Management of severe acute pancreatitis in 2019
Introduction
Acute pancreatitis is described as two or more of the following criteria: abdominal pain suggestive of pancreatitis, serum amylase or lipase level greater than three times the upper limit of normal and characteristic imaging findings (1). Mild acute pancreatitis, or interstitial pancreatitis, is characterized by absence of organ failure or systemic complications. Moderately severe acute pancreatitis is characterized by transient organ failure (resolving within 48 hours) and/or local systemic complications without persistent organ failure (>48 hours). Severe acute pancreatitis is characterized by persistent organ failure that may involve one or more organ systems. This includes shock (systolic blood pressure <90 mmHg), pulmonary insufficiency (PaO2 ≤60 mmHg), acute kidney injury (serum creatinine level >2 mg/dL), and gastrointestinal bleeding. Approximately 15–25% of all patients with acute pancreatitis develop severe pancreatitis with local complications, such as necrosis, pseudocyst, or abscess formation (2).
Nutrition
Patients who develop pancreatitis are often placed NPO until the inflammation has resolved and can withstand an oral diet. Current ligature supports the notion that enteral nutrition is superior to parenteral nutrition. In severe acute pancreatitis a feeding strategy is advised for the patient to eat food orally and when patient cannot tolerate an oral diet after 72 hours, enteral feed are often started (3).
Scoring systems
The ability to predict the severity of pancreatitis may help identify patients at an increased risk for morbidity and mortality. Clinical predictors of severe acute pancreatitis include age >55, alcoholic pancreatitis, the interval to onset, obesity (body mass index >30),and organ failure (4). There are several scoring systems, although most are imperfect due to the tedious nature required for calculation. Glasgow and Ranson scores take 48 hours to complete, can be used only once, and have a low sensitivity and specificity (5).The APACHE II score (Acute Physiology and Chronic Health Examination) has 12 physiologic measures and extra points based upon age and presence of chronic disease. The BISAP (Bedside Index for Severity in Acute Pancreatitis) score can be utilized to identify patients of high risk for 120-day mortality or severe disease in acute pancreatitis based upon each present criterion: BUN >25 mg/dL, acute change in mental status, systemic inflammatory response syndrome (SIRS), age >60 years, and pleural effusions (6). Compared with the Ranson criteria and APACHE II score, the BISAP score outperformed in specificity (91%), but had suboptimal sensitivity (56%) for mortality and severe acute pancreatitis within 3 months (7). Radiological imaging can also be used to determine the severity of acute pancreatitis utilizing the CT severity index (CTSI). The CTSI sums the Balthazar score grading of pancreatitis (extent of pancreatic inflammation and the presence of fluid collections) and CT scan grading of pancreatic necrosis with a sensitivity of 92% and specificity of 100% (8,9).
Classification of pancreatic fluid collections
Conclusive research in pathophysiologic understanding and improvement in diagnostic tools initiated a revision of the Atlanta Criteria in 2012 (10). The revised classification divided acute pancreatitis (4 weeks after pancreatitis episode) into two distinct subtypes, interstitial edematous pancreatitis (IEP) and necrotizing pancreatitis. Interstitial edematous acute pancreatitis is characterized by acute inflammation of the pancreatic parenchyma and peripancreatic tissues, but without tissue necrosis. IEP can lead to acute pancreatic fluid collections (<4 weeks) or pseudocysts (>4 weeks) (11). Necrotizing acute pancreatitis is characterized by inflammation associated with pancreatic parenchymal necrosis and/or peripancreatic necrosis. Necrotizing pancreatitis can lead to acute necrotic collections (<4 weeks) and walled off pancreatic necrosis (WOPN, <4 weeks) (11). The new subdivisions of acute pancreatitis are essential since the management and treatment are tailored to the type of collection present (11).
Procedural considerations for drainage
Up to 80% of PFCs resolve spontaneously and at least 25% will decrease in size with time (12). When the PFCs increase in size, they often become symptomatic and require drainage (13). Indications for drainage include infection, abdominal pain, vomiting, early satiety, persistent jaundice, and weight loss. If drainage is required and the patient is stable, it is suggested that it be performed >4 weeks to allow for encapsulation (14).
