
Endoscopic therapies for Barrett’s esophagus
Introduction
Barrett’s esophagus (BE), defined as the transformation of the squamous epithelium of the distal esophagus to columnar-lined epithelium with intestinal metaplasia, is a premalignant condition that classically develops due to chronic inflammation from gastroesophageal reflux. While BE has only been shown to progress to esophageal adenocarcinoma (EAC) at a rate of roughly 0.2% to 0.6% per year (1), it is essential that individuals with BE who are at highest risk for progression are identified and treated. Progression to invasive esophageal adenocarcinoma has a 5-year survival rate of less than 20%, with a median survival time of less than 1 year (2). Since the incidence of EAC has been steadily increasing since the 1970s, efforts to prevent its development at the premalignant stage should take utmost precedence (3).
Surveillance and treatment guidelines of BE have changed over time and continue to evolve as new treatment and outcome data become available. Management begins by identifying and screening those at increased risk of having BE. Once the diagnosis is confirmed based on endoscopic and histologic findings, the treatment depends on the degree of dysplasia that is identified. Historically, the treatment of choice for patients with high-grade dysplasia or early EAC was esophagectomy. However, given the relatively high rates of post-surgical complications, morbidity, and mortality compared to those of endoscopic therapies, paradigms have shifted in favor of endoscopic techniques as the treatments of choice (4).
Endoscopic eradication therapy (EET) aims to provide safe and effective methods by which to diagnose and fully eliminate premalignant and even malignant mucosa. It encompasses both endoscopic resection as well as endoscopic ablative techniques. This review will discuss the current diagnostic and therapeutic indications for EET, as well as the methods involved in performing these techniques, with a focus on ablative therapies.
Diagnosis of BE
Based on the 2016 American College of Gastroenterology (ACG) guidelines, the diagnosis of BE requires both the presence of salmon-colored mucosa extending ≥1 cm proximal to the gastroesophageal junction as well as confirmation of intestinal metaplasia on biopsy (5). An irregular Z-line alone or suspect lesions that extend <1 cm from the GEJ should not be biopsied, as subjects with biopsy-confirmed IM from these lesions have not been found to have an increased risk of progression to esophageal adenocarcinoma (6).
Intestinal metaplasia has been shown to be significantly heterogeneous on biopsies, even from visibly evident abnormal mucosa. In an observational study of 125 patients diagnosed with BE, intestinal metaplasia was identified in only 34% of biopsy samples (7). It is therefore recommended that a minimum of 8 random biopsies be obtained to maximize diagnostic yield. For patients suspected to have BE but with biopsies negative for intestinal metaplasia, a repeat endoscopy should be considered, which may identify BE in about 30% of these patients based on a longitudinal cohort study (8). The Seattle protocol should be employed when sampling for Barrett’s esophagus. This entails taking four-quadrant biopsies every 1–2 cm from the top of the gastric folds up to the proximal-most extent of the BE (5). Recent guidelines from the American Society of Gastrointestinal Endoscopy (ASGE) did make a conditional recommendation of using WATS-3D brushing in addition to the Seattle protocol biopsies to diagnose patients with suspected BE (9). WATS-3D is wide-area transepithelial sampling with computer-aided three dimensional tissues analysis and consists of an abrasive brush that is used to sample a large circumferential area of a suspected area of BE. This brush obtain microbiopsies in addition to individual cells and was shown to increase the rate of detection of BE from 13.1% to 33% when compared to the Seattle protocol alone (10).
Barrett’s esophagus, if identified, is further classified based on the degree of dysplasia present. The degree of dysplasia will ultimately guide surveillance and treatment decisions. This classification system includes five levels: negative for dysplasia, indefinite for dysplasia, low-grade dysplasia (LGD), high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC). Given the degree of interobserver variability in the interpretation, any level of dysplasia that is identified on histology must be reviewed and confirmed by two pathologists, at least one of whom has specialized experience in GI pathology (11). The risk of progression to cancer varies depending on the presence of dysplasia. In LGD, that annual risk of progression to cancer is about 0.5–0.7%, while it is about 4–8% in HGD (5).
