
A narrative review of the modern surgical management of pediatric choledochal cysts
Introduction
Epidemiology, pathogenesis and classification
Choledochal cysts (CC) are a group of congenital cystic malformations that lead to dilation of the biliary tract. The incidence in the United States is rare at approximately 1 in 100,000 to 150,000 children; it is higher in East Asian populations with reports of 1 in 13,000 children being affected (1). Almost 80% of CC are now discovered prenatally or in infancy, and are surgically addressed by complete resection and reconstruction of the bile drainage into the alimentary system.
Although several theories have been suggested for the etiology of CC, the “long common channel theory” appears to have been most accepted (2). This theory suggests that there is an anomalous union of the pancreatic and biliary ducts that may lead to increased reflux of pancreatic enzymes resulting in enzymatic injury of ductal wall with consequent ductal dilation.
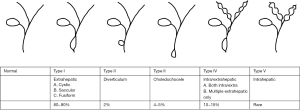
The most common classification utilized is the Todani classification, which expanded on the Alonso-Lej scheme. It groups the types of CC based on the location of the cyst and involved bile duct anatomy (3). Type I CC is characterized by dilation of the common bile duct, and is treated with cyst resection and hepaticoenterostomy. Type II is a diverticulum and can be managed with diverticulectomy; type III, a choledochocele, is managed with endoscopic sphincterotomy. Type IV and V have multiple intra- and extrahepatic dilations and may additionally require liver resection or transplant. Type IVb CC (multiple extrahepatic cysts) are appropriate for cyst resection and hepaticoenterostomy, as is performed for Type I CC. In this review, we address the surgical approach for extrahepatic CC disease in type I and IV disease (Figure 1).
Management rationale
The medical management of CC is limited to preoperative antibiotic treatment in the event of cholangitis or supportive therapy in the setting of pancreatitis. In the rare instance of spontaneous perforation, percutaneous drainage can help stabilize the patient prior to operative resection (4).
The potential of malignant degeneration to gallbladder carcinoma or cholangiocarcinoma is reported to be 6% to 30% in adults with CC, with a life-long malignancy risk of up to 4%, even after excision (5). The risk of malignancy is higher in patients with anomalous pancreatic duct union, the anomaly thought to cause type I and IV CC (6). Therefore, the presence of a CC is indication for resection, and these children require long term follow-up for surveillance. While previous reviews have discussed the pathophysiology and general management of these lesions, there is no comprehensive narrative surrounding modern surgical management of CC. In this review, we focus on minimally invasive surgical techniques, surgical competency, and long-term follow-up in the approach to CC. Articles relevant to these topics were selected from a comprehensive search of PubMed database. Additional articles were identified from the references of selected literature.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tgh-20-235).
Surgical principles
Historically, cystenterostomy or a drainage procedure was the surgical approach for CC. This approach was found to be insufficient due to a high incidence of recurrence of symptoms as well as a relatively high incidence of malignant degeneration (7,8). As a result, cyst excision and bilio-enteric reconstruction are the mainstays of surgical therapy. Two methods of reconstruction have been described: Roux-en-Y hepaticojejunostomy (HJ) and more recently, the hepaticoduodenostomy (HD).
Minimally invasive approaches to CC in the pediatric population
Laparoscopic approach
With the advent of laparoscopy, minimally invasive surgery (MIS) has strongly influenced the surgical management of CC (Figure 2). The first report to present the use of laparoscopic surgery to treat CC was published by Farello in 1995 (9). Since then, myriad articles promoting the feasibility, safety, and outcomes of this approach have followed. The safety of MIS versus open surgery was examined in a systematic review and meta-analysis published in 2015 by Zhen et al. (10). Seven observational, comparative studies were reviewed to compare outcomes between open and laparoscopic surgery for CC excision. This analysis encompassed 1,408 total patients, 611 of whom were managed with laparoscopy (43%). Meta-analysis was performed to detect differences in the following outcomes: operative time, hospital length of stay (LOS), length of time to return of bowel function, intraoperative transfusion, bile leak, abdominal bleeding, anastomotic stenosis, pancreatitis, adhesive small bowel obstruction and total complications. Laparoscopy resulted in significantly longer operative time, with a mean difference (MD) of 57 minutes (MD =56.57; 95% CI, 32.20–80.93; P<0.00001). Conversely, LOS was approximately 2 days shorter in patients treated with laparoscopy (MD =−1.93; 95% CI, −2.51 to −1.36; P<0.00001), and time to recovery of bowel function was faster in patients who underwent laparoscopic surgery (MD =−0.94; 95% CI, −1.33 to −0.55; P<0.00001). Additional data supporting laparoscopy included lower rates of intraoperative blood transfusion (relative risk (RR) =0.20; 95% CI, 0.11–0.38; P<0.00001) and adhesive intestinal obstruction (RR =0.17, 95% CI, 0.04–0.77; P=0.02). Other outcomes did not differ significantly between laparoscopic and open groups. Based upon these data, the authors concluded that laparoscopy is a safe and feasible approach to CC excision. Since the publication of this meta-analysis, additional data comparing laparoscopic and open approaches to pediatric CC management have emerged (11-17). Specific findings from these articles will be discussed below; generally, they support previous conclusions that laparoscopy is safe and effective to manage CC, and confers the benefit of decreased intraoperative bleeding and shorter hospital LOS despite an increase in operative time.

