phase reactant too has been found to be elevated in patients with SBP (19-21). CRP is a chemokine which is secreted by liver and can be elevated in a wide variety of clinical conditions including infection, connective tissue disorders, malignancies and autoimmune conditions.
Table 1
| Author, year, country | # of patients | Underlying disease | Procalcitonin testing method | Cut-off value (ng/mL) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Viallon et al. (in 2000), France | 61 | Cirrhosis | LUMItest | 0.75 | 95 | 98 |
| Spahr et al. (in 2001), Switzerland | 20 | Cirrhosis | LUMItest | 0.5 | 50 | 90 |
| Connert et al. (in 2003), Germany | 100 | Cirrhosis | LUMItest | 0.58 | 92 | 78 |
| Yuan et al. (in 2013), China | 84 | Hepatitis B | Diasorin | 0.48 | 95 | 79 |
| Cekin et al. (in 2013), Turkey | 101 | Cirrhosis | – | 0.42 | 78 | 75 |
| Wu J et al. (in 2014), China | 362 | Cirrhosis | – | 0.462 | 83.7 | 94.9 |
| Lesinska et al. (in 2014), Poland | 32 | Cirrhosis | LUMItest | – | – | – |
| Gharabaghi et al. (in 2015), Iran | 33 | Cirrhosis | – | 0.5 | 75 | 92 |
| Cai et al. (in 2015), China | 78 | Cirrhosis | ELFA VIDAS | 2.0 | 68.8 | 94.2 |
| Wu H et al. (in 2016), China | 88 | Cirrhosis | ECLIA Cobas | 0.78 | 77.5 | 60.4 |
| Abdel-Razik et al. (in 2016), Egypt | 79 | Cirrhosis | ELISA RayBio | 0.94 | 94.3 | 91.8 |
SBP, spontaneous bacterial peritonitis.
To the best of our knowledge, ours is the first prospective study from the United States describing the role of procalcitonin alone or in combination with CRP in diagnosing patients with SBP while adding to the growing body of evidence that non-invasive markers may have a role in the diagnosis of this entity.
We aimed to assess the efficacy of serum procalcitonin level and CRP in predicting the presence of SBP.
Methods
Patient selection
This was a prospective cohort study where cirrhotic patients with suspected SBP admitted to Methodist University Hospital in Memphis, Tennessee, USA between September 2012 and March 2013 were consecutively enrolled in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by The Institutional Review Board of Methodist University Hospital, Memphis, Tennessee, USA (IRB approval number: 12-02006-XP), with exemption for informed consent.
All subjects in the study met the following inclusion criteria: (I) cirrhosis with ascites; (II) clinical suspicion of SBP, based at the minimum on: large volume ascites and new abdominal pain; (III) ascitic fluid WBC count and culture obtained before the use of antibiotics at admission serum PCT and CRP measurements; (IV) absence of infection in other organs or sites or those already on antibiotics.
Paracentesis and procalcitonin, CRP estimation
We developed a protocol, at the Methodist University Hospital Transplant Institute, for the measurement of serum procalcitonin (normal range: 0.5–2.0 ng/mL) and serum CRP (normal range: 0.5–3.0 mg/L) at the time of hospital admission, prior to administering antibiotics. At our center, paracentesis is performed using aseptic technique and ascitic fluid is inoculated into aerobic and anaerobic blood culture bottles at bedside routinely. Fluid specimen is then sent off promptly (within 1 hour) to the laboratory for ascitic fluid analysis.
Ascitic fluid (AF) was collected from hospitalized patients using sterile method and cultured in blood culture bottles, according to the guidelines of the Infectious Diseases Society of America. Bacterial identification and antimicrobial susceptibility testing were carried out as per standard protocol. Based on the results of ascitic fluid PMN count and culture results, patients were classified into 4 groups: classic SBP (WBC count ≥500/mm3 and PMN >250/mm3 in AF with a positive bacterial culture), culture-negative neutrocytic ascites (CNNA; WBC count ≥500/mm3 and PMN >250/mm3 in AF but negative culture), monomicrobial non-neutrocytic bacterascites (MNB; AF with a positive bacterial culture/positive gram stain and WBC count <500/mm3 and PMN <250/mm3). Finally, sterile ascites was defined when there were <250 neutrophils/microliter and ascitic fluid culture was negative (Table 2). A contaminant was defined as a non-pathogenic microorganism that was isolated from ascitic fluid culture. For the purpose of this study, we included variants of classic SBP, CNNA and MNB all under the category of SBP. We also did a subgroup analysis of ‘neutrocytic ascites’ which included classic SBP and CNNA.