Confirmation of dysplasia in this manner is sufficient to adequately diagnose and proceed with treatment options for HGD and EAC. However, due to the histologic similarities between low-grade dysplastic mucosa and inflamed mucosa, patients found to have LGD on biopsy must have first undergone aggressive acid suppression therapy with a PPI before the diagnosis of LGD can formally be made. Thus, patients not on acid suppression prior to a confirmed LGD biopsy must undergo treatment and subsequent repeat endoscopy. Confirmation of LGD with two pathologists at this time would then be sufficient for diagnosis.
In the case of biopsy results classified as indefinite for dysplasia, there is insufficient data to adequately guide practice. A reasonable management algorithm recommended by the ACG is similar to that of LGD above, including the need for acid suppression therapy and repeat endoscopy in 3–6 months. The management thereafter will depend on the results of the repeat biopsy. If the pathology still remains indefinite for dysplasia, it is reasonable to repeat the endoscopy and biopsy in 1 year or to proceed with EET.
The role of endoscopic therapy
The initial management of BE depends on the presence of mucosal irregularities on endoscopy and the degree of dysplasia ultimately identified. Mucosal irregularities in the presence of BE (“nodular BE”) represents the earliest circumstance for which EET is indicated. In patients with known BE, any mucosal irregularities within the BE segment found on surveillance endoscopy should undergo endoscopic resection via endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Given these nodularities have an increased propensity to harbor dysplasia, resection offers both diagnostic and therapeutic value and is therefore crucial in the management of nodular BE. Further treatment beyond endoscopic resection depends on the degree of dysplasia identified and is similar to that of nonnodular BE, but generally includes ablative techniques.
In BE that is biopsy negative for dysplasia, only surveillance is warranted due to generally low malignant potential. Based on a 2012 meta-analysis including 57 studies, the pooled annual incidence of progression of nondysplastic BE to EAC was 0.33% (12). Surveillance endoscopy at 3- to 5-year intervals is therefore recommended.
In BE with confirmed LGD, EET is recommended with the intent to achieve complete eradication of intestinal metaplasia (CE-IM), as EET has been shown to lead to a reduction in progression to HGD or EAC compared to surveillance alone (13). In the case of confirmed LGD, a reasonable alternative to endoscopic ablative therapy may be surveillance at 1-year intervals until two consecutive endoscopies reveal biopsies negative for dysplasia, after which surveillance intervals based on nondysplastic BE may be followed.
EET is also recommended in BE with confirmed HGD, as it has shown to be efficacious and to have a superior side effect profile compared to esophagectomy (14,15). If a neoplastic lesion is found on resected tissue (after EMR or ESD) in a patient with BE, the decision to pursue endoscopic ablative therapy versus more traditional methods of treatment will depend on multiple factors. Variables such as the depth of invasion, the degree of differentiation, the patient’s surgical risk, and the presence of other life-limiting comorbidities may all contribute in making treatment decisions. For instance, EAC lesions that invade the mid to deep submucosa have been shown to have higher incidence of lymphatic spread and therefore will not be eradicated by endoscopic techniques alone (16).
Endoscopic resection
Endoscopic resection represents both the diagnostic and initial treatment modality of choice for managing mucosal irregularities in BE. In addition to allowing for histologic analysis, resection of nodular tissue renders the esophageal lumen amenable to endoscopic ablation and can even be curative for neoplasia confined to the mucosa. The two resection techniques currently used commonly in practice are EMR and ESD. These techniques are more complex than traditional resection methods because dysplastic or neoplastic irregularities are often flat, making them difficult to manipulate. Thus, these techniques both require high-level endoscopic skill and equipment, and each have advantages and disadvantages with regard to adequate resection and treatment outcomes.