The modality of the drainage is dependent on the type of fluid collection, and the method for drainage has evolved with time. Effective management often requires collaboration with a multidisciplinary team of surgeons, radiologists, and gastroenterologists. Before the advancement of endoscopic technology and techniques, surgery was the preferred method. Surgical drainage of PFCs (cystogastrostomy) is performed by creating a tract between the PFC and the stomach or small bowel and has excellent resolution rates (91–97%) (15). However, when external drainage of pancreatic necrosis and necrosectomy is required, there is a high failure rate of 20% to 30% and a complication rate of 4% to 30% including pancreatic duct leaks and abscesses (16).
Percutaneous drainage is an effective intervention in fragile patients, with severe comorbidities or those with immature infected fluid collections who are not candidates for surgical or endoscopic interventions. The limitations of percutaneous drainage include the lack of a safe access route, hemorrhage, and pancreatic fistulas (17). The patients also often require more interventions and have longer hospitalizations (18).
PFCs typically occupy free space between anatomic structures including the stomach and duodenum. The close proximity has promoted the development of endoscopic techniques for drainage with fewer complications (19). For consideration of endoscopic drainage, a PFC must measure at least 3 cm in size, INR <1.5, platelets >50,000/µL, and discontinuation of direct oral anticoagulants for at least 48 hours (20). In the past, conventional endoscopic drainage was performed when a bulge was seen in the lumen of the GI tract. With the advent of endoscopic ultrasound, the fluid collection can be fully characterized in terms of size, wall thickness and regional vasculature.
Several studies demonstrated that a minimally invasive “step up” approach decreased mortality and complications as compared to open surgical interventions (21,22). The shift to endoscopic therapy as the preferred method of PFC management was propelled by a randomized comparative trial by Varadarajulu et al. comparing surgical vs. endoscopic approach to PFC treatment. It was determined that though the two approaches had similar rates of PFC resolution and complications, the total mean cost was lower for patients managed by endoscopy than surgery (23). Since then, various studies evaluated the comparison and overall outcomes between surgical (SD), percutaneous (PD) and endoscopic drainage (ED). Morton et al. compared SD and PD and concluded that SD has fewer complications, less inpatient mortality, reduced hospital stay, and overall cost (24). Larg and colleagues compared surgical versus endoscopic cystgastrostomy and necrosectomy. The success rate was 93.3% and 90% in the laparoscopic and endoscopic groups, respectively (25). Overall, the cost, length of hospital stay, and invasiveness is higher with the surgical approach when compared to an endoscopic approach (26). A study by Bakker et al. demonstrates endoscopic necrosectomy reduces the proinflammatory response compared with surgical necrosectomy (27). However, van Brunschot recently suggested that the step up approach in infected necrotizing pancreatitis by endoscopy was not superior to a surgical approach in reducing major complications (bleeding, perforation) or death (28). Therefore, ongoing evolution of the endoscopic technique requires attention to be focused on decreasing the risk of bleeding associated with regional vasculature and successful stent deployment. Keane and colleagues completed a study between the comparison of ED vs. PD for the treatment of pancreatic fluid collections. The study demonstrated a low risk of stent migration, pneumoperitoneum, esophageal perforations, pneumothorax and aspiration pneumonia with endoscopic drainage (29). However, percutaneous drainage was considered inferior due to residual collections (20% vs. 53%) and the need for reintervention or surgical drainage (4% vs. 6%) (26). While there is not a consensus on how to treat these patients as a whole, each case should be approached in an as minimally invasive manner as possible, and the step up approach is generally accepted.
Methods
History of endoscopic interventions of pancreatic fluid collections
The first endoscopic aspiration was in 1975 with 21 gauge needle drainage in a 31-year-old patient with a pancreatic fluid collection secondary from alcoholic pancreatitis (30).
Ten years later, PFC drainage was reported by the creation of a fistulotomy (31). The hypothesis was that rather than simply aspirating the collection that yielded insufficient drainage, the creation of a larger opening would lead to resolution. Fistulotomies were limited by the rapid, spontaneous closure of the opening before complete cyst resolution, resulting in recurrence and superinfection (32). A nasocystic drainage catheter with normal saline irrigation was utilized to provide sustained drainage, but this technique is cumbersome with poor tolerance of the nasocystic catheter and inferior anchoring methods.
Since superior endoscopic methods were in demand, internal stent placement was performed an alternative to nasocystic catheter drainage (33,34). The stent maintains patency of the fistula without the need for external drainage and patient discomfort. In 1993, 7 or 10 Fr plastic biliary stents for transmural PFC drainage were first reported, with success rates exceeding 90% (33,34).
