
Evaluation of liver transplant candidates with non-alcoholic steatohepatitis
Non-alcoholic fatty liver disease (NAFLD) and liver transplantation (LT)
NAFLD affects 75 to 100 million Americans and up to 25% of the global population (1,2). The prevalence of NAFLD is expected to increase by 60% in the next decade, in parallel with the obesity epidemic, making NAFLD the most common chronic liver disease (3,4). Non-alcoholic steatohepatitis (NASH), affecting 10–30% of patients with NAFLD, is the progressive form of NAFLD that leads to cirrhosis and is associated with cardiovascular (CV) and liver-related morbidity and mortality (5). Hepatic fibrosis is the most important predictor of mortality in NASH (6). One in four NASH patients will progress to cirrhosis over 8 years on average (7). Patients with NASH-related cirrhosis are at increased risk of hepatocellular carcinoma (HCC), occurring at an annual incidence of 0.3–4.3% (8).
NASH is currently the 2nd most common indication for LT in the U.S., but is the fastest growing indication for LT and simultaneous liver-kidney (SLK) transplants and the fastest growing cause of HCC in LT recipients (9-13). NASH is expected to overtake chronic hepatitis C as the most common indication for LT. In the last two decades, waitlist registrations, liver transplants, and SLK transplants for NASH increased 3-fold each (9-11), while the number of LT registrants and recipients with HCC attributable to NASH increased 4- and 8-fold, respectively (12,13).
Due to the rapidly growing incidence of NASH-related cirrhosis and HCC that may require LT, a review on the LT evaluation of patients with NASH is timely. In this paper, we will provide a brief overview of LT evaluation, review unique features of NASH patients that impact their transplant outcomes, and discuss how the LT evaluation may be modified for patients with NASH-related cirrhosis using available evidence.
General overview of the liver transplant evaluation
Demand for LT outstrips supply of available organs. In 2016, there were 11,340 patients in the waitlist for LT, but only 7,841 transplant surgeries were performed (14). The objective of LT evaluation is to determine which patient derives the most benefit from LT with the least risk, thus maximizing the societal benefits of a limited resource (15). Indications for LT in NASH patients are no different from patients with other liver diseases. LT is indicated in patients with cirrhosis complicated by medically refractory hepatic decompensation (e.g., ascites, hepatic encephalopathy, variceal hemorrhage), synthetic dysfunction [i.e., model for end-stage liver disease (MELD) score ≥15], or HCC meeting certain criteria (16,17). With few center-specific differences, the steps involved in LT evaluation are common to all liver diseases. LT evaluation is a multidisciplinary undertaking that involves hepatologists, transplant surgeons, anesthesiologists, cardiologists, infectious disease specialists, social workers, psychiatrists, nutritionists, and financial counselors. Hepatologists optimize medical management of the underlying liver disease and typically determine if LT is indicated. Some contraindications to LT include severe cardiopulmonary disease, uncontrolled sepsis, and extrahepatic malignancy (16). Social workers and psychiatrists evaluate the LT candidate’s social support and for substance use and co-morbid psychopathology, which may negatively affect the candidate’s ability to cope with major surgery and adhere to lifelong immunosuppression and medical care. Dietitians assess the LT candidate’s nutritional status and provide dietary education. The transplant surgeon and anesthesiologist discuss technical issues and risks related to the operation and anesthetic plan. LT candidates are tested for underlying infections that include human immunodeficiency virus, tuberculosis and, in appropriate settings, parasites and fungi. Doppler ultrasound (US) or contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans assess portal vein patency, screen for HCC, and stage HCC, if present, to ensure appropriateness of LT.
Features of NASH that increase transplant risk
LT candidates with NASH have unique clinical features that distinguish them from patients with other liver diseases and potentially increase their risk of having poor LT-related outcomes.
Advanced age
In registry studies, the average age of LT recipients with NASH is 58 years, compared to 52 years in non-NASH LT recipients (10,18). Older LT candidates aged ≥65 years are twice as likely to die in the waitlist or be delisted for being “too sick” [subhazard ratio (SHR) 1.7–2.0] (19). However, a retrospective study shows that LT recipients aged ≥70 years do not have increased risk of mortality [relative risk (RR) 1.00, 95% CI, 0.43–2.31, P=1.00] and graft loss (RR 1.17, 95% CI, 0.54–2.52, P=0.70) after LT (20).
Frailty
Frailty refers to the condition of decreased physiologic reserve, as a result of decline in multiple bodily systems, that predisposes a person to adverse outcomes (21). Although the concept of frailty originated in the geriatric population, it has been validated to predict adverse outcomes in chronic diseases like cirrhosis. In the field of liver disease and transplantation, frailty mostly pertains to physical frailty, which includes functional performance, functional capacity, and disability (22). The prevalence of frailty in patients with cirrhosis is 20–25% (23,24) and approaches 50% in patients undergoing LT evaluation (25,26). In cirrhosis, frailty is associated with more frequent hospitalizations, longer hospital stays, higher healthcare costs, and greater mortality (23,24,27,28). Frailty is a predictor of waitlist morbidity and mortality in LT candidates, independent of patient age and liver disease severity (25,29,30). Frailty is more prevalent in LT candidates with NASH (49–60%) than in those with alcohol-related liver disease (0–34%) or viral hepatitis (20%) (24,31). Obesity further increases waitlist mortality in frail patients (32). In a prospective study of LT candidates with NASH, frailty increases the likelihood of being removed from the waitlist (per 0.1 unit change in frailty index: HR 1.46, 95% CI, 1.06–2.03, P=0.02) (31). Frail patients have poor post-LT outcomes, with significantly higher incidence of mortality, infection, and re-operation (66% vs. 26%, P=0.008) (33).
Sarcopenia
NASH patients are not only typically obese, but also suffer from sarcopenia. Sarcopenia, a state of reduced muscle mass and function, is an objective measure of malnutrition and a major driver of frailty in patients with cirrhosis (22,34). The prevalence of sarcopenia in cirrhosis is 50% and 20–70% in LT candidates with NASH (31,35,36). LT candidates with NASH are likely to be obese and sarcopenic simultaneously (37). Sarcopenic patients with cirrhosis have worse survival (1-year 53% vs. 85%, P<0.005) and higher infection-related mortality (22% vs. 8% of all deaths, P=0.02) than non-sarcopenic patients (38). Sarcopenia is associated with increased waitlist mortality [waitlist mortality 29%; hazard ratio (HR) 2.36, 95% CI, 1.23–4.53, P=0.009], but does not appear to affect post-LT survival (1-year survival 90%) (36,39). In a recent study on LT candidates with NASH, sarcopenia affects neither waitlist mortality (1-year 15%; HR 2.1, 95% CI, 0.7–6.3, P=0.21) nor post-LT survival (1-year survival 85%) (31,40). However, the combination of sarcopenia and obesity has been associated with lower post-LT survival (1-year 66%) (37).