EMR
In EMR, the target lesion is manipulated such that its submucosa is exposed and made amenable to snare resection. One version of this technique, often described as the “lift and cut” method, consists of injecting saline or other lifting agents into the submucosal space at one or more sites around the lesion to lift the submucosa from the muscularis propria. This allows the endoscopist to then pull the lesion into a transparent rubber cap positioned at the end of the endoscope using suction, creating a pseudopolyp that can be ligated across its base with a snare (17).
Another option for EMR, the “ligate and cut” method, involves the use of an endoscopic cap loaded with a rubber band. With this technique, the lesion is sucked into the cap without prior submucosal injection, and the rubber band is deployed over the base of the lesion, allowing for subsequent ligation with a snare. With the advent of multiband mucosectomy, a specialized cap can be loaded with multiple rubber bands, decreasing frequency of endoscope removal for reloading and thus procedural time (18). Indeed, multiple band mucosectomy has been found to be both more efficient and more cost-effective than traditional cap-assisted EMR (19).
Efficacy and complications
Both EMR techniques have been determined to be highly effective and safe in their goal of achieving complete eradication of intestinal metaplasia. This is typically accomplished when EMR is used in tandem with radiofrequency ablation (RFA). However, the sole use of EMR across the entire BE segment, known as stepwise radical EMR (SRER), has been studied as a possible alternative. In a meta-analysis comparing focal EMR + RFA with SRER, the efficacies of achieving CE-IM were similar, with pooled CE-IM rates of 73.1% (95% CI: 63–83.1%) and 79.6% (95% CI: 75.2–84.1%), respectively (20).
However, SRER portends a higher rate of complications compared to focal EMR + RFA. Generally, one of the most common complications of EMR is esophageal stricture, occurring in roughly 10.2% (95% CI: 6.5–13.8.5%) of patients undergoing focal EMR with RFA. In SRER, however, esophageal stricture rate was about 33.5% (95% CI: 18.9–48.1%). Perforations are rare, occurring at a rate of 0.2–1.3% (20).
ESD
With ESD, the target lesion is removed using a specialized endoscopic knife by dissecting through the submucosa. The technique first involves marking the lesion circumferentially using argon plasma coagulation (APC) with a margin of approximately 3–5 mm. Submucosal injection is used to lift the lesion, and the demarcated margin is then cut circumferentially using the ESD knife. Further submucosal solution is injected, at which point the endoscope with ESD knife is slowly advanced through the submucosal space, dissecting through the submucosa until the lesion is freed from the esophageal wall (Figure 1). This step requires careful navigation to maintain the endoscope within the submucosal plane; dissecting too superficially through the mucosa may sacrifice the integrity of the biopsy and dissecting too deeply may lead to penetration through the muscularis propria leading to a perforation (17).
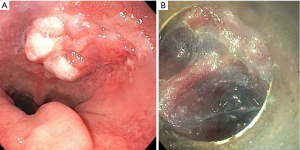
Efficacy and complications
ESD, when performed by endoscopists with significant experience, has been shown to have a higher en bloc resection rate and curative rate of intramucosal EAC when compared to EMR. This is likely due to the unique challenge posed by larger lesions for EMR. Given the endoscopic cap is limited in the size of lesions that can be resected at once, lesions larger than 2 cm often need to be resected in a piecemeal fashion. ESD, conversely, is not limited in this regard and allows lesions of any size to be resected. A recent meta-analysis in Asian populations comparing ESD and EMR in the treatment of intramucosal EAC and showed a pooled en bloc resection rate of 97.1% and 49.3% for ESD and EMR, respectively. This conferred a pooled curative rate of 92.3% for ESD compared to 52.7% for EMR. Although stricture and bleeding rates were not significantly different, ESD did pose a higher risk of perforation and longer operative times compared to EMR (21).