Prior to EUS, conventional drainage was performed by evaluating the luminal wall for a bulge (35). Diagnostic puncture was made into the bulge with aspiration followed contrast injection to localize the PFC. and then immediate wire placement as a one-step procedure. A guidewire is positioned within the collection with subsequent dilation and stent placement.
Technique
The endoscopist should have a strong understanding of both endoscopic ultrasound and ERCP techniques. Patients undergo general anesthesia with intubation for airway management and prevention of aspiration, and all patients receive antibiotics during the procedure. Using a linear array echoendoscope, the fluid collection is localized from the stomach or small intestine. Color flow doppler is used to identify regional vasculature prior to puncture. The distance between the gastrointestinal lumen and the fluid collection should be measured to ensure an optimal puncture site prior to drainage. A 19-gauge FNA needle is used to puncture the stomach or duodenal wall under direct visualization by EUS. The stylet is removed and fluid can be aspirated to send for culture. A guidewire is then advanced through the needled and is coiled within the fluid collection under endosonographic and/or fluoroscopic guidance. The needle is exchanged for a dilating catheter or balloon dilator to create a fistula and facilitate stent placement. This step can be challenging due to positioning and chronic inflammatory changes of the GI tract wall. If necessary, an electrocautery device such as a needle knife or cytostome can be used to create the fistula followed by adequate dilation to 6–8 mm. Depending on the type of fluid collection, an appropriate stent is then deployed.
Stent selection
If the fluid collection is a pure pseudocyst, plastic double pigtail stents (PS) are placed as standard practice due accessibility and low cost (36). Multiple stents can be placed across the fistula to enhance drainage and minimize complications from stent occlusion. This can result in an increase in procedure time, so the use of self-expandable metal stents (SEMS) was described. SEMS create an alternative to balloon catheter dilation of the cystenterostomy tract, and is preferred if there is necrotic material within the collection. The larger lumen of the SEMS improves drainage, and reduces rates of stent occlusion, recurrence, and secondary infections (37). SEMS reduce the risk of leakage between the cyst and enteric lumens, reduce the risk of bleeding by creating a tamponade effect, and have shorter procedure times (37). At the discretion of the endoscopist, one or more double pigtail plastic stents can be placed within the metal stent, which serves as an anchor to prevent stent migration and perforation of the collection wall as it is resolving (38). When comparing the use of plastic stents versus FCSEMS in pancreatic fluid collections, there were no significant difference in success (85% vs. 83%) (39). Overall outcomes and cost effectiveness of SEMs are superior in comparison to plastic stents as seen in a study by Sharaiha et al. where complete resolution of PFCs using double pigtail plastic stent was lower compared to drainage with FCSEMSs (89% vs. 98%; P=0.01) (40). Procedural adverse events were noted in 31% in the plastic stent group and 16% in the FCSEMS group (P=0.006) (40,41).
When endoscopic debridement of a necrotic collection is necessary or if multiple sessions are required for debridement, the stent must be removed first in order to advance the scope into the necrotic cavity. To overcome this limitation, fully covered esophageal self-expanding metal stents (FCESEMS) have been described to provide superior drainage. The large caliber diameter allows for a gastroscope to be advanced into the collection for direct endoscopic irrigation and debridement with various accessories. Sarkaria et al. described this technique for 17 patients with an 88% success rate and adverse event rate of 5% (perforation of fistula tract during dilation, which was managed conservatively) (42).
Tubular stents, whether plastic or metal, have several design flaws for transluminal drainage. There is no mechanism to anchor the collection to the lumen of the GI tract. Thus, there is concern for leakage of contents and premature stent migration, which can require surgical intervention or can be fatal (43). Tubular stents are often longer than what is required. The exposed end of the stent may cause tissue trauma resulting in bleeding or perforation (43). The length of tubular stents also causes a predisposition to clog from cyst debris or food residue.
As endoscopic drainage for pancreatic fluid collections has evolved, experienced endoscopists are aware that dedicated stents are needed facilitate drainage. A lumen apposing stent (LAMS) for transluminal drainage was designed to address the specific concerns with tubular metal stents. Several lumen-apposing metal stents (LAMS), have been designed including the AXIOS stent (Boston Scientific, Marlborough, MA United States), Nagi stent (Taewoong Medical Co, Ilsan, South Korea), and Niti-S SPAXUS stent (TaeWoong Medical Co., Ltd., Ilsan, South Korea) (44).