Obesity, diabetes and metabolic syndrome
NASH is widely considered the hepatic manifestation of the metabolic syndrome (MetS), composed of abdominal obesity, insulin resistance, atherogenic dyslipidemia, and hypertension (41). There is high prevalence of MetS (70.7%), obesity (81.8%), diabetes mellitus (DM) (43.6%), hypertension (HTN) (68.0%), and hyperlipidemia (HLD) (72.1%) in NASH patients (1). Among LT recipients with NASH, the prevalence of obesity, DM and HTN are equally high at 53–68%, 49–73%, 38–75%, respectively (42). Patients with NASH-related cirrhosis are significantly more likely to have MetS than patients with cirrhosis from other liver diseases (43). MetS is associated with poor outcomes in patients and LT recipients with NASH (44-51). Among NHANES-III participants with presumed NAFLD, liver-related mortality is significantly increased by MetS (HR 12.1, 95% CI, 1.1–132.2), insulin resistance (HR 53.6, 95% CI, 9.2–344.3), and obesity (HR 11.2, 95% CI, 2.4–51.5) (44). LT recipients with DM have higher all-cause (HR 1.21, 95% CI, 1.12–1.30) and cardiovascular disease (CVD)-related mortality (HR 1.93, 95% CI, 1.55–2.41) after LT (45). At time of transplant, morbidly obese LT recipients (i.e., Body mass index (BMI) ≥40 kg/m2) tend to be sicker and are more likely to be in intensive care (vs. non-obese: 11.5% vs. 7.6%, P<0.05), on life support (7.7% vs. 4.1%, P<0.05), and mechanically ventilated (6.7% vs. 3.7%, P<0.05) (46). Morbidly obese and diabetic waitlist registrants are 15-20% more likely to be delisted or die in the waitlist (47). Morbidly obese patients spend more time in the waitlist, are less likely to receive MELD exception, and more likely to be turned down for an organ, which combined may account for their increased waitlist mortality (48). Morbidly obese organ recipients have longer LT operative times (8.2 vs. 7.2 hours, P=0.003) and higher incidence of primary non-function (10% vs. 6%, P<0.05), lower short-term (30-day 88% vs. 94%, P<0.05) and long-term (5-year 49% vs. 56%, P<0.05) survival, and higher mortality from CVD, infection and malignancy after transplant (49-51). Correcting for ascites downgrades 20% of LT recipients to lower obesity grades and abolishes the negative effect of BMI on post-LT survival, suggesting that previously described poor outcomes in high BMI patients may be mediated by more severe liver disease and portal hypertension (52). Indeed, recent studies, including a meta-analysis, find no association between obesity and post-LT patient and graft survival unless there is co-morbid DM (53-55). In fact, morbidly obese patients have been shown to derive greater survival benefit from LT than non-obese patients (56).
Chronic kidney disease
LT candidates with NASH have lower glomerular filtration rate (GFR) than patients with chronic hepatitis C infection (55.2±20.0 vs. 61.6±19.9 mL/min/m2) (9), presumably due to higher prevalence of DM and HTN. The prevalence of chronic kidney disease (CKD) in NASH patients is 20-30% (57,58). NAFLD patients are more likely to have co-morbid CKD [odds ratio (OR) 2.12, 95% CI, 1.69–2.99] and develop incident CKD (HR 1.79, 95% CI, 1.65–1.95) than patients without NAFLD (59). The magnitude of association between NAFLD and CKD is unaffected by DM and HTN, suggesting that NAFLD per se may increase CKD risk. Patients with steatohepatitis and advanced fibrosis have greater risk for CKD than patients with simple steatosis (59). CKD predicts CVD-related mortality after LT in patients with NASH (60). SLK transplantation should be offered to patients with NASH-related cirrhosis who have GFR <60 mL/min for ≥90 consecutive days or sustained acute kidney injury, defined as need for renal replacement therapy or GFR <25 mg/min for 6 weeks (61). In patients with end-stage liver disease (ESLD) and CKD, SLK transplantation is associated with better patient and graft survival than LT alone (62).
Cardiovascular disease
Due to their unfavorable metabolic profile, NASH patients are at risk for clinical CVD, including atherosclerosis, valvular heart disease and arrhythmias, and subclinical CVD markers such as greater carotid-intima media thickness, more severe coronary calcification, endothelial dysfunction, and increased arterial stiffness (63,64). A meta-analysis shows increased risk of fatal and non-fatal CV events in patients with NAFLD (OR 1.64, 95% CI, 1.26–2.13, P<0.001) and even greater risk in those with NASH and fibrosis (OR 2.58, 95% CI, 1.78–3.75, P<0.001) (65). LT candidates with NASH conceivably have the most severe disease in the NAFLD spectrum and, thus, the highest CVD risk. The prevalence of angina, peripheral vascular disease, and stroke in LT candidates with NASH are 7%, 2%, and 1%, respectively, all higher than other chronic liver diseases (60). Coronary artery disease (CAD) is present in 10–30% of patients being evaluated for LT, and patients with NASH-related cirrhosis are significantly more likely to have CAD than patients with other liver diseases (43,57,66,67). In a prospective cohort of patients undergoing coronary angiography (CAG), 84.6% of patients with hepatic steatosis have >50% stenosis in at least one coronary artery and 68.3% required a coronary intervention (68). Perioperative morbidity and mortality rates in LT candidates with severe CAD are 80% and 50%, respectively, even if they receive medical therapy or surgical revascularization before LT (69). Although LT outcomes, in general, have improved in the last 2 decades, LT recipients with significant CAD continue to have worse mortality and CV-related morbidity after LT (70). Cardiac-related deaths are more common in cirrhosis due to NASH than chronic hepatitis C (28% vs. 2% of deaths) and a higher percentage of deaths in NASH patients is due to CVD rather than liver disease (25–37% vs. 2–13% of all deaths) (71,72).
Portal vein thrombosis (PVT)
PVT affects 2–26% of cirrhotic patients and appears to occur more frequently in LT candidates with NASH than those with other liver diseases (10% vs. 6%, P<0.001) (73,74). NASH is hypothesized to be a hypercoagulable state due to elevated levels of procoagulant factors (e.g., factor VIII, PAI-1) and reduced levels of endogenous anticoagulants (e.g., protein C) (75). PVT at time of LT is associated with worse perioperative outcomes including greater transfusion requirements (mean 10 vs. 5 units, P<0.001) and higher rates of primary non-function (6.6% vs. 1.4%, P=0.02), post-LT renal dysfunction (20.0% vs. 9.4%, P=0.01) and post-LT mortality (30.0% vs. 12.4%, P<0.001) (76). A recent publication on LT recipients with NASH shows that PVT increases risk of post-LT mortality and graft failure by 30–40% (77).
Hepatocellular carcinoma
HCC has an incidence of 1–4%/year in patients with NASH-related cirrhosis (8,78). Older age, male gender, DM, and HTN are risk factors for HCC development in NASH-related cirrhosis (79). Although the majority of HCC arises in cirrhotic livers, 15% of NASH-related HCC occur without cirrhosis and, interestingly, are also likely to be larger and unresectable (80). About 20% of LT waitlist registrants have co-morbid HCC (9). LT should be considered in HCC meeting Milan criteria as such tumors are associated with low rates of mortality, graft failure, and recurrent HCC (81).
Tailoring liver transplant evaluation for patients with NASH
Although LT candidates with NASH undergo the same steps in LT evaluation as patients with other liver diseases, their LT evaluation may be modified to address some unique characteristics of patients with NASH (Figure 1).
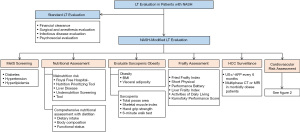
Screening for metabolic syndrome
LT candidates with NASH should be routinely screened for DM, HTN, and HLD due to high prevalence of these co-morbidities. These conditions should be medically optimized before LT according to standards of care, but considering the physiologic changes of ESLD (82). The PPAR-γ agonist pioglitazone and the GLP-1 agonist liraglutide may be considered in non-cirrhotic diabetic (or non-diabetic) patients with NASH as these have been shown to improve steatohepatitis and fibrosis (83,84). Data is lacking on the efficacy and safety of these drugs in cirrhosis. Treating diabetics in cirrhosis is challenging since almost all anti-diabetic agents are metabolized in the liver, which creates a potential for hypoglycemia and hepatotoxicity (85). Insulin is first-line treatment in diabetics with decompensated cirrhosis since its pharmacokinetic profile is not affected by hepatic impairment (82,86). Endocrinology consultation may be required in patients with inadequate glycemic control.
Systemic HTN affects less than 5% of patients with decompensated cirrhosis due to the state of systemic vasodilation found in these patients (87). Diuretics and non-selective beta-blockers are ideal first-line antihypertensive therapies in cirrhotic patients with ascites or varices. NASH patients with co-morbid heart failure and CAD may preferentially be treated with carvedilol, which is also effective in variceal hemorrhage prophylaxis (88). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are potential second-line antihypertensive agents. Some data suggest that inhibiting the renin-angiotensin system attenuates steatohepatitis and fibrosis (89).
NASH may be accompanied by a pro-atherogenic lipid profile, characterized by elevated serum triglycerides (TG) and low-density lipoprotein (LDL) and low high-density lipoprotein (HDL), which has an important role in CVD development (90). Statins may be considered in non-cirrhotic or compensated cirrhotic patients. Randomized trials in patients with compensated NAFLD/NASH show that statins lower serum TG and LDL and reduce risk of CV-related morbidity and mortality without significant hepatotoxicity (in fact, statin-treated patients actually had lower transaminases) (91,92). However, the vast majority of LT candidates with NASH will have decompensated cirrhosis. Statins are not recommended in decompensated cirrhosis due to lack of safety data, potential for hepatic and non-hepatic drug-related toxicity, and their overall poor prognosis that negates statins’ CV benefits (93).