Of note, much of the data analyzing the safety and efficacy of ESD measure outcomes of treatments performed by endoscopists well-trained and highly experienced in the technique, thus potentially limiting generalizability. Therefore, ESD should only be carried out by such individuals with extensive training in the technique. It is important that tools necessary to deal with potential immediate complications are readily available when performing ESD. These may include coagulation devices, through-the-scope clips, over-the-scope clips, and esophageal stents.
Ablative techniques
Endoscopic resection is essential in the initial management of nodular areas of BE to adequately stage and treat the disease, but it is often not sufficient for achieving CE-IM. Endoscopic ablation using photochemical, freezing, or thermal injury aims to eliminate BE by inducing superficial necrosis of the metaplastic tissue, which can effectively eliminate dysplastic potential and allow for re-epithelialization with neo-squamous epithelium (22). These techniques, when used in conjunction with acid suppression therapy to maintain the integrity of the neo-epithelium, can help prevent the recurrence of BE altogether. The more commonly studied and used ablative techniques, including photodynamic therapy (PDT), RFA, cryotherapy ablation, and Hybrid-APC.
PDT
PDT relies on the photochemical properties of certain drugs known as photosensitizers to induce cell death when exposed to light of a certain wavelength. The most commonly used photosensitizer is porfimer sodium, which is given as a bolus injected intravenously 48 hours prior to the procedure. The photosensitizer is then absorbed by most body tissues but, for unclear reasons, is preserved in higher concentrations by neoplastic tissue (23). To activate the photosensitizer, a laser outputting the appropriate wavelength of light is transmitted through an optical diffusing fiber that is passed through the endoscope. The light then causes the photosensitizer to react with oxygen and generate free radicals, leading to cytotoxicity and ultimately cell death in the superficial tissue (24). The specific wavelength and power required to have the desired depth of ablation is based on the photosensitizer and the type of diffusing fiber used. The diffusing fiber may take several forms to allow for homogeneity in light application across the BE segment, such as the cylindrical balloon diffuser.
In terms of photosensitizer selection, porfimer sodium is the most commonly used photosensitizer and accumulates at high concentrations in both the mucosa and submucosa of BE segments. This allows for adequate and reliable penetration to the desired depth at the expense of side effects including skin photosensitivity and intraluminal complications such as strictures. Another photosensitizer which has boasted increasing popularity is 5-aminolevulinic acid (ALA). ALA, which is available in oral form, has a shorter half-life and less systemic absorption, leading to milder skin photosensitivity of shorter duration. It also absorbs more specifically to the mucosa, sparing the submucosa and possibly the intraluminal complications that arise from deep ablation compared to porfimer sodium (25). However, ALA was found to be limited in its utility due to heavy side effect profile include liver toxicity, neuropathy, and sudden death (26) as well as poorer performance compared to other ablative techniques (27).
Efficacy and complications
PDT was one of the first ablative therapies introduced for the treatment of BE. In comparison to acid suppression with a proton pump inhibitor (PPI) alone in a large multicenter study, porfimer sodium PDT was shown to be quite effective. The rate of CE-IM was 52% in those treated with PDT compared to 7% eradication with PPI alone (28). However, PDT has generally been overshadowed by its more recent successors due to its cost and burdensome side effect profile. After 5-year follow-up for patients who underwent PDT, the stricture rate was as high as 36%, and 69% of patients developed a photosensitivity reaction (29). PDT has also been shown to be quite costly and may be nearly 5 times more expensive than RFA, which doesn’t take into consideration the indirect costs of subsequent complications and treatment failures (30).
RFA
RFA uses heat generated by radiofrequency energy to destroy diseased tissue. Generally, the procedure is carried out first with stepwise circumferential ablation followed by focal ablation as needed. The most popular ablation device system in use currently is the Barrx FLEX system, which consists of a variety of circumferential and focal ablation devices.