The AXIOS stent was designed in 2004 and is the most commonly utilized LAMS in the US. The AXIOS is a nitinol braided FCSEMS with bilateral double-walled flanges in a dumbbell configuration that are vertical to the lumen and appose tissue walls to create an anastomosis (37). The respective flanges are designed to reduce stent migration and approximate structures to reduce rates of perforation, leak, and stent erosion (45). These stents have varying diameters, including 10, 15, and 20 mm that promote superior drainage and allow for direct endoscopic necrosectomy (46).
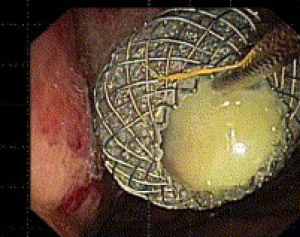
The stents are deployed in a similar fashion as other SEMS. Once the fistula tract has been dilated, the stent is advanced over the guidewire and into the fluid collection. The distal flange of the LAMS is then deployed under endosonographic guidance. The deployment catheter is then gently retracted so that the flange is secured against the wall of the fluid collection. The proximal flange can be deployed in the echoendoscope working channel, and is then released by advancing the catheter hub while simultaneously retracting the echoendoscope away from the bowel wall (Figure 1).
The catheter sheath also has two radiopaque markers indicating each end of the preloaded stent to enable fluoroscopic control of stent position, but the use of fluoroscopy for routine stent deployment is not needed.
Due to the complex nature of each step of EUS guided pancreatic fluid drainage and stent deployment, endoscopists are in need of accessories to streamline this process and reduce the risk for technical complications. In 2013, “hot” AXIOS, the Axios stent with an electrocautery-enhanced delivery system was developed. This system enables the operator to directly access the lumen of the collection with a LAMS-loaded catheter followed by immediate deployment of the stent. The electrocautery-enhanced delivery system reduces the risk of leakage since the tract remains sealed off after puncture by the 10.8 F delivery catheter until the stent is deployed, eliminating exchanging various catheters over a wire (47). The tamponade effect of the fully covered LAMS reduces the risk of bleeding from the transmural tract (48). LAMS is superior to plastic stenting in the setting of WON requiring endoscopic drainage with increased clinical success (LAMS, 80.4% and PS 57.5% (P=0.001), shorter procedure time (LAMS 50.4±26.5 vs. PS 64.6±34.0 minutes, P=0.003), decreased need for surgery (16.1% PS vs. 5.6% LAMS, P=0.02), and lower rate of recurrence with a comparable rate of adverse events (46).
Endoscopic necrosectomy
Endoscopic necrosectomy is the endoscopic manual debridement of tissue from walled off necrotic pancreatic collections. The scope can be advanced directly into the collection and water irrigation and suction are utilized to clear the cavity. Larger pieces of necrotic material can be removed with various accessories including forceps, standard polypectomy snare, Roth nets or a trapezoid basket (49). Lavage with 3% diluted hydrogen peroxide can help to facilitate debridement (50). Discontinuing proton pump inhibitors may foster auto-digestion of the necrotic tissue by gastric acid (51). Nasocystic drains can be used as an adjunct to treatment with the intermittent irrigation of the WON with normal saline to decrease the interval or decrease the need for repeat endoscopic debridement. However, the clinical success of endoscopic transmural drainage using the adjunct of nasocystic drain for PFCs may depend on the presence of necrotic material within the cavity (52). Necrosectomy sessions can be performed every 2–5 days until the majority of non-adherent necrotic material is removed, and/or there is clinical improvement (53). The timing for removal is evaluated between 1–2 months after the initially placement. During this time period the repeat cross-sectional imaging is performed to evaluate for resolution of the pancreatic fluid collection. The stent can then be removed if there is complete resolution of the fluid collection (53).
Variations of this technique are performed based on the clinical status of the patient and the morphologic appearance of the collection. Plastic stents can be utilized, but each session will require that several stents be removed with fistula tract dilation to facilitate advancement of the scope into the collection. After the debridement session is completed, additional stents are placed to keep the fistula patent. Due to the tedious nature of the procedure with the sole use of plastic double pigtail stents, it has been more common placed to use fully covered metal stents as discussed above, most commonly the lumen apposing metal stents. Studies have demonstrated excellent efficacy and safety, so LAMS are quickly becoming favored over plastic stents due to better clinical success rates and few adverse events.
