A narrative review of gastroesophageal reflux in the pediatric patient
Introduction
The understanding and management of gastroesophageal reflux (GER) have significantly changed and developed over time with advancements in medicine and technology. Technological advances have been widely applied in the adult population, but less so in the neonatal and pediatric populations due to a variety of limitations such as size and physiology. In this article, we aim to provide a detailed review of the current literature pertaining to the understanding, evaluation, and management of GER in children. This includes a review of the latest available technologies with regard to diagnosis and treatment as well as a detailed analysis of the evolution of the surgical techniques currently used to treat reflux.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tgh-20-245).
Study methods
A systematic search of PubMed and MEDLINE databases using the terms “gastroesopheageal reflux”, “gastroesophageal reflux disease”, “GERD”, “antacids”, “Nissen”, and “fundoplication” from 1980 to the present was conducted. Both adult and pediatric literature were reviewed. All available, high quality resources written in English or with an available English translation were eligible for inclusion in this review.
Epidemiology, pathogenesis and presentation
GER is the retrograde passage of gastric contents into the esophagus (1-5). This physiologic condition is most common in infants but can be present in older children as well. Current literature suggests GER may have a peak incidence in infants 3–4 months of age (60–70%) with an incidence of 50% in infants less than 2 months and down to 5% of infants by 1 year of age (2,6,7). Gastroesophageal reflux disease (GERD) is a pathologic condition characterized by the reflux of gastric contents into the esophagus and oropharynx, resulting in complications (1-5). These include failure to thrive, recurrent respiratory symptoms or changes in the epithelial lining of the distal esophagus (Barrett’s esophagus).
The first report of a reflux associated esophageal ulceration was in the late 19th century by Quincke (8). Several reports followed and the presence of a hiatal hernia (HH) was thought to be the primary cause of GER. The development of continuous measurement of the lower esophageal sphincter (LES) subsequently led to the understanding that GER was secondary to transient lower esophageal sphincter relaxation (TLESR) and not a lower basal LES pressure (9). In an excellent review of the topic, Herregods et al. provide a summary of the mechanisms resulting in reflux as well as the factors that affect the perception of symptoms (9). The factors leading to reflux are the presence of a HH, lower basal LES pressure, TLESR, acid pocket (i.e., the physiologic layering of acidic gastric secretions at the stomach fundus or gastroesophageal junction above a food bolus), increased distensibility of the esophagogastric junction (EGJ), esophageal clearance, and delayed gastric emptying (DGE) (9). Essentially the first three result in an incompetent barrier between the esophagus and stomach and the latter four lead to prolonged acid exposure through ineffective clearance (9).
Physiologic GER, characterized by mild or intermittent regurgitation, may be seen between 1 week and 6 months of age (10). These episodes become less frequent with age and the associated transition from supine to more upright posture, as well as with the transition to solid food (1,3). Despite these episodes, children with GER continue to feed well and thrive in the absence of other symptoms (2). They may often be referred to as “the happy spitter” by pediatricians. This is in stark contrast to children with GERD who may have failure to thrive or chronic respiratory illnesses.
The presentation of GERD in pediatric patients varies by age. Infants and young children may present with: failure to thrive, irritability, excessive crying, poor food intake and tolerance, sleep disturbances, coughing, wheezing, stridor, recurrent aspiration and pneumonia, recurrent otitis media, grimacing, opisthotonos, or torticollis (2,3,11-13). Older children and adolescents may present with symptoms more similar to that of adults. These include: regurgitation and emesis, nausea, dysphagia, retrosternal or epigastric pain, nausea, chronic cough, wheezing, hoarseness, halitosis, and/or dental erosions (2-4). As gastric contents may or may not reach the oropharynx to induce nausea or vomiting, postprandial emesis itself is not pathognomic and may be frequently ignored due to it ubiquitous nature in infants (1).
It is reasonable to counsel families that symptoms typically improve after 1 year of age in the overwhelming majority of healthy infants. GERD does, however, demonstrate an increased prevalence in children affected by: prematurity, obesity, neurological impairment, congenital heart disease, congenital diaphragmatic hernia, gastrointestinal (GI) tract abnormalities, and chromosomal abnormalities (2,14-21). Refractory GERD refers to GERD symptoms that do not respond to medical treatment after 8 weeks (10).
Diagnostic methods
The diagnosis of GER is primarily clinical. It is important to discern the age of onset of symptoms, feeding/dietary history, pattern of regurgitation including timing and relation to meals, any prior pharmacologic or dietary interventions, patient’s growth trajectory, assessment of possible environmental triggers, and family history. Signs and symptoms that suggest a diagnosis of GERD include: postprandial emesis or discomfort as demonstrated by significant arching of the back and even spasmodic torticollis and dystonia, as are seen in Sandifer syndrome (2,10).
Whereas diagnostic testing and treatment are not typically indicated for GER, further investigation may be warranted in those with severe symptoms or GERD (10). Diagnostic tools for the evaluation of GERD symptoms include: upper GI series, 24-hour pH monitoring, impedance testing, manometry, and endoscopy.
Esophageal pH monitoring
Esophageal pH monitoring involves the use of intraluminal probes passed through the nares to evaluate the pH proximal to the LES, and ideally, the upper esophagus, as well as the stomach (1,2). Alternatively, a wireless esophageal pH capsule can be used (2). The normal pH of the esophageal lumen ranges from four to seven (2). The purpose of monitoring is to detect all pH drops below four lasting for at least 15 seconds (i.e., the number of refluxes), the time required to correct this drop in pH (i.e., reflux clearance), the number of reflux episodes with clearance requiring more than 5 minutes, and the longest reflux episode. These values are used to calculate a reflux index, which reflects the percentage of time over a 24-hour period in which esophageal pH is less than four (1,2). A reflux index greater than 11% in infants or 7% in older children typically qualifies as abnormal (2,22,23). Esophageal pH monitoring has a relatively high sensitivity and specificity for GERD in comparison to other testing modalities, making it a long-standing key diagnostic technique (1,2).