The technique first requires extensive measurements to ensure RFA is delivered safely and only to the desired mucosa. Although newer self-sizing ablation catheters, such as the HALO360 Express, eliminates the need for a separate measuring process, the traditional devices still require measuring to be done prior to introduction of the ablation catheter. First, the esophageal wall is cleaned with tap water through the water jet channel of the endoscope to remove excess mucus, and the location of the esophagogastric junction and proximal extent of BE is recorded. The endoscope is then removed and replaced with a guidewire. The endoscopist must then measure the diameter of the esophagus in a stepwise fashion by inserting a sizing balloon over the guidewire and taking measurements from 6 cm above the BE segment down until the balloon passes into the stomach. The sizing balloon is removed, and based on the measurements, the appropriately sized ablation catheter is selected and advanced over the guidewire followed by the endoscope (Figure 2).
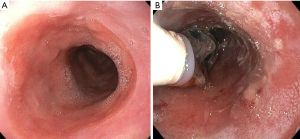
After endoscopic visualization, the catheter is positioned such that the proximal end of the electrode is about 1cm superior to the most proximal extent of the BE segment. The ablation catheter is then inflated, and radiofrequency energy is administered through the device to the apposed BE segment, delivering a reliable and uniform depth of penetration through the diseased tissue. The catheter is then advanced down the BE segment and the process is repeated until the entire segment is treated twice.
If needed, a focal ablation device (either an attachment or a device that is passed through the working channel) can be used to manage small residual islands or tongues of BE. The ablative surface of the device is positioned against the target BE segment and is energized twice with a single application under direct endoscopic visualization. The coagulum is pushed off and the focal ablation device is removed and cleaned. It is then reinserted, and the ablation is repeated. Finally, the ablated regions are rinsed thoroughly and the procedure is complete.
Efficacy and complications
RFA has been studied extensively in the management of BE. Given its efficacy and safety profile, it has for many become the ablative treatment of choice for dysplastic BE. In the landmark randomized controlled trial, Shaheen et al. randomized 127 patients with non-nodular BE to treatment with RFA versus sham endoscopy and were stratified by degree of dysplasia. CE-IM was achieved in 77.4% of patients who underwent RFA versus 2.3% of patients in the sham group, which led to a disease progression rate of 3.6% and 16.3%, respectively. Furthermore, there was no significant difference in CE-IM rates between the subgroups of patients with HGD and those with LGD (14). In a 5-year follow-up study of this population, recurrence of IM following CE-IM were high (>30%), but 69% of them achieved second CE-IM, and nearly all of these recurrences occurred within the first 2 years following initial treatment (31). Regardless, this study among others thereafter demonstrated the importance of adequate surveillance after CE-IM is achieved (32).
Strictures remain the most common complication after treatment with RFA, occurring in 5.6% of patients, although one study reported the risk to be as high as 11.8% (13,33). Bleeding and perforation risk were low as well at 1% and 0.6%, respectively. Factors that conferred a higher risk of adverse events from RFA included prior EMR as well as increased BE length (33).
Although randomized controlled trials are lacking in comparing RFA to newer ablative techniques, a small non-randomized study showed that RFA led to a complete eradication of dysplasia (CE-D) in 88.7% versus 54.5% for PDT. The CE-IM rate was 51.0% for RFA versus 39.4% for PDT. This data, combined with its low complication rate, has made RFA the ablative treatment of choice (30).
Cryotherapy ablation
In cryotherapy ablation, repeated cycles of freezing and thawing of the diseased mucosa trigger both immediate and delayed mechanisms leading to tissue necrosis. The immediate phase involves the rapid formation of intra- and extracellular ice crystals during freezing, which induces apoptosis by interrupting cell membranes. During the thawing phase, further damage is thought to occur to cell membranes as well as thrombosis of local blood vessels, impeding nutrient delivery (34). Delayed cell death has been attributed to an immune-mediated response to the injured tissue, thus destroying any diseased tissue that remained after ablation. The most common cryogens used to achieve this effect are liquid nitrogen and liquid carbon dioxide, with liquid nitrogen being the best-studied modality thus far.