Table 2
| SBP types | PMN ≥250 μL | Culture |
|---|---|---|
| Classic SBP | + | + |
| CNNA | + | − |
| MNB | − | + |
| Sterile | − | − |
CNNA, culture-negative neutrocytic ascites; MNB, monomicrobial non-neutrocytic bacterascites; PMN, polymorphonuclear cells; SBP, spontaneous bacterial peritonitis.
Etiology of cirrhosis was recorded with respect to HBV, HCV, alcoholic, autoimmune cirrhosis and other conditions. Clinical data were obtained retrospectively by thorough review of the patients’ medical charts and included age, presence of ascites, encephalopathy, recent episode of variceal bleeding, bacteremia, AST, ALT, total bilirubin, prothrombin activity (PTA) and other variables.
Statistical analysis
Baseline recipient characteristics were summarized for both patients with SBP and patients without SBP. Mean ± standard deviation (SD) was used for continuous variables, and count with percentages was used for categorical variables. Continuous variables were compared using the Wilcoxon Rank Sum test and categorical variables were analyzed with the Chi-square test or Fisher exact test. The level of procalcitonin and CRP was plotted for SBP and non-SBP patients respectively. The AUROC curve for procalcitonin and CRP were plotted to assess the accuracy of diagnosing SBP.
All analyses were conducted using SAS 9.4 (SAS, Cary, NC) software.
Two-sided P value was considered statistically significant when <0.05.
Results
A total of 45 patients with a mean age (± SD) of 53.8±10.5 years with a mean model for end stage liver disease (MELD) score of 20.8±6.5 were enrolled. Patient population comprised of 31 (68.8% Caucasian), 11 (24.4% African-American) and 3 (6.66% Hispanic) subjects. Most common etiology of underlying cirrhosis was hepatitis C (44%) followed by alcohol-induced cirrhosis (35%) and non-alcoholic steatohepatitis (NASH) (6.6%).
Baseline demographics of patients included in the study are shown in Table 3.
Table 3
| Patient characteristics | All patients (n=45) | SBP (n=14) | No SBP (n=31) | P value (two sided) |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) mean ± SD | 53.8±10.5 | 53.6±12.9 | 53.96±9.4 | 0.62 |
| Gender (male), n (%) | 35 (77.7) | 12 (85.7) | 23 (74.19) | 0.47 |
| Race/ethnicity, n (%) | 0.31 | |||
| Caucasian | 31 | 8 (57.1) | 23 (74.19) | |
| African American | 11 | 4 (28.6) | 7 (22.6) | |
| Hispanics | 3 | 2 (14.3) | 1 (3.23) | |
| Etiology of cirrhosis, n (%) | 0.80 | |||
| Alcohol | 16 (35.56) | 4 (28.6) | 12 (38.8) | |
| Fulminant Hepatic failure | 2 (4.44) | 1 (7.14) | 1 (3.23) | |
| Hep C | 20 (44.44) | 8 (57.1) | 12 (38.8) | |
| NASH | 3 (6.66) | 1 (7.14) | 2 (6.46) | |
| PBC | 2 (4.44) | 0 (0.00) | 2 (6.46) | |
| PSC | 2 (4.44) | 0 (0.00) | 2 (6.46) | |
| MELD Score mean ± SD | 20.8±6.5 | 21.5±5.0 | 20.41±7.16 | 0.35 |
| Ascitic fluid analysis mean ± SD | ||||
| WBC | 556.93±1,437.20 | 1,461.64±2,371 | 148.3±196.3 | 0.001 |
| Neutrophil | 30.35±29.72 | 49.23±30.90 | 16.7±20.39 | 0.007 |
| Lymphocyte | 55.45±28.51 | 47.20±36.00 | 57.88±26.75 | 0.49 |
| Procalcitonin level (ng/dL), mean ± SD | 1.17±1.84 | 2.81±2.59 | 0.43±0.48 | 0.003 |
| CRP, mean ± SD | 32.42±34.34 | 60.30±44.48 | 22.20±23.28 | 0.007 |
CRP, C-reactive protein; Hep C, hepatitis C; MELD, model for end stage liver disease; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; SBP, spontaneous bacterial peritonitis; SD, standard deviation; WBC, white blood cell count.