Several techniques have been described in order to increase the efficacy of endoscopic drainage of PFCs (54). In difficult cases requiring multiple endoscopies for repeat necrosectomy a percutaneous drain may be placed to help maintain adequate drainage (55). Varadarajulu et al. described the use of “Gateway” drainage to decrease the need for surgery and endoscopic necrosectomy by utilizing 2 or 3 transmural tracts created by using EUS guidance between the necrotic cavity and the GI lumen. One tract is used to flush normal saline solution through a nasocystic catheter and multiple stents are used to increase drainage of necrotic contents. Plastic stents can be use in the setting of large collections to obtaining access to deeper portions of the pancreatic fluid collection to facilitate resolution (49). Navarrette et al. described the use of a percutaneous drain as an access point for difficult to access collections that are close to the skin. A fully covered esophageal metal stent is placed through the skin and across the percutaneous fistula tract into the collection for endoscopic necrosectomy (55).
Role of ERCP
Approximately 37–67% of patients with acute pancreatitis will have a pancreatic duct injury (56). Pancreatic duct disruptions can result from acute or chronic pancreatitis, trauma, or surgical injury and the role of ERCP can be successful in detecting this presence with the help of a multidisciplinary approach when considering stenting the PD duct to stop or prevent recurrent collections (57). Trevino and colleagues have shown that transpapillary stenting can lead to successful resolution of pancreatic duct leaks, particularly in partial duct disruptions (58). Shrode et al. demonstrated that there is limited benefit for endoscopic stenting in complete ductal disruptions, especially if the disruption cannot be bridged with the stent (59).
Adverse events
The reported rate of adverse events associated with EUS guided drainage of fluid collections ranges from 4–21%, including bleeding, infection, perforation and stent migration (60). The introduction of LAMS for PFC drainage has substantially decreased the risk of adverse events, but there are reports of bleeding, stent maldepolyment/migration, and buried stents due to a long course (61). Careful patient selection, with appropriate assessment of the size, components and location of the collection, and an ability to predict complications are needed to improve the safety of these stents.
Role in clinical practice
The drainage of PFCs should be reserved for advanced gastroenterologists who have expertise in interventional EUS and ERCP. ASGE guidelines recommend that endoscopic drainage of PFCs be performed only when collections are symptomatic, infected, stagnate collections >8 weeks, or rapidly enlarging cysts after maturation of the cyst wall of PFCs bulge (62). ESGE guidelines recommend performing endoscopic or percutaneous drainage of infected walled-off necrosis as the first interventional method. If there is no improvement following endoscopic transmural drainage of walled-off necrosis, endoscopic necrosectomy or minimally invasive surgery (percutaneous drainage) is preferred over open surgery as the next therapeutic step.
Future considerations
The endoscopic treatment of severe pancreatitis with pancreatic fluid collections has evolved dramatically over the past 10 years. With variations of techniques and the advancement of accessories, the overall success rates will climb. Ongoing research and development of dedicated accessories and tools for drainage and debridement are necessary in order to improve efficacy and decrease adverse event rates.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Amy Tyberg) for the series “Innovation in Endoscopy” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh-2020-08/coif). The series “Innovation in Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Foster BR, Jensen KK, Bakis G, et al. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. RadioGraphics 2016;36:675-87. [Crossref] [PubMed]
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016;59:128-40. [Crossref] [PubMed]
- Lodewijkx PJ, Besselink MG, Witteman BJ, et al. Nutrition in acute pancreatitis: a critical review. Expert Rev Gastroenterol Hepatol 2016;10:571-80. [Crossref] [PubMed]
- Martínez J, Johnson CD, Sanchez-Paya J, et al. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology 2006;6:206-9. [Crossref] [PubMed]