Obesity assessment
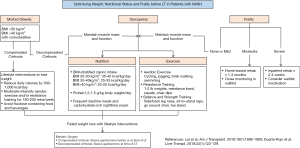
Around 50–70% of LT candidates with NASH are obese (42). BMI is currently the most widely used method to assess obesity. Patients with BMI of 25.0–29.9 kg/m2 are categorized as overweight, while those with BMI of 30.0–34.9 kg/m2, 35.0–39.9 kg/m2, and ≥40.0 kg/m2 are have class I, II, and III obesity, respectively (94). Morbid obesity (BMI ≥40 kg/m2) is associated with higher waitlist mortality, but data is conflicting on whether it impacts post-LT mortality and graft loss (47,53-55). A recent retrospective study suggests a trend towards increased patient mortality (HR 2.36, 95% CI, 0.91–6.09, P=0.07) and graft loss (HR 2.60, 95% CI, 0.99–6.60, P=0.05) in LT recipients with BMI ≥50 kg/m2 (95). Morbid obesity should not necessarily preclude LT and, indeed, upper limits of BMI that contrandicate LT vary widely across transplant centers. However, more stringent patient selection should be observed in obese LT candidates with co-morbid CVD and DM or those with BMI ≥50 kg/m2.
Due to conflicting data on its prognostic value and the confounding effect of ascites, BMI may not be the optimal tool to define obesity and assess obesity-related risk in LT candidates. Body composition and body fat distribution are potentially more important determinants of LT outcomes (96). Visceral adiposity, estimated as abdominal visceral fat area on CT, independently predicts post-LT mortality (HR 1.06 per 10 cm2, 95% CI, 1.04–1.10, P<0.001) (97). The combination of visceral adiposity and sarcopenia portends the worst outcomes, with 1- and 5-year post-LT survival rates of 72% and 37%, respectively (97). Waist circumference and waist:hip ratio are simple, inexpensive ways of measuring central obesity that correlate with radiographic measurements and predict CVD in the general population (98). Although central obesity predicts mortality in kidney transplant recipients (99), the current metrics are inappropriate in LT candidates with ascites. Further research is needed to identify and validate standardized measures of visceral adiposity that are can be used in routine clinical practice and predict LT outcomes (96).
Weight reduction through lifestyle interventions is the cornerstone of management in NASH. Caloric restriction and physical activity resulting in 7–10% weight loss improves liver histology, potentially reverses hepatic fibrosis, and lowers portal pressure (100,101). A prospective study shows that 85% of LT candidates with BMI >35 kg/m2 can achieve their target pre-LT weight through a multidisciplinary approach to lifestyle interventions (102). While there is no consensus on the best weight loss strategy for LT candidates, recommended lifestyle interventions should probably differ if patients have compensated or decompensated NASH-related cirrhosis (96). Patients with compensated cirrhosis may observe traditional lifestyle interventions that include reducing caloric intake by 500–1,000 kcal/day, moderate-intensity aerobic exercise and/or resistance training for 150–200 minutes/week, and avoiding fructose-containing food and beverages (103). Patients with decompensated cirrhosis are at risk for sarcopenia and malnutrition and should focus less on weight loss and more on optimizing nutrition to maintain muscle mass. Recommendations on nutrition and physical activity are reviewed in the section on sarcopenia.
Morbidly obese LT candidates with NASH who are unable to lose weight through lifestyle interventions may be considered for bariatric surgery (BS) in order to reach their center’s prerequisite pre-LT weight. BS may be considered before LT in select patients with compensated cirrhosis. In a case series of 20 morbidly obese patients with ESLD (mean MELD 11), laparoscopic sleeve gastrectomy before LT reduced patients’ weight by 50% on average, resulting in 7 (35%) successful transplants, but with a 25% complication rate (e.g., infections, leak, bleeding) (104). Patients with decompensated cirrhosis should not undergo BS alone due to unacceptably high post-operative mortality (16% vs. 0.9% in compensated cirrhosis and 0.3% in no cirrhosis, P<0.001) (105). BS at time of LT should be considered for patients with decompensated cirrhosis. The largest case series involves 29 patients who underwent combined sleeve gastrectomy and LT (102,105). No deaths or graft losses have been reported after the combined procedure, although a leak from the gastric staple line occurred in 1 patient (102). Compared to non-surgical weight loss, combined sleeve gastrectomy-LT maintains weight loss after LT (weight loss of 34.8% vs. 3.9% of body weight, P<0.001) and resolves MetS, insulin resistance, and hepatic steatosis (106). BS before or at time of LT should only be performed in carefully selected patients by centers with adequate LT and BS volume and expertise due to potential for complications. While BS after LT is feasible, it will not address obesity-related problems in the waitlist and immediate post-LT period and is associated with early post-operative complications due to adhesions (107). The ideal bariatric procedure for obese LT candidates is unknown since there have been no direct comparisons of different bariatric procedures in this population. Sleeve gastrectomy is employed in the majority of studies due to several presumed advantages over Roux-en-Y gastric bypass (108). Sleeve gastrectomy is a less complex procedure, will not affect intestinal absorption of immunosuppressives, and permits endoscopic access to the biliary tree in cases of post-LT biliary complications.
Nutrition and sarcopenia assessment
Malnutrition and sarcopenia are highly prevalent in NASH-related cirrhosis (37). The etiology of malnutrition in cirrhosis is multifactorial and involves impaired dietary intake, decreased nutrient absorption, and altered macronutrient metabolism (109). About 40–90% of LT candidates are undernourished and only 25% meet daily protein requirements (34). Malnutrition in cirrhosis is associated with hepatic decompensation, infections, HCC, and mortality (110,111). Validated nutrition screening tools that are specific to patients with cirrhosis are available. The Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT) is a provider-administered tool that estimates malnutrition risk based on the presence of fluid overload, BMI, unintentional weight loss, reduction in dietary intake, and presence of alcoholic hepatitis (112). RFH-NPT correlates with liver disease severity and predicts hepatic decompensation and transplant-free survival (113). Another cirrhosis-specific nutritional screening tool, the Liver Disease Undernutrition Screening Tool asks patients 6 questions pertaining to nutrient intake, weight loss, subcutaneous fat loss, muscle mass loss, fluid accumulation, and decline in functional status (114). The tool has good positive predictive value (PPV) (>90%) in diagnosing undernutrition, but has poor negative predictive value (NPV) (40%) and has not been validated against clinical outcomes in cirrhosis. LT candidates at high risk for malnutrition and those with decompensated disease should undergo a comprehensive nutritional assessment, preferably by a registered dietitian, that includes assessments of dietary intake, body composition, and functional status (115). Nutritional assessment is recommended at the initial evaluation and at regular intervals until LT.
Sarcopenia is a core component of malnutrition and is a better measure of malnutrition in patients with NASH-related cirrhosis than BMI or weight loss. Sarcopenia is assessed and managed through standardized measurements of muscle mass and function performed at the initial LT evaluation and longitudinally until LT. Cross-sectional imaging is currently the gold standard in quantifying skeletal muscle mass. Sarcopenia can be assessed using the total psoas area at L3 or L4 vertebrae or the skeletal muscle index (SMI) which refers to the cross-sectional area of all muscles at L3 normalized for height (cm2/m2) (30). SMI cutoffs of 50 cm2/m2 in men and 39 cm2/m2 in women have been identified to optimally predict waitlist mortality in ESLD patients awaiting LT (116). Total psoas area and SMI correlate with poor outcomes in the LT population (36,117-120). LT candidates at the lowest percentiles of total psoas area are 2 to 3.5 times and 4.5 times more likely to die and acquire severe infections after LT, respectively (117-119). SMI is predictive of post-LT hospital length of stay (36). In a meta-analysis, sarcopenia increases risk of waitlist (HR 1.72, 95% CI, 0.99–3.00, P=0.05) and post-LT (HR 1.84, 95% CI, 1.11–3.05, P=0.02) mortality, independent of the MELD score (120). Muscle function may be assessed by hand-grip strength test and a 6-minute walk test (34). Cirrhotic patients whose hand-grip strength is more than 2 standard deviations from the mean have lower transplant-free survival (1 year: 69.0% vs. 100.0%) and more frequent hepatic decompensation events (1 year: 65.5% vs. 11.8%) (121). Patients who perform better in 6-minute walk tests have lower waitlist mortality (HR 0.58 per 100 meters, 95% CI, 0.37–0.93, P=0.02) (122). Modifying the MELD score to include sarcopenia enhances the prediction of waitlist mortality in patients with cirrhosis, potentially opening room for improvement in donor and organ allocation (123).