Patients were divided into two groups: those with SBP (n=14) and those without SBP (n=31). There were no statistically significant differences in age, gender, race/ethnicity, etiology of cirrhosis or MELD score (21.5±5.0 vs. 20.41±7.16; P=0.35, Table 3) between the two groups. Differences in serum procalcitonin level (2.81±2.59 vs. 0.43±0.48; P=0.003, Table 3), serum CRP level (60.30±44.48 vs. 22.20±23.28; P=0.007) and ascitic fluid neutrophil counts (49.23±30.90 vs. 16.7±20.39; P=0.007) were highly statistically significant between patients with SBP and those without SBP (Table 3, Figures 1,2).
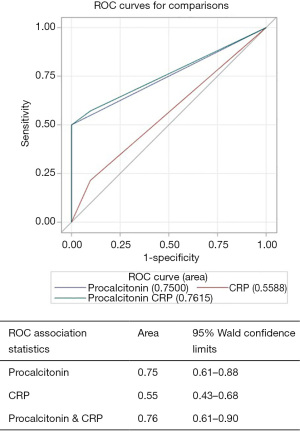
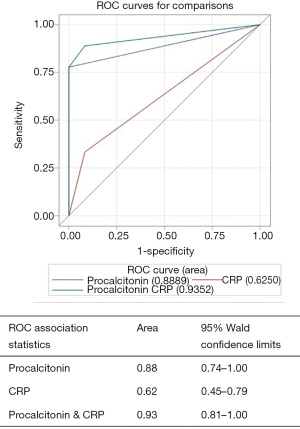
Of the 14 patients diagnosed with SBP, 4 (28.6%) were found to have classic SBP, 5 (35.7%) had CNNA and remaining 5 (35.7%) had MNB. Of the patients with positive ascitic fluid cultures, most common isolated pathogen was E. Coli (44%) followed by Staphylococcus epidermidis (33%), group D Streptococcus (11%) and Streptococcus viridans (11%).
Three out of four patients with classic SBP and 4 out of 5 patients with CNNA were found to have elevated procalcitonin (cut-off 2.0 ng/mL) while all 5 patients with MNB had normal procalcitonin levels (P=0.0476). All 31 patients without SBP had procalcitonin within normal range (≤2 ng/mL).
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of procalcitonin in diagnosing “all SBP” (including classic SBP, CNNA and MNB) and neutrocytic SBP (classic SBP and CNNA) are shown in Tables 4,5. These values for CRP in diagnosing “all SBP” and neutrocytic SBP are shown in Tables 6,7 respectively.
Table 4
| PCT | All SBP | PPV | NPV | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| >2 (positive) | 7 (TP) | 0 (FP) | 100% | 82% | 50% | 100% | 84.4% |
| ≤2 (negative) | 7 (FN) | 31 (TN) | |||||
PPV: TP/(TP + FP); NPV: TN/(TN+FN); sensitivity: TP/(TP + FN); specificity: TN/(TN + FP); accuracy: (TP + TN)/(TP + FP + FN + TN). CNNA, culture negative neutrocytic ascites; FN, false negative; FP, false positive; MNB, monomicrobial non-neutrocytic bacterascites; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; SBP, spontaneous bacterial peritonitis; TN, true negative; TP, true positive.
Table 5
| PCT | Neutrocytic SBP | PPV | NPV | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| >2 (positive) | 7 (TP) | 0 (FP) | 100% | 95% | 78% | 100% | 95.5% |
| ≤2 (negative) | 2 (FN) | 36 (TN) | |||||
PPV: TP/(TP + FP); NPV: TN/(TN+FN); sensitivity: TP/(TP + FN); specificity: TN/(TN + FP); accuracy: (TP + TN)/(TP + FP + FN + TN). CNNA, culture negative neutrocytic ascites; FN, false negative; FP, false positive; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; SBP, spontaneous bacterial peritonitis; TN, true negative; TP, true positive.