Combined multichannel intraluminal impedance and pH monitoring
Combined multichannel intraluminal impedance and pH monitoring allow for the analysis of all refluxes and their pH, as well as the direction of food boluses over 24 hours (1,3). It is estimated that approximately 45% of infants with GERD demonstrate normal pH monitoring, as their GER episodes are more likely to be weakly acidic, normal, or even alkaline (3,24). Combined testing therefore allows for the evaluation of microaspiration of nonacid refluxes, which contribute to recurrent respiratory tract infections in young pediatric patients (1). Despite the increased cost of this combined modality, the significantly increased yield makes combined multichannel impedance and pH monitoring a frequent test of choice in the evaluation of pediatric GERD (3).
Manometry ± pH monitoring
In contrast to adults, manometry is not currently recommended in the evaluation of GERD in children. It can, however, be used to evaluate and exclude esophageal motility disorders such as achalasia that may resemble GERD. In pediatric patients, it typically requires the use of sedation and/or general anesthesia, limiting its routine use (3). When combined with pH monitoring, it allows for the evaluation of pH in pathologic reflux with stepwise normalization by swallowing saliva. It can also be used to analyze acidic, neutral, and alkaline refluxes, allowing for similar conclusions as those derived from impedance testing (1).
Upper GI
Upper GI series is not currently recommended in the evaluation and diagnosis of GERD (1,3,10). It does, however, have utility in select patients to exclude conditions that mimic GERD, such as achalasia, malrotation, and pyloric stenosis (1,3,10). Upper GI allows for visualization of the gastroesophageal junction and the angle of His, demonstration of HHs, as well as esophageal peristalsis. Most importantly, it allows for the evaluation of the location of the duodenojejunal junction and can therefore detect malrotation. Due to short radiologic exposure times, however, the degree of reflux may be under- or overestimated on upper GI (24). Brief radiologic exposure contributes to false negative results and non-pathologic reflux contributes to false positive results (24). Upper GI has poor sensitivity, specificity, and positive predictive value ranging from 29–86%, 21–83%, and 80–82% respectively relative to esophageal pH monitoring (24-31). Indirect signs of pathologic reflux include air reflux and/or a positive water siphon test (1,32).
Endoscopy and histology
The role and timing of endoscopy is controversial. Endoscopy is a more invasive modality that can be used to evaluate for eosinophilic or candida esophagitis, HHs, and/or to relieve esophageal outlet obstruction resulting in stasis, cough, and aspiration (1,10). It also allows for tissue biopsy. Further research is needed to evaluate whether endoscopy is more valuable before or after the initiation of acid suppression therapy. Endoscopy performed prior to initiation of therapy allows for definitive diagnosis at first endoscopy; however, patients typically require repeat endoscopy to evaluate for healing after starting therapy (10).
Medical management
Treatment is not indicated in the majority of cases of pediatric GER (2). Esophageal dysfunction and GER resolve spontaneously in 90% of affected infants (1). In patients who do warrant therapy, the approach is to start with simple modifications and then escalate to medical and finally surgical therapy.
Lifestyle changes
Initial measures for the treatment of GERD include changing the type, volume or frequency of feeds, thickening feeds, and postural therapy. Breastfed infants demonstrate lower rates of GERD than formula-fed infants (2,33,34). Furthermore, the elimination of cow milk protein and other potential allergens from a patient’s diet can reduce GERD (2).
Thickening feeds can increase weight gain per day and reduce the frequency of visible regurgitation episodes in infants, although the patient’s reflux index may remain unchanged (35,36).
The administration of frequent small meals, followed by maintaining an infant in an upright position for 20–30 minutes after feeds can mitigate regurgitation episodes (2). The supine position with the trunk elevated can also be utilized (1). Supervised positioning in the left lateral position is conditionally recommended in awake infants (1,3). The use of prone positioning in infants, however, is always recommended against due to the increased risk of sudden infant death syndrome (SIDS). Emesis that occurs in prone positioning can obstruct the oropharynx and induce apneic spells and apparent life-threatening events (ALTEs) (1-3).
Medical therapy
H2-receptor antagonists
Pharmacotherapy should be considered and reserved for patients whose GERD symptoms do not respond to conservative measures. H2-receptor antagonists and proton pump inhibitors (PPIs) have been proven safe and effective in reducing gastric acid output in pediatric patients (37). Treatment times vary from weeks to months (2).
H2-receptor antagonists reduce gastric acid secretion by competitively inhibiting the interaction between histamine and the H2-receptor on gastric parietal cells (2,22). They also reduce pepsin and the volume of gastric acid (2,22). They have a rapid onset of action. Commonly used H2-receptor antagonists include: cimetidine, ranitidine, famotidine, nizatidine.
In their meta-analysis of eight studies including 276 children, van der Pol et al. found that H2-receptor antagonists were more effective than placebo in promoting histologic healing, increasing gastric pH, and with regard to overall treatment effective in children 0–15 years of age (38).