- Corfield AP, Williamson RCN, McMahon MJ, et al. Prediction of severity in acute pancreatitis: prospective comparison of three prognostic indices. Lancet 1985;2:403-7. [Crossref] [PubMed]
- Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology 2013;144:1272-81. [Crossref] [PubMed]
- Gao W, Yang HX, Ma CE. The Value of BISAP Score for Predicting Mortality and Severity in Acute Pancreatitis: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0130412. [Crossref] [PubMed]
- Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson's score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf) 2018;6:127-31. [Crossref] [PubMed]
- Banday IA, Gattoo I, Khan AM, et al. Modified Computed Tomography Severity Index for Evaluation of Acute Pancreatitis and its Correlation with Clinical Outcome: A Tertiary Care Hospital Based Observational Study. J Clin Diagn Res 2015;9:TC01-5. [Crossref] [PubMed]
- Ignatavicius P, Gulla A, Cernauskis K, et al. How severe is moderately severe acute pancreatitis? Clinical validation of revised 2012 Atlanta Classification. World J Gastroenterol 2017;23:7785-90. [Crossref] [PubMed]
- Banks PA, Freeman ML. Practice Parameters Committee of the American College of G. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379-400. [Crossref] [PubMed]
- Cui ML, Kim KH, Kim HG, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci 2014;59:1055-62. [Crossref] [PubMed]
- Aghdassi A, Simon P, Pickartz T, et al. Endoscopic management of complications of acute pancreatitis: an update on the field. Expert Rev Gastroenterol Hepatol 2018;12:1207-18. [Crossref] [PubMed]
- Andalib I, Dawod E, Kahaleh M. Modern Management of Pancreatic Fluid Collections. J Clin Gastroenterol 2018;52:97-104. [Crossref] [PubMed]
- Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg 2002;235:751-8. [Crossref] [PubMed]
- Nealon WH, Walser E. Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg 2005;241:948-57; discussion 957-60. [Crossref] [PubMed]
- Agalianos C, Passas I, Sideris I, et al. Review of management options for pancreatic pseudocysts. Transl Gastroenterol Hepatol 2018;3:18. [Crossref] [PubMed]
- Akshintala VS, Saxena P, Zaheer A, et al. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc 2014;79:921-8; quiz 83.e2, 83.e5.
- Andren-Sandberg A, Ansorge C, Eiriksson K, et al. Treatment of pancreatic pseudocysts. Scand J Surg 2005;94:165-75. [Crossref] [PubMed]
- Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy 2016;48:385-402. [Crossref] [PubMed]
- Rasch S, Phillip V, Reichel SOpen Surgical versus Minimal Invasive Necrosectomy of the Pancreas-A Retrospective Multicenter Analysis of the German Pancreatitis Study Group, et al. PLoS One 2016;11:e0163651. [Crossref] [PubMed]
- van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-502. [Crossref] [PubMed]
- Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145:583-90.e1. [Crossref] [PubMed]
- Morton JM, Brown A, Galanko JA, et al. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocysts: 1997-2001. J Gastrointest Surg 2005;9:15-20; discussion 21. [Crossref] [PubMed]
- Garg PK, Meena D, Babu D, et al. Endoscopic versus laparoscopic drainage of pseudocyst and walled-off necrosis following acute pancreatitis: a randomized trial. Surg Endosc 2020;34:1157-66. [Crossref] [PubMed]
- Bang JY, Arnoletti JP, Holt BA, et al. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology 2019;156:1027-40.e3. [Crossref] [PubMed]
- Bakker OJ, van Santvoort HC, van Brunschot S, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012;307:1053-61. [Crossref] [PubMed]
- van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51-8. [Crossref] [PubMed]
- Keane MG, Sze SF, Cieplik N, et al. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary centre. Surg Endosc 2016;30:3730-40. [Crossref] [PubMed]
- Rogers BH, Cicurel NJ, Seed RW. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc 1975;21:133-4. [Crossref] [PubMed]
- Kozarek RA, Brayko CM, Harlan J, et al. Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc 1985;31:322-7. [Crossref] [PubMed]
- Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol 2016;22:2256-70. [Crossref] [PubMed]
- Binmoeller KF, Seifert H, Walter A, et al. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 1995;42:219-24. [Crossref] [PubMed]
- Smits ME, Rauws EA, Tytgat GN, et al. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc 1995;42:202-7. [Crossref] [PubMed]
- Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992;38:170-1. [Crossref] [PubMed]