Table 6
| PCT | All SBP | PPV | NPV | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| >3 (positive) | 11 (TP) | 29 (FP) | 27.5% | 100% | 100% | 6.5% | 30.9% |
| ≤3 (negative) | 0 (FN) | 2 (TN) | |||||
PPV: TP/(TP + FP); NPV: TN/(TN+FN); sensitivity: TP/(TP + FN); specificity: TN/(TN + FP); accuracy: (TP + TN)/(TP + FP + FN + TN). CNNA, culture negative neutrocytic ascites; CRP, C-reactive protein; FN, false negative; FP, false positive; MNB, monomicrobial non-neutrocytic bacterascites; NPV, negative predictive value; PPV, positive predictive value; SBP, spontaneous bacterial peritonitis; TN, true negative; TP, true positive.
Table 7
| PCT | Neutrocytic SBP | PPV | NPV | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| >3 (positive) | 6 (TP) | 34 (FP) | 15% | 100% | 100% | 5.56% | 19.05% |
| ≤3 (negative) | 0 (FN) | 2 (TN) | |||||
PPV: TP/(TP + FP); NPV: TN/(TN+FN); sensitivity: TP/(TP + FN); specificity: TN/(TN + FP); accuracy: (TP + TN)/(TP + FP + FN + TN). CNNA, culture negative neutrocytic ascites; CRP, C-reactive protein; FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; SBP, spontaneous bacterial peritonitis; TN, true negative; TP, true positive.
CRP, at a cut-off value of 3 mg/L, alone, was not helpful in distinguishing SBP from non-SBP. Though difference in CRP level was statistically significant between the two groups (60.30±44.48 vs. 22.2±23.28; P=0.007), they were >3 mg/L in an overwhelming majority of patients (95%) irrespective of presence or absence of SBP.
AUROC for diagnosing “all cause SBP” for procalcitonin alone was 0.75 (95% CI, 0.61–0.88), CRP alone was 0.55 (95% CI, 0.43–0.68) and for procalcitonin combined with CRP was 0.76 (95% CI, 0.61–0.90) (Figure 1). Procalcitonin was an even better test for diagnosing “neutrocytic SBP” as AUROC for procalcitonin alone was 0.88 (95% CI, 0.74–1.00), CRP alone was 0.62 (95% CI, 0.45–0.79) and for procalcitonin combined with CRP was 0.93 (95% CI, 0.81–1.00) (Figure 2). As evident from AUROC curves, addition of CRP did not significantly improve the diagnostic yield of procalcitonin in making the diagnosis of SBP. Though AUROC for CRP marginally improved when a much higher cut-off was used for CRP (e.g., >40 mg/L), it still fared poorly when compared to the diagnostic accuracy of procalcitonin.
Discussion
SBP is a common but serious complication of cirrhosis which jeopardizes patient survival as it increases the risk of developing hepatic encephalopathy, gastrointestinal bleeding, renal failure and septic shock (6). It is hypothesized that SBP occurs due to cirrhosis-associated-immune-dysfunction mediated bacterial translocation which is a result of impaired opsonization activity of ascitic fluid, diminished reticulo-endothelial activity, increased intestinal permeability, poor gut motility, decreased phagocytic activity and activation of inflammatory cytokines (22). Most common pathogens that translocate and thus cause SBP include E. Coli, Klebsiella pneumoniae, Enterobacter spp., Enterococcus spp. and others.