A large, international, multicenter study examined the implementation and outcomes of MIS approach to CC excision in adults and children over a span of forty years (11). Of their total cohort of 332 patients, 36 (11%) received an MIS approach (laparoscopic and robotic). Propensity matching revealed longer operative time (open 237 minutes versus MIS 301 minutes) and lower blood loss (open 50 mL versus MIS 18 mL) in the MIS group. There was no difference in cyst size upon pathologic examination (2.5 cm in open and MIS groups), but there were 3 cases of malignancy found in the open group (versus none in the MIS group). Postoperative LOS was 2 days shorter in MIS groups; complications occurred in 35% of the entire cohort within 30 days from surgery, with no differences in severity or frequency in complication in patients treated with open or MIS techniques. This study is unique in its presentation of CC management across the entire spectrum of patient age, and it supports the minimally invasive approach, especially for children. It is limited by the overall low implementation of MIS management of CC (approximately 11%), lacks long term follow-up data and may be biased by selection and retrospective nature (11). Longer follow-up and a large cohort are available from Qiao et al., who published their multicenter experience with 956 consecutive patients treated with laparoscopic CC excision, with a mean follow-up of 5.7 years (18). Complications were low overall, with 0.6% overall rate in anastomotic stricture, 1.5% bile leak, 0.8% Roux loop obstruction. There were two mortalities, including one in the immediate postoperative period due to bleeding. The operative time decreased as time from introduction of laparoscopy progressed, and centers who implemented the MIS approach later approached shorter operative times more quickly, perhaps from the collective experience of the study group.
Liem et al. reported the largest single-institution series of laparoscopic management of CC in pediatric patients to date (19). In their series, 400 patients were treated over a four year period with age ranges between one month and 16 years. Conversion to open and biliary leakage were rare. Compared to the HJ group, patients who underwent HD reconstruction had shorter operative times (220±60 vs. 165±51 min), but similar LOS (approximately 6 days each). One of the major criticisms of the HD reconstruction has been a potentially higher risk of cholangitis. In this series, the rates of cholangitis were 1.5% and 0.6% for the HD and HJ groups, respectively (P=0.24).
Laparoscopic excision in neonates
Several studies have examined the safety of laparoscopic CC excision specifically in neonates. Liu et al. reported their experience with laparoscopic management of 9 neonates who presented with symptomatic CC (20). Four ports were used to perform the excision, and reconstruction was achieved with retrocolic Roux-en-Y hepaticojejunostomy in all cases, with the jejunojejunostomy performed extracorporeally. The average age of the cohort was 24 days, with average weight of 3.7 kg and all presented with Todani type I lesions. There were no conversions to open and operative time was a mean of 216 minutes, which is comparable with other studies. Over a mean follow-up period of 32 months, there were no complications observed. The authors highlight the early consequences of untreated CC in symptomatic infants, given that biliary obstruction is generally high grade, and suggest that their data supports the feasibility and safety of early, MIS intervention (20).