The use of H2-receptor antagonists is limited by several factors. First, decreased gastric acid and hypochlorhydria can allow for gastric bacterial colonization. This can lead to an increased risk of certain infections, such as community-acquired pneumonia, enteric infections, and Clostridium difficile (1,2). Second, H2-receptor antagonists have side effects that include abdominal pain, diarrhea, headache, somnolence, and dizziness (2). Third, patients will develop a tolerance to H2-receptor antagonists with time (2,3).
In April 2020, the U.S. Food and Drug Administration (FDA) requested the immediate withdrawal of ranitidine from the market in light of ongoing investigations of contaminants in the medication. N-Nitrosodimethylamine (NDMA) is a probable human carcinogen that has been found in ranitidine. Concentrations appear to increase over time when stored above room temperature and could therefore result in dangerous levels of ingestion (39).
Proton pump inhibitors
PPIs inhibit gastric acid secretion by selectively blocking the hydrogen-potassium-adenosine triphosphatase (H+-K+-ATPase) pumps found on the gastric parietal cell membrane (2,22). They effectively ameliorate dyspepsia, and both prevent and accelerate the healing of esophagitis and esophageal injury (2,3). They are well tolerated and more effective than H2-receptor antagonists, making them the drug of choice for the treatment of pediatric GERD (2). Furthermore, patients are less likely to develop a tolerance compared to H2-receptor antagonists (3). Common agents include: omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole (2).
In their study of 30 infants aged 3–12 months, Moore et al. concluded that omeprazole was more effective than placebo in reducing esophageal acid exposure. Irritability improved with time in both groups (40). The International Pediatric Omeprazole Study Group concluded that omeprazole is effective for the treatment of erosive esophagitis and GERD symptoms at dosages of 0.7–3.5 mg/kg/day. In this study, 54 of 57 patients demonstrated healing of their erosive esophagitis, defined as macroscopically normal esophageal mucosa on endoscopy performed 3 months after initiation of treatment. Ninety-three percent of patients reported absent or mild GERD symptoms after treatment (41).
However in another systematic review of 12 studies, van der Pol et al. concluded that although well tolerated, PPIs are not effective in reducing GERD symptoms in infants and that additional placebo-controlled trials in older children are needed (42).
Side effects of PPIs include: abdominal pain, diarrhea, nausea, somnolence, dizziness, headaches. Similar to H2-receptor antagonists, patients are at increased risk of hypochlorhydria contributing to gastric bacterial colonization, community acquired pneumonia, and enteric infections including Clostridium difficile (1-3).
In a study of 1,815 individuals, Imhann et al. demonstrated significant changes to the gut microbiome with PPI use. This included a significant decrease in Shannon’s diversity, changes in 20% of the bacterial taxa, and over-representation of oral bacteria in the fecal microbiome (43), There are currently ongoing observational studies in the adult population examining whether these changes to the gut microbiome may increase the risk of gastric or colon cancer with prolonged PPI use. Although PPIs have been shown to increase serum gastrin concentration, they have not yet been shown to increase the risk of gastric or esophageal cancer, enterochromaffin-like cell hyperplasia, or carcinoid tumors (44).
The hypochlorhydria thought to contribute to increased risk of enteric infections has also been hypothesized to limit calcium and magnesium absorption. In a meta-analysis of 18 studies, Zhou et al. demonstrated that PPI use increased the risk of hip, spine, and any-site fractures in both short-term use (i.e., less than 1 year) and longer use (i.e., greater than 1 year) groups (45).
Antacids
Antacids neutralize gastric acid, minimizing exposure of the esophageal mucosa to gastric acid (2). They are not effective in the treatment of GERD in infants, but may be used for short-term symptomatic use in older children and young adults (2). This drug class includes: aluminum hydroxide, calcium carbonate, and magnesium hydroxide (2).
Prokinetics
Prokinetic agents improve esophageal contractility, increase LES pressure, and promote gastric emptying (4). These drugs, which include metoclopramide, cisapride, domperidone, and baclofen, have not however been proven to be effective in the treatment of pediatric GERD (24,46). Furthermore, they have a significant side effect profile that includes: extrapyramidal effects, prolactinemia, galactorrhea, ventricular arrhythmias, QT prolongation, drowsiness, and restlessness (1-3).
Endoscopic management of GERD
New endoscopic techniques are emerging for the treatment of pediatric GERD. The benefit of these techniques is that they would be able to be performed in an outpatient setting. Endoscopic fundoplication includes three techniques: endoscopic suturing devices, endoscopic radiofrequency, and endoscopic implantation of inert material (1). Endoscopic suturing devices function by constructing mucosal valves to prevent reflux (47,48). Gerson et al.’s meta-analysis of 233 patients over three randomized controlled trials demonstrated that transoral incisionless fundoplication (TIF) improves esophageal pH, decreases PPI utilization, and improves quality of life at 3-year follow-up (49). In one of these trials, the TEMPO trial, which consisted of 63 patients, troublesome regurgitation was eliminated in 88% of patients at 1-year follow-up, 90% at 3-year follow-up, and 86% at 5-year follow-up after TIF (50). Endoscopic radiofrequency techniques use metallic prongs and radiofrequency energy to create thermal lesions in the muscle wall of the LES (47,51). Fass et al.’s meta-analysis of 28 studies and 2,468 patients evaluated the effectiveness of endoscopic radiofrequency procedures (Stretta). Stretta improved patients’ health-related quality of life score by –14.6 (–16.48, 12.73) (P<0.001) and improved pooled heartburn standardized score by –1.53 (–1.97, –1.09) (P<0.001). Stretta reduced the incidence of erosive esophagitis by 24% and reduced PPI use from 100% of patients at baseline to 49% at follow-up (52). Endoscopic implantation of inert material decreases reflux events by reducing the distensibility of the LES (53,54). Johnson et al. and Cohen et al.’s sequential studies evaluated the effectiveness of Enteryx implantation at 12 and 24 months, respectively. Johnson et al. demonstrated a 50% or greater reduction in PPI use in 84% of their 144 patients at 12-month follow-up (53). At 24 months, Cohen et al. (n=64 patients) demonstrated a 50% or greater reduction in PPI use in 72% of patients and PPI cessation in 67% of patients (54). Esophageal acid exposure (i.e., total time pH was less than 4) was reduced by 31% at 12 months (53,54). These techniques are still being developed and tested.