Sarcopenic LT candidates should consume adequate amounts of calories and protein and exercise regularly. Calorie intake in NASH-related cirrhosis, where obesity is prevalent, should be stratified by BMI using ideal body weight: 20–25 kcal/kg/day for BMI ≥40 kg/m2, 25–35 kcal/kg/day for BMI 30–40 kg/m2, and 35–40 kcal/kg/day for BMI 20-30 kg/m2 (124). The recommended protein intake is 1.2–1.5 g/kg/day (124). Small frequent meals during waking hours and a carbohydrate-rich nighttime snack should be encouraged to avoid starvation which increases muscle and lipid breakdown (124,125). Supervised moderate-intensity aerobic exercise improves exercise endurance, muscle mass and strength, and quality of life without associated adverse events (126,127). LT candidates may be advised to engage in 30- to 60-minute sessions of light to moderate aerobic exercise and low-weight resistance training daily, under a physiotherapist’s supervision if possible, to achieve ≥150 minutes of physical activity weekly for at least 3 months (128). Balance training and stretching are recommended, especially in severely sarcopenic patients, to strengthen core muscles and improve range of motion (128). Exercise should match patients’ baseline function and effort level since fatigue is a major barrier (129). It is worth noting that trials of exercise in cirrhosis exclude patients with decompensated cirrhosis who make up the majority of LT candidates. There is concern that exercise increases risk of variceal bleeding since a previous showed that exercise increases portal pressure by up to 30% (130). However, recent randomized trials prove that light-moderate physical exercise actually reduces portal pressure (1.5–2.5 mmHg on average) and are not associated with variceal bleeding (101,131). However, it is probably prudent to screen and eradicate high-risk varices before embarking on an exercise program. Pre-exercise evaluation is rarely required since LT candidates already require cardiopulmonary work-up and clearance as part of LT evaluation.
Frailty assessment
The increasing number of successful LT in elderly patients suggests that perhaps frailty, instead of chronologic age, may be a better criterion of transplant candidacy. Several validated frailty assessment tools are available. The Fried Frailty Instrument (FFI) assesses patients’ gait speed and hand grip strength, physical activity, and self-reported exhaustion and unintentional weight loss (23). The Short Physical Performance Battery (SPPB) measures patients’ performance on repeated chair stands, balance testing and 13-foot walk (25). Both tools predict waitlist mortality and unplanned hospitalizations in outpatients being evaluated for LT (23,25). Recently, a Liver Frailty Index (LFI), composed of hand grip strength, chair stands and balance time, was developed specifically for LT candidates and has been shown to improve waitlist mortality prediction when combined with the MELD score (132). Activities of daily living (ADL) and the Karnofsky Performance Score (KPS) are easy-to-use tools that are also validated to predict mortality in LT candidates (133,134). Transplant centers are recommended to include frailty screening using standardized tools in LT evaluation (22). A one-time assessment of frailty should not contraindicate LT; instead, frailty should be integrated with the rest of the evaluation to guide transplant decision-making (22). Frailty assessments over time are recommended to assess for a decline in physical frailty, which predicts waitlist mortality (135). No single frailty assessment tool is recommended for routine use. Tools should be selected based on the clinical setting (outpatient vs. inpatient, transplant vs. non-transplant), available time and resources, and the impact of the test result on clinical decision-making (22).
Frailty is potentially reversible. While all LT candidates should receive guidance on nutrition and physical activity, the degree of frailty guides the intensity of recommended interventions. A short course of inpatient rehabilitation, with possible inactivation from the waitlist and close follow-up every 2–4 weeks, may be considered for severely frail LT candidates, while supervised home-based exercise programs and follow-up every 1-3 months may be prescribed for the less frail (22).
Screening for alcohol use
Although NAFLD by definition excludes significant alcohol use (≥21 and ≥14 standard drinks per week in men and women, respectively), 60% of patients with NAFLD will consume alcohol in their lifetime (136). Some data suggest that light alcohol drinkers are less likely than lifetime abstainers to have histologic NASH or advanced fibrosis (137,138). However, the typical LT candidate with decompensated cirrhosis is not represented in these studies. Any alcohol use increases risk of HCC in patients with NASH-related cirrhosis (HR 3.8, 95% CI, 1.6–8.9, P<0.01) and the risk is not modified by volume of alcohol consumed (139). Despite lack of direct data, light alcohol use can be inferred to increase risk of hepatic decompensation in NASH-related cirrhosis since light alcohol use increases portal pressures and accelerates fibrosis progression (140,141). Hence, LT candidates with NASH should be screened for alcohol use and advised to abstain completely.
HCC surveillance
LT candidates with NASH should be enrolled in an HCC surveillance program. NASH patients are less likely to undergo HCC screening and receive treatment for HCC than patients with alcohol- or hepatitis C-related liver disease (142). Patients found to have large or multifocal HCC exceeding Milan or UCSF criteria, macrovascular tumor invasion, or extrahepatic disease should not undergo LT due to high rates of post-LT mortality and HCC recurrence (83,143). HCC surveillance also permits identification of patients eligible for MELD exception points and those who may benefit from tumor downstaging to facilitate LT (61,144). Abdominal US, with or without alpha fetoprotein (AFP), every 6 months is the recommended modality for HCC surveillance (145). US has pooled sensitivity of 84% in detecting HCC of all stages, but only 47% for early HCC (146). AFP, at a diagnostic cut-off of 20 ng/mL, has sensitivity and specificity of 90% and 85%, respectively, in detecting NASH-related HCC (147). Combining AFP with US increases sensitivity (from 45% to 63%), but lowers specificity (from 92% to 84%) for detection of early HCC (146). HCC surveillance is associated with a 30–40% reduction in mortality risk and higher likelihood of early stage HCC and receipt of curative treatment (148,149).
Obesity complicates HCC surveillance in LT candidates with NASH as it increases the likelihood of false negative US exams. In patients with BMI >35 kg/m2, up to 35–40% of US are inadequate to exclude HCC, compared with <10% in patients with normal BMI (150). Guidelines recommend considering multiphase contrast-enhanced CT or MRI for HCC surveillance in patients likely to have an inadequate US such as the morbidly obese (145). Triple phase CT and gadoxetic acid-enhanced MRI have sensitivities of 85-90% and 88% and specificities of 85–90% and 94%, respectively, in detecting HCC (151,152). Head-to-head comparisons of US-, CT- and MRI-based HCC surveillance show that CT has slightly lower sensitivity (66.7% vs. 71.4%) and specificity (94.4% vs. 97.5%), while MRI has higher HCC detection rate (86.0% vs. 27.9%, P<0.001) and lower false positive rate (3.0% vs. 5.6%, P=0.004) than US (153,154). Routine use of CT and MRI is generally limited by cost, although a new study suggests that MRI may be more cost-effective than US for patients at highest risk for HCC (155). To address long MRI scan times and high costs, an abbreviated MRI protocol has been developed that has >80% sensitivity and >90% specificity for HCC detection (156). However, only a small percentage of patients included in these studies have NAFLD/NASH. Hence, there is insufficient data at present to recommend routine CT- or MRI-based HCC surveillance for patients with NASH-related cirrhosis.
Cardiovascular risk assessment
Serious perioperative CV complications are a threat to LT candidates with NASH-related cirrhosis due to the presence of co-morbid CVD, CV risk factors, and CV physiologic derangements in decompensated cirrhosis that include high cardiac output, systemic vasodilation, blunted inotropic and chronotropic responses to stress, and diastolic dysfunction (43,60,157,158). CV risk assessment includes an appraisal of clinical CV risk factors and a battery of diagnostic cardiac testing. The objective of CV risk assessment is to identify patients with very severe CVD who should not undergo LT and patients who may benefit from CV risk-reducing interventions that will facilitate safe LT (Figures 2,3).