Ascitic fluid analysis is an essential part of making the diagnosis of SBP. However, obtaining and analyzing ascitic fluid by doing paracentesis itself is fraught with its own set of challenges. Given the time required to perform a paracentesis and the need for ultrasound machine to minimize the risk of bowel perforation, many physicians are reluctant to incorporate this procedure in their busy clinic practices. As most cirrhotic patients have coagulopathy and some degree of thrombocytopenia, many physicians are hesitant to do this procedure despite the strong evidence of the safety of paracentesis in face of these laboratory abnormalities (6,23-25). Proper handling of ascitic fluid is of paramount importance. As bacterial culture is usually desired, at least 10–20 mL of ascitic fluid should be collected during diagnostic paracentesis (26) and promptly inoculated into blood culture bottles at the bedside as taking ascitic fluid-filled syringe to laboratory has been shown to lower the sensitivity of bacterial detection in the specimen (6,27). Though ascites-specific dipstick based on leukocyte esterase for detecting SBP has been developed (6,28), it is not in widespread use and thus diagnosis of SBP still depends on manual cell count which is laborious, time-consuming and introduces operator-bias. Thus, given these challenges associated with diagnostic paracentesis, it would be desirable to have a widely available, non-invasive test with rapid turnaround time and high sensitivity/positive predictive value which can help make an accurate diagnosis of SBP.
Procalcitonin, a precursor of thyroid hormone calcitonin, is a 116 amino acid protein which was first found to be associated with clinically significant infections in humans approximately 25 years ago (29). Although it is most widely used as a surrogate marker of bacterial infection, it has also been found to be elevated in a variety of other non-infectious conditions such as burns (29), postoperative state (30), neuroendocrine tumors of lung and gastrointestinal tract (31,32), hemophagocytic lymph histiocytosis (33) and various other types of cancers (34). Procalcitonin is secreted by almost all parenchymal tissues in the body in response to TNF-alpha during bacterial infection and begins to rise within 4 hours of bacterial infection (11,35). It has a long half-life of 25–30 hours and is undetectable in healthy individuals.
Procalcitonin has been studied in the setting of SBP and serum procalcitonin values have almost invariably been found to be significantly higher in patients with SBP than those without it with the exception of study done by Lesinska et al. (7-18) (Table 1). In a recent meta-analysis, procalcitonin was found to have a sensitivity of 0.82 and specificity of 0.86 with AUROC of 0.92 for diagnosis of SBP (16). Procalcitonin levels in patients with culture positive SBP versus culture negative SBP (CNNA) were variable where some studies reported statistically significantly elevated procalcitonin in the former group (10) while others did not (9).
MNB patients often pose challenge in a clinical situation when pretest probability of SBP is high but neutrophil count is low in fluid studies. We often wonder if it is a false result related to sample contamination or is it an attenuated response due to weakened immune system, unrecognized outpatient prophylactic antibiotic use or a characteristic unique to certain bacteria. Due to these reasons, we also performed subgroup testing excluding MNB patients and comparing only neutrocytic ascites (classic SBP and CNNA) versus controls which showed even higher sensitivity and specificity of procalcitonin in diagnosing SBP. All our 5 MNB patients had normal procalcitonin levels, suggesting possible contamination or subclinical infection.
C-reactive protein (CRP) is an acute phase reactant of hepatic origin which is secreted in response to IL-6 during systemic inflammatory response (11). It may be elevated in a wide variety of conditions such as infections, collagen vascular diseases, cancers, coronary artery disease, obstructive sleep apnea and others. Both serum and ascitic fluid CRP levels have been studied and found to be elevated in patients with SBP (20,21,36) though some studies did not find any significant difference in CRP levels in patients with SBP and those without it (14). Levels of hs-CRP correlated with increased mortality in patients with SBP (19). A cutoff of 10 mg/L was suggested for CRP (AUC: 0.93) in a large study of patients with cirrhosis associated bacterial infections (37).
There have been a few studies that looked at combining procalcitonin and CRP in making the diagnosis of SBP and they have been well summarized in the meta-analysis done by Lin et al. (9). They reported pooled sensitivity and specificity for procalcitonin to be 79% (95% CI: 64–89%) and 89% (95% CI: 82–94%) respectively. Pooled sensitivity and specificity for CRP was 77% (95% CI: 69–84%) and 85% (95% CI: 76–90%) (11). It concluded that positive likelihood ratio for procalcitonin was high enough for it to qualify as a rule-in test (11). Correspondingly, negative likelihood ratio for CRP was low enough for it to be accepted as a rule-out test (11).