Surgical management
Absolute indications for surgical intervention in the treatment of GERD include apnea or near-SIDS secondary to GERD, pneumonitis with lung changes, and esophagitis with ulceration or stricture. Relative indications include failure of symptoms to resolve with medical management, failure to thrive, recurrent pneumonia, asthma and/or chronic cough secondary to GERD, and chronic emesis (4).
Surgical principles
Classically, surgical fundoplication involves mobilization of the gastroesophageal junction, forming approximately 2 to 3 centimeters of intra-abdominal esophagus around which the fundus of the stomach is wrapped. This procedure is performed over an intraesophageal dilator/bougie to prevent narrowing of the GE junction and stomach that would lead to post-operative dysphagia. In patients with a HH, this procedure is accompanied by hiatoplasty: approximation of the crura, and/or anchoring of the wrap to the diaphragm, to prevent herniation into the thorax.
Minimally invasive surgical approaches to GER
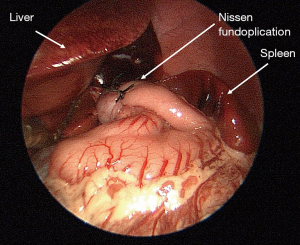
Laparoscopic Nissen fundoplication is commonly regarded as the operation of choice for the management of refractory GERD (Figure 1) (1,4). The Nissen fundoplication is a 360-degree wrap, relative to the partial dorsal wrap known as the Toupet technique or the partial ventral wrap known as the Thal technique (1). Gastrostomy tube placement can be performed concurrently with fundoplication, as is frequently seen in neurologically impaired children who both suffer from GERD and require long-term feeding access (1,4). The success rate of fundoplication is cited as 60–90% (10). Complications, when they do occur, include: persistent GERD symptoms, surgical site infection, bleeding, GI perforation, dysphagia, stricture, breakdown of the wrap, pneumothorax, dyspepsia, vagus nerve injury, dumping syndrome, and obstruction (2,4,55,56).
Kubiak et al. performed a prospective randomized study comparing long-term outcomes of laparoscopic Nissen vs. laparoscopic Thal fundoplication in children from 1998 to 2007. They demonstrated that patients, particularly those with neurological disorders, who undergo laparoscopic Nissen fundoplication have decreased recurrence rates in the long-term relative to those who undergo laparoscopic Thal fundoplication (57).
Ru et al. performed a systematic meta-analysis using nine studies to compare open versus laparoscopic Nissen fundoplication. Of 916 patients, 557 underwent laparoscopic Nissen fundoplication and 359 patients underwent open Nissen fundoplication. Although the laparoscopic group demonstrated longer operative times, there were less total post-operative complications compared to the open group (58). Patients in the laparoscopic group demonstrated less retching postoperatively (58). This was thought to be due to reduced trauma to the stomach when using a minimally invasive approach, causing less impairment to the autonomic pathways to the stomach (58). Thatch et al., whose study was included in Ru et al.’s meta-analysis, further demonstrated that patients in the laparoscopic group had decreased post-operative opiate requirements (59). Ru et al. also concluded that there were no significant differences with regard to time to full feed, surgical site infections, or need for redo fundoplication between the two groups (58). Laparoscopic Nissen fundoplication is a safe and feasible surgical treatment of GERD in children (58).
Techniques for performing pediatric laparoscopic Nissen fundoplication have been modified throughout the last 20 years. The initial approach involved an extensive hiatal dissection with complete division of the phrenoesophageal attachments and skeletonization of the crura before performing a hiatoplasty as described above.
The technique has since evolved and Holcomb & St Peter published a series of technical considerations for performing a laparoscopic Nissen fundoplication. The authors provide a list of “error traps” to mitigate the impact of lack of surgeon experience and hazard unawareness that may lead to human error. The first error trap described is failure to ligate and divide the short gastric vessels (60). They elaborate that failure to do so creates excessive tension on the wrap, contributing to post-operative dysphagia. They state that this departure from the previously described Rosetti modification typically takes less than five minutes and does not devascularize the greater curvature of the stomach (61-65). Furthermore, it improves visualization of the esophageal dissection and mobility of the wrap (60). The second error trap is failure to correctly identify the retroesophageal space and instead bringing the fundus up the free space inferior to the left gastric artery (60). The correct space is inferior to and abutting the crus, and cephalad to the left gastric artery (60). The third error trap described is division of the phrenoesophageal membrane and extensive esophageal mobilization (60). Minimal esophageal mobilization decreases the risk of transmigration of the wrap into the lower mediastinum (60,66,67). The fourth error trap is failure to use a bougie when performing the fundoplication, increasing the risk of dysphagia and/or need for dilation. The authors recommend a 2 centimeter wrap for infants and a 2.5 to 3 centimeter wrap for older children (60). The fifth and final error trap described is bringing too much fundus under the esophagus, contributing to dysphagia (60). The suture line should be placed at approximately 11 o’clock to allow for an even distribution of the wrap anterior and posterior to the esophagus and to reduce the risk of dysphagia post-operatively (60).