Clinical risk factors
Traditional coronary risk factors include age >45 years for males and >55 years for females, hypercholesterolemia, HTN, DM, tobacco use, and family history of early CAD. CAD risk increases in parallel with number of risk factors (159,160).
12-lead electrocardiogram (12-L EKG)
12-L EKG identifies cardiac arrhythmias and may detect asymptomatic CAD. The presence of Q waves pre-LT predicts acute coronary syndrome and cardiac arrhythmias after LT (161). Half of patients with cirrhosis will have QTc prolongation ≥440 milliseconds, which is associated with decreased survival (162). Diagnosis of QTc prolongation should prompt a search for and treatment of reversible causes such as electrolyte disturbances and QTc-prolonging medications, although QTc prolongation is potentially part of the overall CV disturbance in cirrhosis (163).
Contrast-enhanced echocardiography (CE-TTE)
CE-TTE assesses left and right ventricular size and function, valvular function, intracardiac or intrapulmonary shunting, and pulmonary artery (PA) pressure. Even mildly depressed left ventricular (LV) ejection fraction should prompt an evaluation for underlying cardiomyopathy or CAD since patients with decompensated cirrhosis typically have a hyperdynamic circulation (164). LV systolic dysfunction is not an absolute contraindication for LT, but requires aggressive medical management to reduce risk of perioperative CV complications (159,165). Diastolic dysfunction and impaired systolic response to stress are frequently found in cirrhosis and are potentially reversible with LT (166). LT is contraindicated in patients with moderate to severe tricuspid regurgitation due to increased risk of post-LT mortality (167,168), although successful simultaneous LT and tricuspid valve repair has been previously reported (169). A retrospective study does not find increased mortality in LT candidates with aortic stenosis (170). None of the patients in this study has severe aortic stenosis and LT is typically not offered to patients with severe aortic stenosis. However, successful LT followed by aortic valve replacement has previously been reported (171). CE-TTE should evaluate for clinically significant LV outflow tract obstruction due to LV hypertrophy and hyperdynamic circulation as this may cause intraoperative hypotension (172).
CE-TTE is useful in diagnosing hepatopulmonary syndrome (HPS) and portopulmonary hypertension (POPH). HPS is characterized by hypoxemia due to intrapulmonary shunting in patients, while POPH refers to pulmonary arterial hypertension in the setting of portal hypertension after excluding alternative etiologies (173). Intrapulmonary shunting is indicated by the appearance of agitated saline bubbles in the left atrium after 3–5 cardiac cycles (174). Increased PA pressure on TTE is 97% sensitive, but only 77% specific, in diagnosing POPH (175). Although 20% of LT candidates have elevated PA pressures, <5% are due to POPH and the rest are due to volume overload or cirrhotic cardiomyopathy (176). Patients with PA systolic pressure ≥45 mmHg should undergo right heart catheterization to confirm POPH (16). Vasodilators should be considered in moderate (mean PA pressure 35–50 mmHg) or severe (mean PA pressure ≥50 mmHg) POPH. Persistent moderate and severe POPH despite vasodilator therapy are contraindications to LT, with post-LT mortality approaching 100% (177).
Non-invasive stress testing
Abnormal findings on 12-L EKG and CE-TTE, cardiac symptoms, or the presence of multiple coronary risk factors warrant testing for obstructive CAD (159,178). First-line non-invasive testing for CAD includes dobutamine stress echocardiography (DSE) and single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI). Coronary artery calcium (CAC) score and coronary CT angiography (CCTA) are newer modalities that have been proposed for pre-LT CV risk assessment due to modest accuracy in predicting post-LT CV events (179,180). Non-invasive stress testing has important limitations in the ESLD population. First, most LT candidates are unable to exercise due to a combination of sarcopenia, anemia, and ascites. Second, patients with decompensated cirrhosis may not reach target heart rate due to chronotropic incompetence or beta-blockade, hence limiting the accuracy of DSE. Third, systemic vasodilation increases false negatives, while coronary microvascular dysfunction increases false positives in SPECT MPI (181). With coronary angiography (CAG) as gold standard, DSE and SPECT have poor sensitivity (<35%) and PPV (20%), but modest specificity (60–90%) and NPV (75–90%) (182-184). Two systematic reviews conclude that pre-LT non-invasive stress testing does not satisfactorily predict post-LT CV events and all-cause mortality (185,186). Current data indicate that non-invasive stress testing is potentially no better than conventional clinical risk scoring in predicting major adverse cardiac events and need for invasive cardiac testing in LT candidates. The role of stress testing in pre-LT CV risk assessment should be decided by individual transplant centers, based on local experience and expertise (84).
Coronary angiography
CAG is recommended in patients with abnormal non-invasive stress testing and may be considered as first-line cardiac testing in patients with high pre-test probability of CAD who are unable to undergo non-invasive testing. In one center, up to 70% of LT candidates undergo CAG as first-line screening for CAD on the basis of coronary risk factors alone, and this approach predictably increased percutaneous coronary intervention (PCI) rates (8% vs. 1%) and interestingly reduced 1-year mortality (6% vs. 10–16%, P<0.001) and myocardial infarction rates (0.6% vs. 1.7%, P<0.001) (187). PCI should be considered in symptomatic patients or in asymptomatic patients with significant CAD (e.g., ≥70% occlusion) where the extent of disease precludes LT. Drug-eluting stents should be avoided because of the need for prolonged dual antiplatelet therapy, which delays LT and increases bleeding risk in already coagulopathic patients. Major bleeding occurs more frequently after CAG in LT candidates compared to matched controls without ESLD (14.8% vs. 3.8%, P=0.014) (188). ESLD patients are theoretically at risk for contrast-induced nephropathy due to their usually tenuous renal function, although the incidence of acute kidney injury after CAG is <5% (189). Simultaneous coronary artery bypass grafting (CABG) and LT can be considered and has been reported without major post-operative complications (190). No study to date has evaluated if routine pre-LT CAG and revascularization in asymptomatic LT candidates with NASH improves outcomes. However, pre-operative coronary revascularization before major vascular surgery, which ostensibly carries higher perioperative cardiac mortality than LT, does not improve survival (191). Moreover, the incidence of early post-LT CV mortality in registry studies (1.2%) is comparable to that in other major surgeries where CAG and revascularization are not recommended in asymptomatic patients because of lack of survival benefit, associated risks, and potential for procedural delays (178,192).
Should CV risk assessment be different in NASH?
Although CVD is highly prevalent in LT candidates with NASH, there is insufficient evidence to support utilizing a different approach to pre-LT CV risk assessment in LT candidates with NASH. Assessment for CV risk factors, 12-L EKG and CE-TTE should be routinely performed. Non-invasive stress testing should likely be performed due to high likelihood of multiple CV risk factors, including NASH itself, and silent CAD. There is currently no evidence to support routine CAG in asymptomatic patients with NASH despite their increased CV risk. CAG is probably best reserved for symptomatic patients, patients with abnormal non-invasive stress test, or patients with multiple coronary risk factors who are unable to undergo non-invasive stress testing.