The limitation of the current study is its small sample size that may have led to potential selection bias. Unmeasured confounding variables, and missingness in the data may have potentially influenced the diagnostic accuracy of the procalcitonin and CRP. Additionally, the time of collection since the onset of SBP, duration of illness, severity of underlying liver disease, and associated comorbidities could have a potential influence on the accuracy of results. These confounders may need to be assessed in a larger prospective study. In conclusion, while ascitic fluid neutrophil count still remains the gold standard, results of our study suggest that procalcitonin may be a helpful adjunct in making the diagnosis of SBP in patients with decompensated cirrhosis. In our study, we did not find any significant increase in diagnostic accuracy for SBP when CRP is added to procalcitonin.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://tgh.amegroups.com/article/view/10.21037/tgh-19-297/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-19-297/coif). SKS reports grants from Gilead Sciences, Conatus Pharma, Intercept Pharma, Genfit, Bayer, Exact Sciences, Biotest, Shire NASH and Enanta, outside the submitted work; he reports Speaker’s Bureau from Intercept Pharma, Alexion and Dova, outside the submitted work; he is on Advisory Board of Bayer and Biotest, outside the submitted work. SKS serves as an Editor-in-Chief of Translational Gastroenterology and Hepatology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Institutional Review Board of Methodist University Hospital, Memphis, Tennessee, USA (IRB approval number: 12-02006-XP) with exemption for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis--in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol 2001;96:1232-6. [PubMed]
- Mostafa MS, El-Seidi EA, Kassem AM, et al. Detection of ascitic fluid infections in patients with liver cirrhosis and ascites. Arab J Gastroenterol 2011;12:20-4. [Crossref] [PubMed]
- Facciorusso A, Antonino M, Orsitto E, Sacco R. Primary and secondary prophylaxis of spontaneous bacterial peritonitis: current state of the art. Expert Rev Gastroenterol Hepatol 2019;13:751-9. [Crossref] [PubMed]
- Facciorusso A, Papagiouvanni I, Cela M, Buccino VR, Sacco R. Comparative efficacy of long-term antibiotic treatments in the primary prophylaxis of spontaneous bacterial peritonitis. Liver Int 2019;39:1448-58. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Runyon BA. AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651-3. [Crossref] [PubMed]
- Abdel-Razik A, Mousa N, Elhammady D, et al. Ascitic Fluid Calprotectin and Serum Procalcitonin as Accurate Diagnostic Markers for Spontaneous Bacterial Peritonitis. Gut Liver 2016;10:624-31. [Crossref] [PubMed]
- Asadi Gharabaghi M, Allameh SF, Foroutan H, et al. Blood Procalcitonin Predicts Spontaneous Bacterial Peritonitis in Patients with Cirrhosis and Ascites. Middle East J Dig Dis 2015;7:189-90. [PubMed]
- Cai ZH, Fan CL, Zheng JF, et al. Measurement of serum procalcitonin levels for the early diagnosis of spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis. BMC Infect Dis 2015;15:55. [Crossref] [PubMed]
- Cekin Y, Cekin AH, Duman A, et al. The role of serum procalcitonin levels in predicting ascitic fluid infection in hospitalized cirrhotic and non-cirrhotic patients. Int J Med Sci 2013;10:1367-74. [Crossref] [PubMed]
- Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80:72-8. [Crossref] [PubMed]
- Su DH, Zhuo C, Liao K, et al. Value of serum procalcitonin levels in predicting spontaneous bacterial peritonitis. Hepatogastroenterology 2013;60:641-6. [PubMed]
- Viallon A, Zeni F, Pouzet V, et al. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med 2000;26:1082-8. [Crossref] [PubMed]
- Wu H, Chen L, Sun Y, et al. The role of serum procalcitonin and C-reactive protein levels in predicting spontaneous bacterial peritonitis in patients with advanced liver cirrhosis. Pak J Med Sci 2016;32:1484-8. [Crossref] [PubMed]