With regards to the management of infants diagnosed with CC prenatally, Matsumoto et al. describe implementation of laparoscopy for excision and bilioenteric reconstruction (12). They present 7 patients in an open surgery cohort and 6 in a laparoscopic cohort. Laparoscopy was performed in 4 asymptomatic infants when they reached 5 kg, and was performed earlier in 2 patients who were symptomatic from biliary obstruction (jaundice, acholic stools and hepatic stones). This small series again showed a significant difference in operative time between laparoscopic and open groups, with a mean of 92 additional minutes needed to complete laparoscopic excision. Blood loss was lower in the laparoscopic group, and they also had an earlier return to full feedings. There were no complications recorded after 33 months of follow up in the laparoscopic group.
Ryu et al. presented the largest series of neonatal patients, with 22 who received laparoscopic CC excision, compared to 21 who underwent open surgery (15). The surgical approach was similar to the method presented by Liu et al. (20). These authors show no significant difference in operative time, and similar outcomes with regards to hospital days and time to full feeding. Additionally, arterial carbon dioxide (PaCO2) level was measured intraoperatively and was not significantly higher in the laparoscopic patients, resolving concerns of hypercapnia in young patients receiving carbon dioxide insufflation. Parents were questioned regarding their satisfaction regarding the surgical wound and scar, and satisfaction scores were significantly higher in the parents of infants treated with laparoscopic CC excision (15).
Taken together, these articles suggest that laparoscopic CC excision is feasible and safe in neonates, whether symptomatic or asymptomatic and diagnosed on pre- or postnatal imaging. Limitations and benefits of MIS approach mirror those of older children, including longer surgical time, decreased blood loss, faster return of bowel function, and improved cosmesis.
Single-incision laparoscopy
Further sophistication of the laparoscopic approach in CC management has been developed via single-incision techniques (21-24). The first description of single-incision laparoscopy for CC excision was published by Diao et al. in 2012. Nineteen patients underwent single-incision laparoscopic hepaticojejunostomy, two of whom were converted to conventional laparoscopy. Outcomes were compared to historical controls of patients who had received traditional laparoscopic hepaticojejunostomy for CC excision (n=218). Operative time was similar, as were hospital LOS and time to full feeds (22). Building upon their initial data, a matched case-control study was then performed to compare single-incision and conventional/four-port laparoscopy, with 75 patients included in each arm (these data overlap with and include patients from their initial report). Again, the authors found no difference in operative time, bleeding, hospital LOS, time to return of bowel function nor immediate or long-term outcomes. They report that single-incision patients had remarkable postoperative cosmesis with no visible scarring (25). A similar retrospective study from another institution examined single-incision outcomes in 17 patients who were matched to standard laparoscopy controls from a time period prior to implementation of single-incision laparoscopy. Again, this showed equivalent outcomes with possible improved cosmesis in single-incision cases (26). For all of the above studies, single-incision approaches were facilitated with transabdominal stay sutures for retraction, and jejunojejunostomy was created extracorporeally with an end-to-side hepaticojejunostomy for biliary drainage. Overall, these data suggest that single-incision laparoscopy is an appropriate approach to pediatric CC excision, though the benefit of this technique is mainly cosmetic and appears to rely upon extensively on surgeon experience.
Diao et al. expound upon their single-incision experience specific to neonates in additional studies (focusing on both CC and cystic biliary atresia) (27), and this group’s approach to giant CC (24) and perforated CC (21) are also available in the literature. Operative time and postoperative outcomes were acceptable and similar to their other reports, except in the group with perforation. Out of the cohort of 133 patients with perforated CC who underwent single-incision laparoscopic excision and hepaticojejunostomy, 15 (11%) were converted to open due to adhesions and bleeding. Three patients received blood transfusions and 2 had duodenal injuries which were repaired intraoperatively. The authors did employ a staged approach to children who presented with perforation and generalized peritonitis (n=52, 39%), with external biliary drainage performed prior to definitive cyst excision and bilioenteric reconstruction (21). This article highlights the difficult reality of perforated CC, since these children present with a much higher degree of intraabdominal inflammation and associated adhesions in the surgical zone, which confers higher operative risk. We surmise that the lowest-risk strategy to manage children with perforated CC is a patient-tailored approach with reliance upon preoperative drainage.