- DiMaio CJ. Management of complications of acute pancreatitis. Curr Opin Gastroenterol 2018;34:336-42. [Crossref] [PubMed]
- Binmoeller KF, Nett A. The Evolution of Endoscopic Cystgastrostomy. Gastrointest Endosc Clin N Am 2018;28:143-56. [Crossref] [PubMed]
- Talreja JP, Shami VM, Ku J, et al. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc 2008;68:1199-203. [Crossref] [PubMed]
- Bang JY, Hawes R, Bartolucci A, et al. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc 2015;27:486-98. [Crossref] [PubMed]
- Sharaiha RZ, DeFilippis EM, Kedia P, et al. Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc 2015;82:822-7. [Crossref] [PubMed]
- Bang JY, Varadarajulu S. Metal versus Plastic Stent for Transmural Drainage of Pancreatic Fluid Collections. Clin Endosc 2013;46:500-2. [Crossref] [PubMed]
- Sarkaria S, Sethi A, Rondon C, et al. Pancreatic necrosectomy using covered esophageal stents: a novel approach. J Clin Gastroenterol 2014;48:145-52. [Crossref] [PubMed]
- Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy 2010;42:E126-7. [Crossref] [PubMed]
- Stier MW, Waxman I. Lumen-Apposing Metal Stents: Which One and Why? Gastrointest Endosc Clin N Am 2018;28:207-17. [Crossref] [PubMed]
- Rodrigues-Pinto E, Baron TH. Evaluation of the AXIOS stent for the treatment of pancreatic fluid collections. Expert Rev Med Devices 2016;13:793-805. [Crossref] [PubMed]
- Chen YI, Yang J, Friedland S, et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: a large international multicenter study. Endosc Int Open 2019;7:E347-54. [Crossref] [PubMed]
- Yamamoto N, Isayama H, Kawakami H, et al. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc 2013;77:809-14. [Crossref] [PubMed]
- Saftoiu A, Ciobanu L, Seicean A, et al. Arterial bleeding during EUS-guided pseudocyst drainage stopped by placement of a covered self-expandable metal stent. BMC Gastroenterol 2013;13:93. [Crossref] [PubMed]
- Saumoy M, Kumta NA, Tyberg A, et al. Transcutaneous Endoscopic Necrosectomy for Walled-off Pancreatic Necrosis in the Paracolic Gutter. J Clin Gastroenterol 2018;52:458-63. [Crossref] [PubMed]
- Siddiqui AA, Easler J, Strongin A, et al. Hydrogen peroxide-assisted endoscopic necrosectomy for walled-off pancreatic necrosis: a dual center pilot experience. Dig Dis Sci 2014;59:687-90. [Crossref] [PubMed]
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016;16:66-72. [Crossref] [PubMed]
- Kitamura K, Yamamiya A, Ishii Y, et al. Clinical outcomes of endoscopic ultrasonography-guided transmural drainage using plastic stent and nasocystic drain for pancreatic and peripancreatic collections. Hepatobiliary Pancreat Dis Int 2019;18:96-9. [Crossref] [PubMed]
- Gornals JB, Consiglieri CF, Busquets J, et al. Endoscopic necrosectomy of walled-off pancreatic necrosis using a lumen-apposing metal stent and irrigation technique. Surg Endosc 2016;30:2592-602. [Crossref] [PubMed]
- Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc 2011;74:74-80. [Crossref] [PubMed]
- Navarrete C, Castillo C, Caracci M, et al. Wide percutaneous access to pancreatic necrosis with self-expandable stent: new application (with video). Gastrointest Endosc 2011;73:609-10. [Crossref] [PubMed]
- Lau ST, Simchuk EJ, Kozarek RA, et al. A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg 2001;181:411-5. [Crossref] [PubMed]
- Canlas KR, Branch MS. Role of endoscopic retrograde cholangiopancreatography in acute pancreatitis. World J Gastroenterol 2007;13:6314-20. [Crossref] [PubMed]
- Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol 2010;25:526-31. [Crossref] [PubMed]
- Shrode CW, Macdonough P, Gaidhane M, et al. Multimodality endoscopic treatment of pancreatic duct disruption with stenting and pseudocyst drainage: how efficacious is it? Dig Liver Dis 2013;45:129-33. [Crossref] [PubMed]
- Vazquez-Sequeiros E, Baron TH, Perez-Miranda M, et al. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: results of a Spanish nationwide registry. Gastrointest Endosc 2016;84:450-7.e2. [Crossref] [PubMed]
- Yang D, Perbtani YB, Mramba LK, et al. Safety and rate of delayed adverse events with lumen-apposing metal stents (LAMS) for pancreatic fluid collections: a multicenter study. Endosc Int Open 2018;6:E1267-75. [Crossref] [PubMed]
- Muthusamy VR, Chandrasekhara V, Acosta RD, et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc 2016;83:481-8. [Crossref] [PubMed]
Cite this article as: Copelin E, Widmer J. Management of severe acute pancreatitis in 2019. Transl Gastroenterol Hepatol 2022;7:16.