- Wu J, Jiang F, Zeng T, et al. Role of serum procalcitonin assay for diagnosis of spontaneous bacterial peritonitis in end-stage liver diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2014;36:37-41. [PubMed]
- Yang Y, Li L, Qu C, et al. Diagnostic Accuracy of Serum Procalcitonin for Spontaneous Bacterial Peritonitis Due to End-stage Liver Disease: A Meta-analysis. Medicine (Baltimore) 2015;94:e2077. [Crossref] [PubMed]
- Yuan LY, Ke ZQ, Wang M, et al. Procalcitonin and C-reactive protein in the diagnosis and prediction of spontaneous bacterial peritonitis associated with chronic severe hepatitis B. Ann Lab Med 2013;33:449-54. [Crossref] [PubMed]
- Lesińska M, Hartleb M, Gutkowski K, et al. Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1beta) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv Med Sci 2014;59:52-6. [Crossref] [PubMed]
- Cho Y, Park SY, Lee JH, et al. High-sensitivity C-reactive protein level is an independent predictor of poor prognosis in cirrhotic patients with spontaneous bacterial peritonitis. J Clin Gastroenterol 2014;48:444-9. [Crossref] [PubMed]
- Preto-Zamperlini M, Farhat SC, Perondi MB, et al. Elevated C-reactive protein and spontaneous bacterial peritonitis in children with chronic liver disease and ascites. J Pediatr Gastroenterol Nutr 2014;58:96-8. [Crossref] [PubMed]
- Wehmeyer MH, Krohm S, Kastein F, et al. Prediction of spontaneous bacterial peritonitis in cirrhotic ascites by a simple scoring system. Scand J Gastroenterol 2014;49:595-603. [Crossref] [PubMed]
- Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol 2016;8:307-21. [Crossref] [PubMed]
- Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology 2004;40:484-8. [Crossref] [PubMed]
- Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther 2005;21:525-9. [Crossref] [PubMed]
- Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med 1986;146:2259-61. [Crossref] [PubMed]
- Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology 1988;95:1351-5. [Crossref] [PubMed]
- Runyon BA, Antillon MR, Akriviadis EA, et al. Bedside inoculation of blood culture bottles with ascitic fluid is superior to delayed inoculation in the detection of spontaneous bacterial peritonitis. J Clin Microbiol 1990;28:2811-2. [Crossref] [PubMed]
- Mendler MH, Agarwal A, Trimzi M, et al. A new highly sensitive point of care screen for spontaneous bacterial peritonitis using the leukocyte esterase method. J Hepatol 2010;53:477-83. [Crossref] [PubMed]
- Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515-8. [Crossref] [PubMed]
- Zant R, Stocker C, Schlapbach LJ, et al. Procalcitonin in the Early Course Post Pediatric Cardiac Surgery. Pediatr Crit Care Med 2016;17:624-9. [Crossref] [PubMed]
- Chen L, Zhang Y, Lin Y, et al. The role of elevated serum procalcitonin in neuroendocrine neoplasms of digestive system. Clin Biochem 2017;50:982-7. [Crossref] [PubMed]
- Patout M, Salaun M, Brunel V, et al. Diagnostic and prognostic value of serum procalcitonin concentrations in primary lung cancers. Clin Biochem 2014;47:263-7. [Crossref] [PubMed]
- Denimal D, Menegaut L, Rossi C, et al. Extreme hyperferritinemia in the setting of acute myeloid leukaemia: a case report of hemophagocytic lymphohistiocytosis. Biochem Med (Zagreb) 2016;26:255-9. [Crossref] [PubMed]
- Chaftari AM, Hachem R, Reitzel R, et al. Role of Procalcitonin and Interleukin-6 in Predicting Cancer, and Its Progression Independent of Infection. PLoS One 2015;10:e0130999. [Crossref] [PubMed]
- Twilla JD, Nair SP, Talwar M, et al. Severity of Systemic Inflammatory Response Syndrome Affects Outcomes in Decompensated Cirrhotics with Spontaneous Bacterial Peritonitis. Am J Gastroenterol 2016;111:1043-5. [Crossref] [PubMed]
- Yildirim B, Sari R, Isci N. Patients with spontaneous bacterial peritonitis, and malignant and cirrhotic ascites. J Natl Med Assoc 2005;97:276-80. [PubMed]
- Papp M, Vitalis Z, Altorjay I, et al. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int 2012;32:603-11. [Crossref] [PubMed]
Cite this article as: Verma R, Satapathy SK, Bilal M. Procalcitonin and C-reactive protein in the diagnosis of spontaneous bacterial peritonitis. Transl Gastroenterol Hepatol 2022;7:36.