St Peter et al.’s two-center, prospective, randomized trial utilized 177 patients to compare transmigration rates after laparoscopic Nissen fundoplication in patients who underwent circumferential division of phrenoesophageal attachments [i.e., extensive esophageal mobilization (MAX group)] versus no violation of the phrenoesophageal membrane [i.e., minimal esophageal mobilization (MIN group)]. Patients in the MAX group demonstrated increased transmigration rates at 30% vs. 7.8% in the MIN group (P=0.002) (66). Furthermore, patients who underwent extensive esophageal mobilization had higher rates of reoperation at 18.4% vs. 3.3% in the MIN group (66).
St Peter’s research group later examined long-term outcomes of minimal vs. extensive esophageal mobilization during laparoscopic Nissen fundoplication. In this study by Desai et al., at 6.5-year median follow-up, re-herniation was noted in 22.7–36.5% of patients in the MAX group vs. 2.8–12.2% in the MIN group (67). Time to diagnosis of herniation was 14.7±9.5 months in the MAX group compared to the more prolonged time to diagnosis of 30.2±23.6 months in the MIN group (67). There was no significant different between the two groups with regard to reflux symptoms or PPI use (67).
Muensterer et al. has described success with single incision pediatric endosurgery that leaves patients without any appreciable scar (68). While this is a novel technique, it is technically quite challenging and therefore has not been widely adopted. Furthermore, in Gasior et al.’s study evaluating patient and parent satisfaction after single incision versus multi-port appendectomy, there was no significant different between the two groups regarding scar assessment at long-term 25-month follow-up (69).
There is currently minimal data available in the pediatric population evaluating the role of robotic Nissen fundoplication. In the adult population, Markar et al.’s meta-analysis comparing robotic vs. laparoscopic Nissen fundoplication demonstrated similar results between the two groups. There was no significant difference in need for re-operation or post-operative dysphagia between the robotic and laparoscopic groups (70). There was also no significant difference with regard to hospital stay or operative complications (70). There were, however, increased operative times and procedure costs in the robotic group (70).
There is also currently limited data regarding the potential role for magnetic sphincter augmentation (MSA) in pediatric patients with GERD. Clinical trials evaluating the efficacy of MSA in the adult population, however, have demonstrated improvement of GERD symptoms, as well as decreased rates of gas-bloat, esophageal acid exposure, and PPI use after intervention (71,72). Ganz et al.’s prospective clinical trial, which included 85 patients at 14 centers in the United States and the Netherlands, evaluated the efficacy and safety profile of MSA over a 5-year follow-up period. In their patient population, PPI used decreased from 100% to 15.3%, moderate or severe regurgitation rates decreased from 57% to 1.2%, and gas-bloat decreased from 52% to 8.3% at 5 years. There were no reported device erosions, migrations, or malfunctions (71). Rona et al. demonstrated that MSA remains efficacious even in the presence of a large HH (73).
Complications of surgical management
Surgical antireflux procedures are relatively safe when performed by experienced providers (74). In one of the largest published series regarding laparoscopic Nissen fundoplication, with 2008 operations performed over 20 years, Rothenberg reported an intraoperative complication rate of 0.13% in the primary group and 2.2% in the redo fundoplication group. Intraoperative complications include bleeding, gastric or esophageal perforation, pneumothorax, or vagal nerve injury (75).
Dysphagia & gas-bloat syndrome
Modifications to the original Nissen fundoplication have been made over time to address the unique complications of dysphagia and gas-bloat syndrome (76). “Gas-bloat syndrome” refers to bloating with inability to belch seen in some patients after Nissen fundoplication (76). Post-operative dysphagia rates are known to be higher in patients who undergo 360-degree wraps (57,76). The use of a 270-degree wrap, however, was not successful in reducing rates of gas-bloat. Rates of post-operative dysphagia and gas-bloat, however, both decreased with the advent of the “floppy Nissen”, the creation of a loose 360-degree wrap around a bougie (60,76,77). The floppy Nissen prevented pathologic reflux and decreased the rates of post-operative dysphagia and gas-bloat without prohibiting belching or physiologic vomiting (78). Patients who continue to demonstrate post-operative dysphagia despite the creation of a floppy Nissen typically demonstrate resolution of their symptoms within 2 years of their operation (57,76).
Delayed gastric emptying
DGE has been reported after surgical fundoplication; however, this is more commonly a pre-existing condition, as opposed to a complication, of fundoplication (79). This condition is seen in both neurologically normal and neurologically impaired children, but it is more common in the latter (79). Furthermore, many patients in both the pediatric and adult populations demonstrate improvement of DGE after fundoplication, as demonstrated by multiple studies comparing pre- and post-operative gastric emptying scans (57,79). Pyloroplasty has not been shown to have an effect on rates of DGE when performed in conjunction with Nissen fundoplication. In Maxson et al.’s study of 40 patients, the Nissen with pyloroplasty group demonstrated an increased rate of post-operative complication rates at 23.8% vs. 5% in the Nissen only group (80).
Wrap failure/migration
One of the most common long-term complications of laparoscopic Nissen fundoplication is failure or migration of the wrap. Wrap failure most commonly occurs 16 months after fundoplication and is typically diagnosed due to recurrent GERD symptoms (79). Fundoplication failure can be classified by four types:
Type 1: Complete or nearly complete disruption of the fundoplication wrap.
Type 2: Malpositioned fundoplication with sliding of the hernia in the setting of an intact wrap.
Type 3: Slipped Nissen fundoplication (slippage of the stomach above the wrap).