In conclusion, LT evaluation in patients with NASH is a multidisciplinary undertaking that takes into consideration the unique demographic and clinical features of patients with NASH-related cirrhosis that impact LT outcomes. Nutritional status, sarcopenia, frailty, and CV and metabolic co-morbidities and risk factors should be assessed during LT evaluation and optimized in preparation for LT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sanjaya K. Satapathy, David Bernstein, Nitzan Roth) for the series “Liver Transplantation in NASH and ALD” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tgh.amegroups.com/article/view/10.21037/tgh.2020.03.04/coif). The series “Liver Transplantation in NASH and ALD” was commissioned by the editorial office without any funding or sponsorship. AA reports personal fees from Intercept Pharmaceutical, Gilead Sciences, Genfit, Expert Connect, NASHNET, SanyalBio, MEDACorp and Sterotherapeutics, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Rinella ME. Nonalcoholic Fatty Liver Disease: A Systematic Review. JAMA 2015;313:2263-73. [Crossref] [PubMed]
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123-33. [Crossref] [PubMed]
- Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology 2016;150:1778-85. [Crossref] [PubMed]
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [Crossref] [PubMed]
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557-65. [Crossref] [PubMed]
- Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-9. [Crossref] [PubMed]
- White DL, Kanwal F, El-Serag HB. Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clin Gastroenterol Hepatol 2012;10:1342-59.e2. [Crossref] [PubMed]
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55. [Crossref] [PubMed]
- Cholankeril G, Wong RJ, Hu M, et al. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig Dis Sci 2017;62:2915-22. [Crossref] [PubMed]
- Singal AK, Hasanin M, Kaif M, et al. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous LiverKidney Transplantation in the United States. Transplantation 2016;100:607-12. [Crossref] [PubMed]
- Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019;17:748-55.e3. [Crossref] [PubMed]
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188-95. [Crossref] [PubMed]
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant 2018;18:172-253. [Crossref] [PubMed]
- O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology 2008;134:1764-76. [Crossref] [PubMed]
- Martin P, DiMartini A, Feng S, et al. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the AASLD and the American Society of Transplantation. Hepatology 2014;59:1144-65. [Crossref] [PubMed]
- Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005;5:307-13. [Crossref] [PubMed]
- Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl 2012;18:29-37. [Crossref] [PubMed]
- Su F, Yu L, Berry K, et al. Aging of Liver Transplant Registrants and Recipients: Trends and Impact on Waitlist Outcomes, Post-Transplantation Outcomes, and Transplant-Related Survival Benefit. Gastroenterology 2016;150:441-53.e6. [Crossref] [PubMed]
- Aduen JF, Sujay B, Dickson RC, et al. Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc 2009;84:973-8. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896-906. [Crossref] [PubMed]
- Tandon P, Tangri N, Thomas L, et al. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients with Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am J Gastroenterol 2016;111:1759-67. [Crossref] [PubMed]
- Lai JC, Volk ML, Strasburg D, et al. Performance-Based Measures Associate with Frailty in Patients with End-Stage Liver Disease. Transplantation 2016;100:2656-60. [Crossref] [PubMed]
- Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870-9. [Crossref] [PubMed]
- Derck JE, Thelen AE, Cron DC, et al. Quality of life in liver transplant candidates: Frailty is a better indicator than severity of liver disease. Transplantation 2015;99:340-4. [Crossref] [PubMed]
- Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol 2016;111:1768-75. [Crossref] [PubMed]
- Tapper EB, Finkelstein D, Mittleman MA, et al. Standard Assessments of Frailty are Validated Predictors of Mortality in Hospitalized Patients with Cirrhosis. Hepatology 2015;62:584-90. [Crossref] [PubMed]
- Sinclair M, Poltavskiy E, Dodge JL, et al. Frailty is independently associated with increased hospitalization days in patients on the liver transplant waitlist. World J Gastroenterol 2017;23:899-905. [Crossref] [PubMed]
- Haugen CE, McAdams-DeMarco M, Holscher CM, et al. Multicenter Study of Age, Frailty and Waitlist Mortality Among Liver Transplant Candidates. Ann Surg 2020;271:1132-6. [Crossref] [PubMed]
- Bhanji RA, Narayanan P, Moynagh MR, et al. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transpl 2019;25:14-24. [Crossref] [PubMed]
- Haugen CE, McAdams-DeMarco M, Verna EC, et al. Association Between Liver Transplant Wait-list Mortality and Frailty Based on Body Mass Index. JAMA Surg 2019;154:1103-9. [Crossref] [PubMed]
- Verna E, Chan C, Pisa J, et al. Frailty, physical performance and sarcopenia measures in patients awaiting liver transplantation predict mortality and post‐operative complications. Am J Transplant 2014;14:742. [Crossref]
- Mazurak VC, Tandon P, Montano-Loza AJ. Nutrition and the transplant candidate. Liver Transpl 2017;23:1451-64. [Crossref] [PubMed]
- Kim G, Kang SH, Kim MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 2017;12:e0186990. [Crossref] [PubMed]
- Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640-8. [Crossref] [PubMed]
- Carias S, Castellanos AL, Vilchez V, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31:628-33. [Crossref] [PubMed]
- Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166-73. [Crossref] [PubMed]
- Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant waitlist: its prevalence and independent prognostic value. Liver Transpl 2012;18:1209-16. [Crossref] [PubMed]
- Aby ES, Lee E, Saggi SS, et al. Pretransplant Sarcopenia in Patients with NASH Cirrhosis Does Not Impact Rehospitalization or Mortality. J Clin Gastroenterol 2019;53:680-5. [Crossref] [PubMed]
- Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute / American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433-8. [Crossref] [PubMed]
- Wang X, Li J, Riaz DR, et al. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:394-402.e1. [Crossref] [PubMed]
- Kadayifci A, Tan V, Ursell PC, et al. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: A comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J Hepatol 2008;49:595-9. [Crossref] [PubMed]
- Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 2010;59:1410-5. [Crossref] [PubMed]
- Younossi ZM, Stepanova M, Saab S, et al. The impact of type 2 diabetes and obesity on the long-term outcomes of more than 85 000 liver transplant recipients in the US. Aliment Pharmacol Ther 2014;40:686-94. [Crossref] [PubMed]
- Singhal A, Wilson GC, Wima K, et al. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int 2015;28:148-55. [Crossref] [PubMed]
- Kardashian AA, Dodge JL, Roberts J, et al. Weighing the risks: Morbid obesity and diabetes are associated with increased risk of death on the liver transplant waiting list. Liver Int 2018;38:553-63. [Crossref] [PubMed]
- Segev DL, Thompson RE, Locke JE, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg 2008;248:863-70. [Crossref] [PubMed]
- LaMattina JC, Foley DP, Fernandez LA, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant 2012;26:910-8. [Crossref] [PubMed]
- Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology 2002;35:105-9. [Crossref] [PubMed]
- Dick AA, Spitzer AL, Seifert CF, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl 2009;15:968-77. [Crossref] [PubMed]
- Leonard J, Heimbach JK, Malinchoc M, et al. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant 2008;8:667-72. [Crossref] [PubMed]
- Bambha KM, Dodge JL, Gralla J, et al. Low, rather than high, body mass index confers increased risk for post-liver transplant death and graft loss: Risk modulated by model for end-stage liver disease. Liver Transpl 2015;21:1286-94. [Crossref] [PubMed]
- Wong RJ, Cheung R, Perumpail RB, et al. Diabetes mellitus, and not obesity, is associated with lower survival following liver transplantation. Dig Dis Sci 2015;60:1036-44. [Crossref] [PubMed]
- Saab S, Lalezari D, Pruthi P, et al. The impact of obesity on patient survival in liver transplant recipients: a meta-analysis. Liver Int 2015;35:164-70. [Crossref] [PubMed]
- Schlansky B, Naugler WE, Orloff SL, et al. Higher Mortality and Survival Benefit in Obese Patients Awaiting Liver Transplantation. Transplantation 2016;100:2648-55. [Crossref] [PubMed]
- Vanwagner LB, Bhave M, Te HS, et al. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012;56:1741-50. [Crossref] [PubMed]
- Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with non-alcoholic steatohepatitis. Clin J Am Soc Nephrol 2010;5:2166-71. [Crossref] [PubMed]
- Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680. [Crossref] [PubMed]
- VanWagner LB, Lapin B, Skari AI, et al. Impact of Renal Impairment on Cardiovascular Disease Mortality After Liver Transplantation for Nonalcoholic Steatohepatitis Cirrhosis. Liver Int 2015;35:2575-83. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network. Policy 9: Allocation of Livers and Liver-Intestines. Effective date 10/24/2019.