Other groups have subsequently published larger series describing the feasibility of single-incision laparoscopy for CC excision. In a group of 86 patients, this technique was successfully used, with only 1% of cases requiring placement of additional ports (28). Overall, the data regarding single-incision laparoscopy for CC excision suggests that it is a feasible approach, with its main benefit being the improved cosmesis from trans-umbilical incision only. There are no randomized trials comparing single-incision with conventional laparoscopy, and existing studies are limited by small size, with questionable extrapolation of technique to other centers where existing experience may be lower. There is a lack of long-term follow-up to determine if cosmetic benefit remains present years after surgery. Additional, more rigorous comparisons with longer follow-up will better elucidate the usefulness of single-incision surgery for CC excision.
Robotic CC excision
With regards to the application of robotic surgery to CC management, the earliest reports which described application of robotics to pediatric biliary surgery were published in 2006 (case report) and 2007 (small case series) (29,30). The case report from Woo et al. presented successful robotic excision of type I CC in a 5-year-old girl. The small case series from Meehan et al. presented 2 Kasai portoenterostomies and 2 CC excisions. Each of these studies were reconstructed with completely intracorporeal Roux-en-Y hepaticojejunostomy, and there were no reported complications. Subsequently, a larger case series of 14 children was published which highlighted the importance of surgeon skill versus the robotic learning curve (31). This group reported that the first 3 patients in their cohort experienced complications; these included conversion to open, anastomotic stricture requiring revisional surgery, and anastomotic leak treated with drainage. After the primary surgeon underwent robotic-specific skills training, the subsequent 11 cases were performed without incident (mean follow-up of 14.2 months), but operative time was long with a median of 570 minutes. This did decrease from 816 minutes in the first case to 432 minutes in the last case. Of note, the jejunojejunostomy was performed extracorporeally and prior to robot docking while the hepaticojejunostomy was created with the robotic instruments.
Though initial reports regarding the potential for robotic intervention in the management of pediatric CC were unclear with regards to its role, a more robust, comparative study further elucidated the utility of robotics in this context. Kim et al. retrospectively compared open (n=43, 54%) and robotic (n=36, 46%) approaches. This group also struggled with higher complication rates immediately after the introduction of robotic surgery (5 complications within 30 days in robotic group versus none in open group). These complications included anastomotic stricture, bile leak and intestinal obstruction. Robotic outcomes improved along with surgeon experience It is notable that the robotic surgery patients were older (57 versus 30 months) and larger (19 versus 12 kg) (32).
More recent studies have shown improved outcomes compared to the early era of robotic CC excision. Pham et al. argue that the augmented dexterity conferred from robotic technology would lend itself to enhanced technique over laparoscopy (33). While this group did not compare their robotic experience to open or laparoscopic outcomes, their retrospective case series did present acceptable outcomes from robotic CC excision. Of the 39 patients they enrolled, none had any postoperative complications. Their operative time was significantly decreased compared to earlier data, at a mean of only 193 minutes. This group highlights the collaboration of a highly skilled robotic operative assistant and careful measurement of robotic arm interactions with adjustments as the study proceeded to optimize technique. Finally, a matched retrospective study comparing robotic versus laparoscopic anastomosis in pediatric CC excision was published by Koga et al. (34). These authors used a laparoscopic setup to perform initial excision and extracorporeal jejunojejunostomy. Subsequently, 27 patients underwent laparoscopic hepaticojejunostomy while 10 received robotic hepaticojejunostomy. Robotic anastomoses were performed more quickly (70 versus 100 minutes) and surgeons perceived them to be easier to perform compared to laparoscopic anastomoses. However, the time benefit was lost with robotic setup and docking, since overall operative time was not significantly different between groups (618 minutes in robotic and 654 minutes in laparoscopic group). Overall, the operative time in this study was very high at an average of 10 to 11 hours per surgery.
Holistic examination of the data regarding robotic CC excision in children makes it clear that more research is needed to examine the utility of this approach. The most recent and larger studies do show acceptable complication rates, and robotic CC excision does not appear inferior with regards to complications if performed by experienced surgeons. On the other hand, concerns regarding the long operative times may be reinforced if forthcoming data shows similar trends; additionally, cost-effectiveness information should be reported given known expense of robotic platforms.