Type 4: Transhiatal herniation of the wrap (81,82).
Failure of the wrap occurs in 2% of neurologically normal children and in approximately 12% of neurologically impaired children (83). In Rothenberg’s series, he reports 4.6% and 6.8% wrap failure rates for primary and redo fundoplications, respectively. The highest incidence of wrap failure occurred in patients younger than 6 months of age. The most commonly cited causes of wrap failure were HH, followed by slipped Nissen (75).
Conclusions
GER is common in pediatric patients, particularly in neonates and infants. It is characterized by functional disturbances of the esophagus with prolonged transient LES relaxations. It resolves spontaneously in approximately 90% of affected infants (1). Conservative management is typically sufficient in managing symptoms. When reflux persists or causes life-threatening conditions or consequences despite lifestyle changes and optimal medical therapy, surgical fundoplication is indicated. Further studies are required to determine the role of novel endoscopic approaches to reflux in the pediatric patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology for the series “Current Topics in Pediatric General Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tgh-20-245
Peer Review File: Available at http://dx.doi.org/10.21037/tgh-20-245
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh-20-245). The series “Current Topics in Pediatric General Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. SRP served as the unpaid Guest Editor of the series. Dr. SRP reports personal fees from Transenterix, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hollwarth ME. Gastroesophageal reflux disease. Pediatric Surgery. Seventh Edition ed: Saunders, an imprint of Elsevier, 2012.
- Leung AK, Hon KL. Gastroesophageal reflux in children: an updated review. Drugs Context 2019;8:212591 [Crossref] [PubMed]
- Rybak A, Pesce M, Thapar N, et al. Gastro-esophageal reflux in children. Int J Mol Sci 2017;18:1671. [Crossref] [PubMed]
- Esposito C, Roberti A, Turra F, et al. Management of gastroesophageal reflux disease in pediatric patients: a literature review. Pediatric Health Med Ther 2015;6:1-8. [Crossref] [PubMed]
- Shin MS. Esophageal pH and combined impedance-pH monitoring in children. Pediatr Gastroenterol Hepatol Nutr 2014;17:13-22. [Crossref] [PubMed]
- Dranove JE. Focus on diagnosis: new technologies for the diagnosis of gastroesophageal reflux disease. Pediatr Rev 2008;29:317-20. [Crossref] [PubMed]
- Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med 1997;151:569-72. [Crossref] [PubMed]
- Quincke H. Ulcus oesophagi ex digestione. Dtsch Arch Klin Med 1879;24:72-9.
- Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil 2015;27:1202-13. [Crossref] [PubMed]
- Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:516-54. [Crossref] [PubMed]
- Mousa H, Hassan M. Gastroesophageal reflux disease. Pediatr Clin North Am 2017;64:487-505. [Crossref] [PubMed]
- Martin RJ, Hibbs AM. Gastroesophageal reflux in premature infants. Waltham: UpToDate, 2013.
- Lightdale JR, Gremse DA. Section on Gastroenterology, Hepatology, and Nutrition. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics 2013;131:e1684-95. [Crossref] [PubMed]
- Arcos-Machancoses JV, Ruiz Hernández C, Martin de Carpi J, et al. A systematic review with meta-analysis of the prevalence of gastroesophageal reflux in congenital diaphragmatic hernia pediatric survivors. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Blanco FC, Davenport KP, Kane TD. Pediatric gastroesophageal reflux disease. Surg Clin North Am 2012;92:541-58. viii. [Crossref] [PubMed]
- Kawahara H, Tazuke Y, Soh H, et al. Characteristics of gastroesophageal reflux in pediatric patients with neurological impairment. Pediatr Surg Int 2017;33:1073-9. [Crossref] [PubMed]
- Kim S, Koh H, Lee JS. Gastroesophageal reflux in neurologically impaired children: what are the risk factors? Gut Liver 2017;11:232-6. [Crossref] [PubMed]
- Lauriti G, Lisi G, Lelli Chiesa P, et al. Gastroesophageal reflux in children with neurological impairment: a systematic review and meta-analysis. Pediatr Surg Int 2018;34:1139-49. [Crossref] [PubMed]
- Leung AK. Ebstein's anomaly and gastroesophageal reflux. J Natl Med Assoc 1986;78:151-2. [PubMed]
- Leung AK, Rudd NL. A case of ring (9)/del(9p) mosaicism associated with gastroesophageal reflux. Am J Med Genet 1988;29:43-8. [Crossref] [PubMed]
- Pashankar DS, Corbin Z, Shah SK, et al. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. J Clin Gastroenterol 2009;43:410-3. [Crossref] [PubMed]
- Czinn SJ, Blanchard S. Gastroesophageal reflux disease in neonates and infants: when and how to treat. Paediatr Drugs 2013;15:19-27. [Crossref] [PubMed]
- van der Pol RJ, Smits MJ, Venmans L, et al. Diagnostic accuracy of tests in pediatric gastroesophageal reflux disease. J Pediatr 2013;162:983-7.e74. [Crossref] [PubMed]
- Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 2009;49:498-547. [Crossref] [PubMed]
- Seibert JJ, Byrne WJ, Euler AR, et al. Gastroesophageal reflux--the acid test: scintigraphy or the pH probe? AJR Am J Roentgenol 1983;140:1087-90. [Crossref] [PubMed]