- Hmoud B, Kuo YF, Wiesner RH, et al. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation 2015;99:823-8. [Crossref] [PubMed]
- Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol 2018;68:335-52. [Crossref] [PubMed]
- Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; Should we care? Atherosclerosis 2013;230:258-67. [Crossref] [PubMed]
- Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589-600. [Crossref] [PubMed]
- Carey WD, Dumot JA, Pimentel RR, et al. The prevalence of coronary artery diseases in liver transplant candidates over age 50. Transplantation 1995;59:859-64. [Crossref] [PubMed]
- McAvoy NC, Kochar N, McKillop G, et al. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transpl 2008;14:1725-31. [Crossref] [PubMed]
- Wong VW, Wong GL, Yeung JC, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology 2016;63:754-63. [Crossref] [PubMed]
- Plotkin JS, Scott VL, Pinna A, et al. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg 1996;2:426-30. [Crossref] [PubMed]
- Diedrich DA, Findlay JY, Harrison BA, et al. Influence of coronary artery disease on outcomes after liver transplantation. Transplant Proc 2008;40:3554-7. [Crossref] [PubMed]
- Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682-9. [Crossref] [PubMed]
- Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357-65. [Crossref] [PubMed]
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691-7. [Crossref] [PubMed]
- Stine JG, Shah NL, Argo CK, et al. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl 2015;21:1016-21. [Crossref] [PubMed]
- Tripodi A, Fracanzani AL, Primignani M, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol 2014;61:148-54. [Crossref] [PubMed]
- Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69:1873-81. [Crossref] [PubMed]
- Agbim U, Jiang Y, Kedia SK, et al. Impact of Nonmalignant Portal Vein Thrombosis in Transplant Recipients with Nonalcoholic Steatohepatitis. Liver Transpl 2019;25:68-78. [Crossref] [PubMed]
- Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018;155:1828-37.e2. [Crossref] [PubMed]
- Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:428-33. [Crossref] [PubMed]
- Leung C, Yeoh SW, Patrick D, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015;21:1189-96. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Tsochatzis E, Coilly A, Nadalin S, et al. International Liver Transplantation Consensus Statement on End-stage Liver Disease Due to Nonalcoholic Steatohepatitis and Liver Transplantation. Transplantation 2019;103:45-56. [Crossref] [PubMed]
- Cusi K, Orsak B, Bril F, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Int Med 2016;165:305-15. [Crossref] [PubMed]
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-90. [Crossref] [PubMed]
- García-Compeán D, González-González JA, Lavalle-González FJ, et al. The treatment of diabetes mellitus of patients with chronic liver disease. Ann Hepatol 2015;14:780-8. [Crossref] [PubMed]
- Holmes G, Galitz L, Hu P, et al. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br J Clin Pharmacol 2005;60:469-76. [Crossref] [PubMed]
- Henriksen JH, Fuglsang S, Bendtsen F, et al. Arterial hypertension in cirrhosis: arterial compliance, volume distribution, and central haemodynamics. Gut 2006;55:380-7. [Crossref] [PubMed]
- Malandris K, Paschos P, Katsoula A, et al. Carvedilol for prevention of variceal bleeding: a systematic review and meta-analysis. Ann Gastroenterol 2019;32:287-97. [Crossref] [PubMed]
- Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int 2015;35:979-85. [Crossref] [PubMed]
- Loria P, Marchesini G, Nascimbeni F, et al. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99-109. [Crossref] [PubMed]
- Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high‐dose pravastatin in hypercholesterolemic patients with well‐compensated chronic liver disease: Results of a prospective, randomized, double‐blind, placebo‐controlled, multicenter trial. Hepatology 2007;46:1453-63. [Crossref] [PubMed]
- Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 2010;376:1916-22. [Crossref] [PubMed]
- Speliotes EK, Balakrishnan M, Friedman LS, et al. Treatment of Dyslipidemia in Common Liver Diseases. Clin Gastroenterol Hepatol 2018;16:1189-96. [Crossref] [PubMed]
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985-3023. [Crossref] [PubMed]
- Daniel K, Hause J, Osman F, et al. Liver transplant recipients with BMI 40 to <50 kg/m2 have excellent patient and graft survival. Hepatology 2019;70:64A.
- Spengler EK, O’Leary JG, Te HS, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017;101:2288-96. [Crossref] [PubMed]
- Terjimanian MN, Harbaugh CM, Hussain A, et al. Abdominal Adiposity, Body Composition and Survival after Liver Transplantation. Clin Transplant 2016;30:289-94. [Crossref] [PubMed]
- Cornier MA, Despres JP, Davis N, et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 2011;124:1996-2019. [Crossref] [PubMed]
- Kovesdy CP, Czira ME, Rudas A, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant 2010;10:2644-51. [Crossref] [PubMed]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367-78. [Crossref] [PubMed]
- Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017;65:1293-305. [Crossref] [PubMed]
- Heimbach JK, Watt KD, Poterucha JJ, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant 2013;13:363-8. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref]
- Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis 2013;9:653-8. [Crossref] [PubMed]
- Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:897-901. [Crossref] [PubMed]
- Zamora-Valdes D, Watt KD, Kellogg TA, et al. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018;68:485-95. [Crossref] [PubMed]
- Lin MY, Tavakol MM, Sarin A, et al. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc 2013;27:81-5. [Crossref] [PubMed]
- Lazzati A, Iannelli A, Schneck AS, et al. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg 2015;25:134-42. [Crossref] [PubMed]
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol 2012;10:117-25. [Crossref] [PubMed]
- Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi). Hepatology 1996;23:1041-6. [Crossref] [PubMed]
- Huisman EJ, Trip EJ, Siersema PD, et al. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol 2011;23:982-9. [Crossref] [PubMed]
- Arora S, Mattina C, McAnenny C, et al. The development and validation of a nutritional prioritizing tool for use in patients with chronic liver disease. J Hepatol 2012;56:S241. [Crossref]
- Borhofen SM, Gerner C, Lehmann J, et al. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig Dis Sci 2016;61:1735-43. [Crossref] [PubMed]
- Booi AN, Menendez J, Norton HJ, et al. Validation of a Screening Tool to Identify Undernutrition in Ambulatory Patients with Liver Cirrhosis. Nutr Clin Pract 2015;30:683-9. [Crossref] [PubMed]
- Tandon P, Raman M, Mourtzakis M, et al. A practical approach to nutritional screening and assessment in cirrhosis Hepatology 2017;65:1044-57. [Crossref] [PubMed]
- Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625-33. [Crossref] [PubMed]
- Masuda T, Shirabe K, Ikegami T, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014;20:401-7. [Crossref] [PubMed]
- Englesbe MJ, Patel SP, He K, et al. Sarcopenia and Post-Liver Transplant Mortality. J Am Coll Surg 2010;211:271-8. [Crossref] [PubMed]
- Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl 2013;19:1396-402. [Crossref] [PubMed]
- van Vugt JL, Levolger S, de Bruin RW, et al. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant 2016;16:2277-92. [Crossref] [PubMed]
- Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic patients. Nutrition 2005;21:113-7. [Crossref] [PubMed]
- Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373-8. [Crossref] [PubMed]
- Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [Crossref] [PubMed]
- Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013;58:325-36. [Crossref] [PubMed]
- Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012;27:430-1. [Crossref] [PubMed]
- Román E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966-75. [Crossref] [PubMed]
- Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920-6.e2. [Crossref] [PubMed]
- Duarte-Rojo A, Ruiz-Margain A, Montaño-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122-39. [Crossref] [PubMed]
- Ney M, Gramlich L, Mathiesen V, et al. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol 2017;23:97-104. [PubMed]
- García-Pagàn JC, Santos C, Barbera JA, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996;111:1300-6. [Crossref] [PubMed]
- Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, et al. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol 2016;7:e180. [Crossref] [PubMed]
- Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564-74. [Crossref] [PubMed]
- Samoylova ML, Covinsky KE, Haftek M, et al. Disability in patients with end-stage liver disease: Results from the functional assessment in liver transplantation study. Liver Transpl 2017;23:292-8. [Crossref] [PubMed]