Maintenance of surgical competency
CC are a classic example of a rare, anatomically complex congenital anomaly, which presents challenges to achieve sufficient maintenance of surgeon skill and experience. Surgical experience was perceived to be highly significant to successful innovation of new MIS approaches to CC excision, such as in single-incision laparoscopy. Additionally, data showed that both complications and operative time in robotic CC excision are significantly higher compared to laparoscopy at the onset, when overall experience with this technique is low. However, over time, surgeon experience increased with commensurate decrease in complication rates and operative times, which demonstrates a clear learning curve in the MIS management of pediatric CC (31). A learning curve is a measure of improvement in the technical performance of a certain procedure over time and is often defined in terms of the number of cases required to gain proficiency before a technique is considered optimally safe and effective to perform. In this section, we will examine the MIS learning curve in pediatric CC excision, discuss the data regarding challenges in skill maintenance of pediatric surgeons for this rare disease, and present proposed strategies to increase skill in light of low case volumes.
Though it is known that adverse events decrease with increasing experience, Wen et al. in 2017 set out to precisely define the number of cases to achieve the learning curve for laparoscopic CC excision (35). By prospectively reviewing 104 patients undergoing laparoscopic CC excision and Roux-en-Y hepaticojejunostomy performed by a single surgeon, they found that the learning curve for CC excision was approximately 37 cases. The cases were then evaluated by early experience (less than 37 cases) and late experience (the following 67 cases). Overall, they found a decrease in operative time (352 vs. 240 min; P<0.001), LOS (9.4 vs. 7.8 days; P=0.01) and complication rate (12.5% vs. 1.5%; P=0.02) with increasing numbers of procedures performed. A multivariate analysis was performed which found that completion of the learning curve (reaching 37 cases) and the amount of patient adhesions were independent predictors of operative time, perioperative adverse outcomes and LOS.
Wen et al. also evaluated the learning curve of the two major and most difficult steps of the procedure, cyst resection and creation of the hepaticojejunostomy, and found them to be 29 and 42 cases, respectively, concluding that the anastomosis time was key and could be shortened with practice. In addition to experience, the authors state that technical skills are reasons for failure of laparoscopic CC excision and recommend training and simulation as well as supervision by more senior surgeons. Due to the association between outcomes and adhesions, the authors also suggest that surgeons who are early along in their learning curve implement careful patient selection, opting for patients with shorter disease duration.
In order to proceed along the learning curve, exposure and repetition are necessary. In direct opposition to this for diseases such as CC is the relative stable case volume and the increasing numbers of pediatric surgeons (36). The relationship between case volumes and outcomes has been widely studied and there is a positive correlation between higher case volumes and patient outcomes (37). As previously stated, CC is a rare condition in the United States, affecting approximately 1 in 100,000 to 150,000 children, making CC case volume and exposure difficult, particularly in areas of geographic isolation. In fact, from 2009 to 2013, 60% of board-certified pediatric surgeons reported no exposure (zero cases) to CC or biliary atresia (38).
While attending pediatric surgeons may find it difficult to maintain laparoscopic CC excision skills, trainees face an additional challenge. Trainees in both general surgery and pediatric surgery are required to perform “index cases,” and despite an 88% increase in pediatric surgery trainees, there has been no change in complex case volume (such as hepatic lobectomy, biliary atresia or CC excision) in pediatric surgery from 2005 to 2017 (36). Additionally, upon review of pediatric surgery fellow case records from 2000 to 2017, Park et al. found that there was at least 1 trainee per year who reported 0 complex CC or biliary atresia cases during their entire two years of pediatric surgery fellowship.
Sharing of cases may represent a helpful adjunct to improve exposure in lieu of increased clinical volume. A study by Johnson et al. in 2019 evaluated the implementation of a team-based approach to surgical care in order to increase exposure to rare index cases in a geographically isolated setting (37). A retrospective review of cases performed by at least 2 attending surgeons was conducted. The authors found that 100% of their CC excisions were team surgeries and, by utilizing a team-based approach, their case volume exceeded the national average for CC excision (1.5 vs. 0.9 per surgeon) (37). Of note, they also reported resident satisfaction with exposure to cases using a team-based approach. These findings suggest a team surgical approach may be a potential solution to increase experience per surgeon in support of maintenance of the skillset required for rare congenital diseases such as CC excision.