- Stephen TC, Younoszai MK, Massey MP, et al. Diagnosis of gastroesophageal reflux in pediatrics. J Ky Med Assoc 1994;92:188-91. [PubMed]
- Meyers WF, Roberts CC, Johnson DG, et al. Value of tests for evaluation of gastroesophageal reflux in children. J Pediatr Surg 1985;20:515-20. [Crossref] [PubMed]
- Thompson JK, Koehler RE, Richter JE. Detection of gastroesophageal reflux: value of barium studies compared with 24-hr pH monitoring. AJR Am J Roentgenol 1994;162:621-6. [Crossref] [PubMed]
- Gupta JP, Kumar A, Jain AK, et al. Gastro-esophageal reflux disease (GERD): an appraisal of different tests for diagnosis. J Assoc Physicians India 1990;38:699-702. [PubMed]
- Chen MY, Ott DJ, Sinclair JW, et al. Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic findings. Radiology 1992;185:483-6. [Crossref] [PubMed]
- Aksglaede K, Pedersen JB, Lange A, et al. Gastro-esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring. Acta Radiol 2003;44:136-8. [Crossref] [PubMed]
- Dane B, Doshi A, Khan A, et al. Utility of water siphon maneuver for eliciting gastroesophageal reflux during barium esophagography: correlation with histologic findings. AJR Am J Roentgenol 2018;211:335-9. [Crossref] [PubMed]
- Campanozzi A, Boccia G, Pensabene L, et al. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics 2009;123:779-83. [Crossref] [PubMed]
- Leung AK, Sauve RS. Breast is best for babies. J Natl Med Assoc 2005;97:1010-9. [PubMed]
- Horvath A, Dziechciarz P, Szajewska H. The effect of thickened-feed interventions on gastroesophageal reflux in infants: systematic review and meta-analysis of randomized, controlled trials. Pediatrics 2008;122:e1268-77. [Crossref] [PubMed]
- Carroll AE, Garrison MM, Christakis DA. A systematic review of nonpharmacological and nonsurgical therapies for gastroesophageal reflux in infants. Arch Pediatr Adolesc Med 2002;156:109-13. [Crossref] [PubMed]
- Mattos ÂZ, Marchese GM, Fonseca BB, et al. Antisecretory treatment for pediatric gastroesophageal reflux disease - a systematic review. Arq Gastroenterol 2017;54:271-80. [Crossref] [PubMed]
- van der Pol R, Langendam M, Benninga M, et al. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatrics 2014;168:947-54. [Crossref] [PubMed]
- FDA Requests Removal of All Ranitidine Products (Zantac) from the Market [press release]. April 1, 2020.
- Moore DJ, Tao BS, Lines DR, et al. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr 2003;143:219-23. [Crossref] [PubMed]
- Hassall E, Israel D, Shepherd R, et al. Omeprazole for treatment of chronic erosive esophagitis in children: a multicenter study of efficacy, safety, tolerability and dose requirements. International Pediatric Omeprazole Study Group. J Pediatr 2000;137:800-7. [Crossref] [PubMed]
- van der Pol RJ, Smits MJ, van Wijk MP, et al. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics 2011;127:925-35. [Crossref] [PubMed]
- Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740-8. [Crossref] [PubMed]
- Thomson AB, Sauve MD, Kassam N, et al. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol 2010;16:2323-30. [Crossref] [PubMed]
- Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int 2016;27:339-47. [Crossref] [PubMed]
- Ciciora SL, Woodley FW. Optimizing the use of medications and other therapies in infant gastroesophageal reflux. Paediatr Drugs 2018;20:523-37. [Crossref] [PubMed]
- Oleynikov D, Oelschlager B. New alternatives in the management of gastroesophageal reflux disease. Am J Surg 2003;186:106-11. [Crossref] [PubMed]
- Chang KJ. Endoscopic foregut surgery and interventions: the future is now. The state-of-the-art and my personal journey. World J Gastroenterol 2019;25:1-41. [Crossref] [PubMed]
- Gerson L, Stouch B, Lobontiu A. transoral incisionless fundoplication (TIF 2.0): a meta-analysis of three randomized, controlled clinical trials. Chirurgia (Bucur) 2018;113:173-84. [Crossref] [PubMed]
- Trad KS, Barnes WE, Prevou ER, et al. The TEMPO trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov 2018;25:149-57. [Crossref] [PubMed]
- Liu DC, Somme S, Mavrelis PG, et al. Stretta as the initial antireflux procedure in children. J Pediatr Surg 2005;40:148-51; discussion 151-2. [Crossref] [PubMed]
- Fass R, Cahn F, Scotti DJ, et al. Systematic review and meta-analysis of controlled and prospective cohort efficacy studies of endoscopic radiofrequency for treatment of gastroesophageal reflux disease. Surg Endosc 2017;31:4865-82. [Crossref] [PubMed]
- Johnson DA, Ganz R, Aisenberg J, et al. Endoscopic implantation of enteryx for treatment of GERD: 12-month results of a prospective, multicenter trial. Am J Gastroenterol 2003;98:1921-30. [Crossref] [PubMed]
- Cohen LB, Johnson DA, Ganz RA, et al. Enteryx implantation for GERD: expanded multicenter trial results and interim postapproval follow-up to 24 months. Gastrointest Endosc 2005;61:650-8. [Crossref] [PubMed]
- Forbes D. Mewling and puking: infantile gastroesophageal reflux in the 21st century. J Paediatr Child Health 2013;49:259-63. [Crossref] [PubMed]