- Orman ES, Ghabril M, Chalasani N. Poor Performance Status Is Associated With Increased Mortality in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2016;14:1189-95.e1. [Crossref] [PubMed]
- Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology 2016;63:574-80. [Crossref] [PubMed]
- VanWagner LB, Ning H, Allen NB, et al. Alcohol Use and Cardiovascular Disease Risk in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;153:1260-72.e3. [Crossref] [PubMed]
- Hagström H, Nasr P, Ekstedt M, et al. Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol 2017;52:159-65. [Crossref] [PubMed]
- Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol 2012;57:384-91. [Crossref] [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [Crossref] [PubMed]
- Luca A, Garcia-Pagan JC, Bosch J, et al. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology 1997;112:1284-9. [Crossref] [PubMed]
- Ajmera V, Belt P, Wilson LA, et al. Among Patients With Nonalcoholic Fatty Liver Disease, Modest Alcohol Use Is Associated With Less Improvement in Histologic Steatosis and Steatohepatitis. Clin Gastroenterol Hepatol 2018;16:1511-20.e5. [Crossref] [PubMed]
- Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015;13:594-601.e1. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Sinha J, Mehta N, Dodge JL, et al. Are There Upper Limits in Tumor Burden for Down-Staging of Hepatocellular Carcinoma to Liver Transplant? Analysis of the All-Comers Protocol. Hepatology 2019;70:1185-96. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706-17.e1. [Crossref] [PubMed]
- Gopal P, Yopp AC, Waljee AK. Factors that affect accuracy of α-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:870-7. [Crossref] [PubMed]
- Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol 2016;65:1148-54. [Crossref] [PubMed]
- Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976-87.e4. [Crossref] [PubMed]
- Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169-77. [Crossref] [PubMed]
- Andersson KL, Salomon JA, Goldie SJ, et al. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418-24. [Crossref] [PubMed]
- Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MRI imaging - a systematic review and meta-analysis. Radiology 2015;275:97-109. [Crossref] [PubMed]
- Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography - a randomised study. Aliment Pharmacol Ther 2013;38:303-12. [Crossref] [PubMed]
- Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol 2017;3:456-63. [Crossref] [PubMed]
- Kim HL, An J, Park JA, et al. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology 2019;69:1599-613. [Crossref] [PubMed]
- Marks RM, Ryan A, Heba ER, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 2015;204:527-35. [Crossref] [PubMed]
- Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart 2002;87:9-15. [Crossref] [PubMed]
- Dec GW, Kondo N, Farrell ML, et al. Cardiovascular complications following liver transplantation. Clin Transplant 1995;9:463-71. [PubMed]
- Raval Z, Harinstein ME, Skaro AI, et al. Cardiovascular Risk Assessment of the Liver Transplant Candidate. J Am Coll Cardiol 2011;58:223-31. [Crossref] [PubMed]
- Canto JG. Kiefe Cim, Rogers WJ. Number of Coronary Heart Disease Risk Factors and Mortality in Patients With First Myocardial Infarction. JAMA 2011;306:2120-7. [Crossref] [PubMed]
- Josefsson A, Fu M, Björnsson E, et al. Prevalence of pre-transplant electrocardiographic abnormalities and post-transplant cardiac events in patients with liver cirrhosis. BMC Gastroenterol 2014;14:65. [Crossref] [PubMed]
- Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998;27:28-34. [Crossref] [PubMed]
- Mohamed R, Forsey PR, Davies MK, et al. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology 1996;23:1128-34. [Crossref] [PubMed]
- Garg A, Armstrong WF. Echocardiography in liver transplant candidates. JACC Cardiovasc Imaging 2013;6:105-19. [Crossref] [PubMed]
- Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;53:e1-90. [Crossref] [PubMed]
- Torregrosa M, Aguade S, Dos L, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol 2005;42:68-74. [Crossref] [PubMed]
- Bushyhead D, Kirkpatrick JN, Goldberg D. Pretransplant echocardiographic parameters as markers of posttransplant outcomes in liver transplant recipients. Liver Transpl 2016;22:316-23. [Crossref] [PubMed]
- Kia L, Shah SJ, Wang E, et al. Role of pretransplant echocardiographic evaluation in predicting outcomes following liver transplantation. Am J Transplant 2013;13:2395-401. [Crossref] [PubMed]
- Lima B, Nowicki ER, Miller CM, et al. Outcomes of simultaneous liver transplantation and elective cardiac surgical procedures. Ann Thorac Surg 2011;92:1580-4. [Crossref] [PubMed]
- Nicolau-Raducu R, Marshall T, Patel H, et al. Long-Term Cardiac Morbidity and Mortality in Patients with Aortic Valve Disease Following Liver Transplantation: A Case Matching Study. Semin Cardiothorac Vasc Anesth 2017;21:345-51. [Crossref] [PubMed]
- Adachi T, Murakawa M, Uetsuki N, Segawa H. Living related donor liver transplantation in a patient with severe aortic stenosis. Br J Anaesth 1999;83:488-90. [Crossref] [PubMed]
- Maraj S, Jacobs LE, Maraj R, et al. Inducible left ventricular outflow tract gradient during dobutamine stress echocardiography: an association with intraoperative hypotension but not a contraindication to liver transplantation. Echocardiography 2004;21:681-5. [Crossref] [PubMed]
- Krowka MJ, Fallon MB, Kawut SM, et al. International Liver Transplant Society Practice Guidelines: Diagnosis and Management of Hepatopulmonary Syndrome and Portopulmonary Hypertension. Transplantation 2016;100:1440-52. [Crossref] [PubMed]
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004;363:1461-8. [Crossref] [PubMed]
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl 2000;6:453-8. [Crossref] [PubMed]
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 1996;71:543-51. [Crossref] [PubMed]
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl 2000;6:443-50. [Crossref] [PubMed]
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:2215-45. [Crossref] [PubMed]
- Kong YG, Kang JW, Kim YK, et al. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br J Anaesth 2015;114:437-43. [Crossref] [PubMed]
- Jodocy D, Abbrederis S, Graziadei IW, et al. Coronary computer tomographic angiography for preoperative risk stratification in patients undergoing liver transplantation. Eur J Radiol 2012;81:2260-4. [Crossref] [PubMed]
- Yilmaz Y, Kurt R, Yonal O, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Atherosclerosis 2010;211:182-6. [Crossref] [PubMed]
- Harinstein ME, Flaherty JD, Ansari AH, et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant 2008;8:1523-8. [Crossref] [PubMed]
- Davidson CJ, Gheorghiade M, Flaherty JD, et al. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am J Cardiol 2002;89:359-60. [Crossref] [PubMed]
- Bhutani S, Tobis J, Geovrgyan R, et al. Accuracy of Stress Myocardial Perfusion Imaging to Diagnose Coronary Artery Disease in End Stage Liver Disease Patients. Am J Cardiol 2013;111:1057-61. [Crossref] [PubMed]
- Konerman MA, Fritze D, Weinberg RL, et al. Incidence of and risk assessment for adverse cardiovascular outcomes after liver transplantation: a systematic review. Transplantation 2017;101:1645-57. [Crossref] [PubMed]
- Soldera J, Camazzola F, Rodríguez S, et al. Dobutamine stress echocardiography, myocardial perfusion scintigraphy, invasive coronary angiography, and post-liver transplantation events: systematic review and meta-analysis. Clin Transplant 2018;32:e13222. [Crossref] [PubMed]
- Maddur H, Bourdillon PD, Liangpunsakul S, et al. Role of cardiac catheterization and percutaneous coronary intervention in the preoperative assessment and management of patients before orthotopic liver transplantation. Liver Transpl 2014;20:664-72. [Crossref] [PubMed]
- Sharma M, Yong C, Majure D, et al. Safety of Cardiac Catheterization in Patients With End-Stage Liver Disease Awaiting Liver Transplantation. Am J Cardiol 2009;103:742-6. [Crossref] [PubMed]
- Jacobs E, Singh V, Damluji A, et al. Safety of transradial cardiac catheterization in patients with end‐stage liver disease. Catheter Cardiovasc Interv 2014;83:360-6. [Crossref] [PubMed]
- Axelrod D, Koffron A, Dewolf A, et al. Safety and efficacy of combined orthotopic liver transplantation and coronary artery bypass grafting. Liver Transpl 2004;10:1386-90. [Crossref] [PubMed]
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-Artery Revascularization before Elective Major Vascular Surgery. N Engl J Med 2004;351:2795-804. [Crossref] [PubMed]
- VanWagner LB, Lapin B, Levitsky J, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl 2014;20:1306-16. [Crossref] [PubMed]
Cite this article as: Esteban JPG, Asgharpour A. Evaluation of liver transplant candidates with non-alcoholic steatohepatitis. Transl Gastroenterol Hepatol 2022;7:24.