Simulation also plays a key role in acquiring and maintaining surgical skills. Surgical simulation focuses on the technical and cognitive aspects of a procedure and is a method for both trainees and surgeons to improve or maintain their surgical skills (38). Previous studies have found that simulation training leads to improvement in operative times, technical skills and patient-outcomes. Burdall et al. evaluated the use of a 3D-printed laparoscopic model for CC excision (39). Their model was tested by a group of ten pediatric surgeons, with interest in MIS, who rated the model 5.6/10 in tactile likeness, 6.3/10 in complexity and 7.4/10 in usefulness, suggesting a benefit of laparoscopic simulation with modeling. The authors also described additional benefits of 3D printing and simulation such as the ability to model variations in structure and allow for patient specific practice and operative planning.
Overall, simulation and team-based operations are feasible tactics to counter low case volumes and promote skills maintenance in pediatric surgeons who manage CC. What remains to be studied is whether or not these strategies may be linked to measurable improvements in surgeon skill or patient outcomes. Data is also needed to determine if robotic skills are transferrable from other pediatric or biliary conditions; aforementioned studies suggest that this may be true for CC and biliary atresia. Collectively, these data highlight a common challenge of the surgical management of complex, rare anomalies in children.
Long-term outcomes and transition to adult care
Prognosis overall is excellent for CC managed with surgical resection. The risk of malignancy, even after complete operative resection, remains higher than the general population and can be as high as 4% (1,5). Groups have advocated for surveillance with serial CA 19-9 and abdominal ultrasound. However, more research is needed to devise the optimal follow-up and surveillance strategies is needed.
Post-operative complications not related to malignancy include anastomotic stricture, cholangitis, pancreatitis, bowel obstruction, and need for reoperation. In a retrospective review of 54 children who underwent laparoscopic CC excision and biliary-enteric reconstruction with HD versus Roux-en-Y hepaticojejunostomy anastomoses, it was found that there were no statistical differences in the rate of anastomotic leakage requiring reoperation, anastomotic stricture, cholangitis, abdominal pain or gastritis, or need for reoperation (40). A Japanese single institution cohort of 110 patients with CC found that late complications were observed within 20 years and included intrahepatic bile duct dilation (0.9%), intrahepatic bile duct stones (2.7%), and adhesive bowel obstructions (3.6%). They did not have any malignancies in their series (41). While these data shine light on the long-term outcomes of patients who received CC excision, they are relatively small in size and scope. Higher volume, multi-institutional data is needed, to include quality of life information and follow-up well into adulthood, and would greatly benefit the understanding of this disease and its management.
Conclusions
This review focuses on the modern surgical management of pediatric CC, specifically types I and IV. It has been well-established that all children with a diagnosis of CC require excision and hepatic drainage given the malignancy risk associated with this disease process. However, significant innovation has emerged with regard to the surgical approach of CC excision, specifically in MIS techniques. It is clear that the open surgical management of pediatric CC is no longer the standard of care; robust data confirms the safety of MIS to manage these anomalies. This is not surprising, as the field of pediatric surgery has always pushed the envelope of less invasive approaches in order to minimize the surgical trauma that our small, delicate patients must endure. Overall, the selection of MIS (laparoscopic, single incision versus robotic) or even of open techniques should be tailored to the individual patient. Amongst these approaches, a dearth of data supporting implementation of robotic technology in this disease process calls for caution in employing this specific modality, and larger, comparative studies should be carried out to further explore the utility of robotic interventions in pediatric CC. Pediatric surgeons with higher accumulations of experience with MIS management of CC should lead the care of these patients in order to achieve less invasive approaches while still ensuring optimal outcomes, with a defined need to study surgical competency and skills maintenance in the operative management of this rare disease. Finally, multi-institutional studies with follow-up into adulthood are required in order to fully understand the spectrum of this disease and its implications for long-term wellbeing after successful CC excision.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Gastroenterology and Hepatology for the series “Current Topics in Pediatric General Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tgh-20-235
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh-20-235). The series “Current Topics in Pediatric General Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. SRP served as the unpaid Guest Editor of the series. Dr. SRP reports being a consultant for robotic surgery company named Transenterix from April 2019 to April 2020. The authors have no other conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soares KC, Arnaoutakis DJ, Kamel I, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg 2014;219:1167-80. [Crossref] [PubMed]
- Yamata A. KY, Miyano T. Choledochal Cysts. Ashcraft’s Pediatric Surgery. 5 ed. Philadelphia: Saunders Elsevier; 2010:557.
- Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977;134:263-9. [Crossref] [PubMed]
- She WH, Chung HY, Lan LC, et al. Management of choledochal cyst: 30 years of experience and results in a single center. J Pediatr Surg 2009;44:2307-11. [Crossref] [PubMed]
- Madadi-Sanjani O, Wirth TC, Kuebler JF, et al. Choledochal Cyst and Malignancy: A Plea for Lifelong Follow-Up. Eur J Pediatr Surg 2019;29:143-9. [Crossref] [PubMed]
- Kim JW, Moon SH, Park DH, et al. Course of choledochal cysts according to the type of treatment. Scand J Gastroenterol 2010;45:739-45. [Crossref] [PubMed]
- Komuro H, Makino SI, Yasuda Y, et al. Pancreatic complications in choledochal cyst and their surgical outcomes. World J Surg 2001;25:1519-23. [Crossref] [PubMed]
- Visser BC, Suh I, Way LW, et al. Congenital choledochal cysts in adults. Arch Surg 2004;139:855-60; discussion 860-2. [Crossref] [PubMed]
- Farello GA, Cerofolini A, Rebonato M, et al. Congenital choledochal cyst: video-guided laparoscopic treatment. Surg Laparosc Endosc 1995;5:354-8. [PubMed]
- Zhen C, Xia Z, Long L, et al. Laparoscopic excision versus open excision for the treatment of choledochal cysts: a systematic review and meta-analysis. Int Surg 2015;100:115-22. [Crossref] [PubMed]
- Margonis GA, Spolverato G, Kim Y, et al. Minimally invasive resection of choledochal cyst: a feasible and safe surgical option. J Gastrointest Surg 2015;19:858-65. [Crossref] [PubMed]
- Matsumoto M, Urushihara N, Fukumoto K, et al. Laparoscopic management for prenatally diagnosed choledochal cysts. Surg Today 2016;46:1410-4. [Crossref] [PubMed]
- Miyano G, Koyama M, Miyake H, et al. Comparison of laparoscopic hepaticojejunostomy and open hepaticojejunostomy. Can stenosis of the hilar hepatic duct affect postoperative outcome? Asian J Endosc Surg 2017;10:295-300. [Crossref] [PubMed]
- Qu X, Cui L, Xu J. Laparoscopic Surgery in the treatment of children with Choledochal Cyst. Pak J Med Sci 2019;35:807-11. [Crossref] [PubMed]
- Ryu HS, Lee JY, Kim DY, et al. Minimally-invasive neonatal surgery: laparoscopic excision of choledochal cysts in neonates. Ann Surg Treat Res 2019;97:21-6. [Crossref] [PubMed]
- Song G, Jiang X, Wang J, et al. Comparative clinical study of laparoscopic and open surgery in children with choledochal cysts. Saudi Med J 2017;38:476-81. [Crossref] [PubMed]
- Yu BH, Lin F. Clinical effects in resection of congenital choledochal cyst of children and jejunum Roux-Y anastomosis by laparoscope. Eur Rev Med Pharmacol Sci 2016;20:4530-4. [PubMed]
- Qiao G, Li L, Li S, et al. Laparoscopic cyst excision and Roux-Y hepaticojejunostomy for children with choledochal cysts in China: a multicenter study. Surg Endosc 2015;29:140-4. [Crossref] [PubMed]
- Liem NT, Pham HD. Early and intermediate outcomes of laparoscopic surgery for choledochal cysts with 400 patients. J Laparoendosc Adv Surg Tech A 2012;22:599-603. [Crossref] [PubMed]
- Liu SL, Li L, Hou WY, et al. Laparoscopic excision of choledochal cyst and Roux-en-Y hepaticojejunostomy in symptomatic neonates. J Pediatr Surg 2009;44:508-11. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Single-incision laparoscopic hepaticojejunostomy for children with perforated choledochal cysts. Surg Endosc 2018;32:3402-9. [Crossref] [PubMed]
- Diao M, Li L, Dong N, et al. Single-incision laparoscopic Roux-en-Y hepaticojejunostomy using conventional instruments for children with choledochal cysts. Surg Endosc 2012;26:1784-90. [Crossref] [PubMed]
- Diao M, Li L, Li Q, et al. Single-incision versus conventional laparoscopic cyst excision and Roux-Y hepaticojejunostomy for children with choledochal cysts: a case-control study. World J Surg 2013;37:1707-13. [Crossref] [PubMed]