- Slater BJ, Rothenberg SS. Gastroesophageal reflux. Semin Pediatr Surg 2017;26:56-60. [Crossref] [PubMed]
- Kubiak R, Andrews J, Grant HW. Long-term outcome of laparoscopic nissen fundoplication compared with laparoscopic thal fundoplication in children: a prospective, randomized study. Ann Surg 2011;253:44-9. [Crossref] [PubMed]
- Ru W, Wu P, Feng S, et al. Laparoscopic versus open Nissen fundoplication in children: a systematic review and meta-analysis. J Pediatr Surg 2016;51:1731-6. [Crossref] [PubMed]
- Thatch KA, Yoo EY, Arthur LG 3rd, et al. A comparison of laparoscopic and open Nissen fundoplication and gastrostomy placement in the neonatal intensive care unit population. J Pediatr Surg 2010;45:346-9. [Crossref] [PubMed]
- Holcomb GW 3rd, St Peter SD. Error traps and safety steps when performing a laparoscopic Nissen fundoplication. Semin Pediatr Surg 2019;28:160-3. [Crossref] [PubMed]
- Hunter JG, Swanstrom L, Waring JP. Dysphagia after laparoscopic antireflux surgery. The impact of operative technique. Ann Surg 1996;224:51-7. [Crossref] [PubMed]
- Wu JS, Dunnegan DL, Luttmann DR, et al. The influence of surgical technique on clinical outcome of laparoscopic Nissen fundoplication. Surg Endosc 1996;10:1164-69; discussion 1169-70. [Crossref] [PubMed]
- Dallemagne B, Weerts JM, Jehaes C, et al. Causes of failures of laparoscopic antireflux operations. Surg Endosc 1996;10:305-10. [Crossref] [PubMed]
- Laycock WS, Trus TL, Hunter JG. New technology for the division of short gastric vessels during laparoscopic Nissen fundoplication. A prospective randomized trial. Surg Endosc 1996;10:71-3. [Crossref] [PubMed]
- Lopez M, Kalfa N, Forgues D, et al. Laparoscopic redo fundoplication in children: failure causes and feasibility. J Pediatr Surg 2008;43:1885-90. [Crossref] [PubMed]
- St Peter SD, Barnhart DC, Ostlie DJ, et al. Minimal vs extensive esophageal mobilization during laparoscopic fundoplication: a prospective randomized trial. J Pediatr Surg 2011;46:163-8. [Crossref] [PubMed]
- Desai AA, Alemayehu H, Holcomb GW 3rd, et al. Minimal vs. maximal esophageal dissection and mobilization during laparoscopic fundoplication: long-term follow-up from a prospective, randomized trial. J Pediatr Surg 2015;50:111-4. [Crossref] [PubMed]
- Muensterer OJ, Perger L, Hansen EN, et al. Single-incision pediatric endosurgical Nissen fundoplication. J Laparoendosc Adv Surg Tech A 2011;21:641-5. [Crossref] [PubMed]
- Gasior AC, Knott EM, Holcomb GW 3rd, et al. Patient and parental scar assessment after single incision versus standard 3-port laparoscopic appendectomy: long-term follow-up from a prospective randomized trial. J Pediatr Surg 2014;49:120-2; discussion 122. [Crossref] [PubMed]
- Markar SR, Karthikesalingam AP, Hagen ME, et al. Robotic vs. laparoscopic Nissen fundoplication for gastro-oesophageal reflux disease: systematic review and meta-analysis. Int J Med Robot 2010;6:125-31. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368:719-27. [Crossref] [PubMed]
- Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc 2017;31:2096-102. [Crossref] [PubMed]
- Kane TD, Brown MF, Chen MK, et al. Position paper on laparoscopic antireflux operations in infants and children for gastroesophageal reflux disease. American Pediatric Surgery Association. J Pediatr Surg 2009;44:1034-40. [Crossref] [PubMed]
- Rothenberg SS. Two decades of experience with laparoscopic nissen fundoplication in infants and children: a critical evaluation of indications, technique, and results. J Laparoendosc Adv Surg Tech A 2013;23:791-4. [Crossref] [PubMed]
- Davis CS, Baldea A, Johns JR, et al. The evolution and long-term results of laparoscopic antireflux surgery for the treatment of gastroesophageal reflux disease. JSLS 2010;14:332-41. [Crossref] [PubMed]
- Donahue PE, Bombeck CT. The modified Nissen fundoplication-reflux prevention without gas bloat. Chir Gastroenterol 1977;11:15-7.
- Donahue PE, Samelson S, Nyhus LM, et al. The floppy Nissen fundoplication. Effective long-term control of pathologic reflux. Arch Surg 1985;120:663-8. [Crossref] [PubMed]
- Rothenberg SS. The first decade's experience with laparoscopic Nissen fundoplication in infants and children. J Pediatr Surg 2005;40:142-6; discussion 147. [Crossref] [PubMed]
- Maxson RT, Harp S, Jackson RJ, et al. Delayed gastric emptying in neurologically impaired children with gastroesophageal reflux: the role of pyloroplasty. J Pediatr Surg 1994;29:726-9. [Crossref] [PubMed]
- Makdisi G, Nichols FC 3rd, Cassivi SD, et al. Laparoscopic repair for failed antireflux procedures. Ann Thorac Surg 2014;98:1261-6. [Crossref] [PubMed]
- Hinder R. Gastroesophageal reflux disease. Digestive tract surgery A text and atlas. Philadelphia: Lippincott-Raven Publishers, 1996:19.
- Capito C, Leclair MD, Piloquet H, et al. Long-term outcome of laparoscopic Nissen-Rossetti fundoplication for neurologically impaired and normal children. Surg Endosc 2008;22:875-80. [Crossref] [PubMed]
Cite this article as: Jacobson JC, Pandya SR. A narrative review of gastroesophageal reflux in the pediatric patient. Transl Gastroenterol Hepatol 2021;6:34.