Liquid nitrogen spray cryotherapy uses low pressure liquid nitrogen to induce freezing. In this technique, a dual-lumen orogastric decompression tube is first passed into the stomach over a guidewire. This tube allows for both passive and active venting to evacuate nitrogen that evaporates during the procedure, intended to prevent gastric distention. A plastic cap is placed on the tip of the endoscope to minimize splash-back onto the lens, and the endoscope is advanced alongside the decompression tube. The cryotherapy catheter is then passed through the endoscope and positioned between 0.5 and 1 cm from the BE mucosa. The spray is then applied to a hemi-circumferential swath of tissue until a white frost has formed, at which point a timer is started and spray is continued for predetermined duration of time. The optimal freeze time duration and number of freeze-thaw cycles necessary to achieve adequate depth of ablation has not been standardized, but generally ranges between 2 to 4 cycles, each with 10 to 20 seconds of freeze time followed by at least 45 seconds to allow for thaw. Based on the successes of several clinical trials, however, it appears reasonable to select 2 cycles of 20 seconds of freeze time as a standard (35,36). This process is repeated until all segments of BE mucosa undergo the selected treatment dosimetry.
The other main form of cryotherapy used liquid carbon dioxide as a cryogen. Polar Wand had been the leading device and, similar to the liquid nitrogen spray cryotherapy procedure, it involved cycles of direct application of cryogen to the mucosa for a period of time followed by thawing. However, Polar Wand was discontinued by its manufacturer in 2016.
A novel cryotherapy device that has boasted increased popularity and is currently being studied is the cryotherapy balloon. When positioned at the level of interest, the cryotherapy catheter balloon is designed to automatically self-size to the diameter of the esophagus, at which point the cryogen is expelled from the catheter through an aperture that is within the balloon itself, directed perpendicularly to the balloon wall. This creates a focal area of cryoablation to the mucosa adjacent to the balloon. The evaporated gas is expelled from within the balloon and catheter, so a decompression tube is not necessary (34).
Efficacy and complications
While data is still lacking in comparing cryotherapy to other ablative techniques, recent trials have shown promising results with regard to its efficacy and safety. In a recent meta-analysis of 9 studies assessing liquid nitrogen spray cryotherapy, the pooled rate of CE-IM in those who were treatment-naïve prior to undergoing cryotherapy was 53.7%, noting moderate heterogeneity among studies. Including patients who underwent prior treatments with RFA, pooled rates of CE-IM, complete eradication of dysplasia, and complete eradication of HGD were 56.5%, 83.5%, and 86.5%, respectively. The pooled rate of BE recurrence was 12.7%, although the studies included were limited in terms of follow-up time (36). This data indicates that although eradication rates are generally lower for cryotherapy than those reported for RFA, there may at least be a role for cryotherapy in the setting of RFA treatment failure, although more data is needed to characterize this effect.
A recent prospective clinical trial by Canto et al., 41 consecutive patients with confirmed BE with dysplasia or intramucosal adenocarcinoma were treated with a cryoballoon focal ablation system. A median of 3 ablation sessions were needed. At 1 year the CE-D rate was 95% and the CE-IM rate was 88%. 9.7% of patients did develop dysphagia due to stricture and required endoscopic dilation for relief (37).
In terms of safety, Shaheen et al. carried out a multi-center retrospective cohort study which reported a stricture rate of 3% and post-operative chest pain in 2% of patients, without any other serious adverse events (38). In comparison with patients undergoing RFA, patients who undergo spray cryotherapy also report a lower degree of post-procedural pain, although most reported pain resolved within 3 weeks regardless of the modality used (39).
Ultimately, it appears that liquid nitrogen spray cryotherapy is a safe and effective alternative to RFA. Cryotherapy balloon technology may further improve the safety profile and reduce treatment duration of this technique. However, more data in the form of head-to-head trials are needed before cryotherapy can be formally recommended at the same degree of confidence as RFA.