- Diao M, Li L, Li Q, et al. Challenges and strategies for single-incision laparoscopic Roux-en-Y hepaticojejunostomy in managing giant choledochal cysts. Int J Surg 2014;12:412-7. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Coagulopathy in a subtype of choledochal cyst and management strategy. World J Gastroenterol 2014;20:10606-12. [Crossref] [PubMed]
- Tang Y, Li F, He G. Comparison of Single-Incision and Conventional Laparoscopic Cyst Excision and Roux-en-Y Hepaticojejunostomy for Children with Choledochal Cysts. Indian J Surg 2016;78:259-64. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Single-incision laparoscopic hepaticojejunostomy using conventional instruments for neonates with extrahepatic biliary cystic lesions. Surg Innov 2013;20:214-8. [Crossref] [PubMed]
- Son TN, Liem NT, Hoan VX. Transumbilical laparoendoscopic single-site surgery with conventional instruments for choledochal cyst in children: early results of 86 cases. J Laparoendosc Adv Surg Tech A 2014;24:907-10. [Crossref] [PubMed]
- Woo R, Le D, Albanese CT, et al. Robot-assisted laparoscopic resection of a type I choledochal cyst in a child. J Laparoendosc Adv Surg Tech A 2006;16:179-83. [Crossref] [PubMed]
- Meehan JJ, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg 2007;42:2110-4. [Crossref] [PubMed]
- Chang EY, Hong YJ, Chang HK, et al. Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech A 2012;22:609-14. [Crossref] [PubMed]
- Kim NY, Chang EY, Hong YJ, et al. Retrospective assessment of the validity of robotic surgery in comparison to open surgery for pediatric choledochal cyst. Yonsei Med J 2015;56:737-43. [Crossref] [PubMed]
- Pham HD, Okata Y, Vu HM, et al. Robotic-assisted surgery for choledochal cyst in children: early experience at Vietnam National Children's Hospital. Pediatr Surg Int 2019;35:1211-6. [Crossref] [PubMed]
- Koga H, Murakami H, Ochi T, et al. Comparison of robotic versus laparoscopic hepaticojejunostomy for choledochal cyst in children: a first report. Pediatr Surg Int 2019;35:1421-5. [Crossref] [PubMed]
- Wen Z, Liang H, Liang J, et al. Evaluation of the learning curve of laparoscopic choledochal cyst excision and Roux-en-Y hepaticojejunostomy in children: CUSUM analysis of a single surgeon's experience. Surg Endosc 2017;31:778-87. [Crossref] [PubMed]
- Park CJ, Armenia SJ, Cowles RA. Trends in Routine and Complex Hepatobiliary Surgery Among General and Pediatric Surgical Residents: What is the Next Generation Learning and is it Enough? J Surg Educ 2019;76:1005-14. [Crossref] [PubMed]
- Johnson SM, Lee WG, Puapong DP, et al. The Pediatric Surgical Team: a Model for Increased Surgeon Index Case Exposure. J Pediatr Surg 2019;54:1878-83. [Crossref] [PubMed]
- Abdullah F, Salazar JH, Gause CD, et al. Understanding the Operative Experience of the Practicing Pediatric Surgeon: Implications for Training and Maintaining Competency. JAMA Surg 2016;151:735-41. [Crossref] [PubMed]
- Burdall OC, Makin E, Davenport M, et al. 3D printing to simulate laparoscopic choledochal surgery. J Pediatr Surg 2016;51:828-31. [Crossref] [PubMed]
- Yeung F, Fung ACH, Chung PHY, et al. Short-term and long-term outcomes after Roux-en-Y hepaticojejunostomy versus hepaticoduodenostomy following laparoscopic excision of choledochal cyst in children. Surg Endosc 2020;34:2172-7. [Crossref] [PubMed]
- Mukai M, Kaji T, Masuya R, et al. Long-term outcomes of surgery for choledochal cysts: a single-institution study focusing on follow-up and late complications. Surg Today 2018;48:835-40. [Crossref] [PubMed]
Cite this article as: Jones RE, Zagory JA, Clark RA, Pandya SR. A narrative review of the modern surgical management of pediatric choledochal cysts. Transl Gastroenterol Hepatol 2021;6:37.