Hybrid-APC
APC utilizes thermal energy to induce tissue necrosis. This energy is generated when argon gas is released from a probe and ionized by a high voltage spark. The ionized gas seeks the nearest tissue as a ground, creating a relatively reliable field and depth of tissue destruction. APC had been used historically in the treatment of BE due to the simplicity and efficacy of treatment. However, it lost popularity after a multicenter trial reported that 10% of patients who underwent APC ablation suffered major complications including bleeding, strictures, and perforation (40). This was thought to be due to the extensive depth of tissue damage caused by APC. Thus, it has since fallen out of favor in light of the more novel ablative techniques with better safety profiles.
However, in 2015, Manner et al. introduced a modified technique dubbed “Hybrid-APC” that attempts to preserve the efficacy of traditional APC while ameliorating adverse effects. The treatment involves creating a safety “cushion” under the BE mucosa using submucosal injection prior to treatment with APC. APC is then carried out in the traditional fashion to ablate the affected BE segment. Upon completion, the coagulum is pushed off with the endoscope (40).
Efficacy and complications
Hybrid-APC is still in its infancy. In its inaugural trial, 60 patients with residual non-neoplastic BE after endoscopic resection were treated with the hybrid-APC treatment. Of the 60 initial patients, 10 were ultimately excluded due to poor wound healing after initial treatment, attributed to be secondary to damage from acid reflux at the gastroesophageal junction. Of the remaining 50 patients, 78% achieved CE-IM, 2% of patients developed strictures, and 22% experienced other minor adverse effects such as pain and odynophagia (41). Several limitations existed for this study including short-term follow up of only 3 months after macroscopically complete ablation, poor generalizability, and loss of several patients to histologic follow up. These limitations make it difficult to interpret the results, requiring further study to ultimately validate these findings.
Conclusions
With the development of newer technologies and techniques, as well as the outcome data to support them, the role of endoscopic therapy in the management of BE continues to expand and become clearer. EMR remains the initial diagnostic and therapeutic intervention of choice in the management of nodular BE, although ESD has become more widespread in its use for achieving en bloc resection for larger lesions despite higher stricture rate. In the setting of non-dysplastic BE, surveillance alone remains the treatment of choice given its minimal propensity for progression. In patients with confirmed LGD, HGD, or intramucosal EAC on biopsy, ablative treatment is recommended after endoscopic resection of nodularity, with the goal to achieve complete eradication of intestinal metaplasia.
Among ablative techniques, RFA continues to have the most data to support its safety, efficacy, and treatment durability. PDT, although effective, is limited by its high cost and stricture rate, and therefore has fallen out of favor. However, cryotherapy ablation has shown promise in its ability to induce durable CE-IM while minimizing complications, with some studies proposing a potential role for cryotherapy after RFA treatment failure. Cryotherapy, as well as the novel Hybrid-APC technique, would benefit greatly from robust head-to-head trials to better guide the endoscopist’s choice of treatment modality. Regardless of modality used, patients should continue in a strict surveillance protocol after EET due to the unpredictable risk of recurrence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Amy Tyberg) for the series “Innovation in Endoscopy” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh.2020.02.04). The series “Innovation in Endoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schoofs N, Bisschops R, Prenen H. Progression of Barrett's esophagus toward esophageal adenocarcinoma: an overview. Ann Gastroenterol 2017;30:1-6. [PubMed]
- Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015;149:302-17.e1. [Crossref] [PubMed]
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. [Crossref] [PubMed]
- Wu J, Pan YM, Wang TT, et al. Endotherapy versus surgery for early neoplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc 2014;79:233-41.e2. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50; quiz 1. [Crossref] [PubMed]
- Jung KW, Talley NJ, Romero Y, et al. Epidemiology and Natural History of Intestinal Metaplasia of the Gastroesophageal Junction and Barrettʼs Esophagus: A Population-Based Study. Am J Gastroenterol 2011;106:1447-55. [Crossref] [PubMed]
- Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett's esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol 2007;102:1154-61. [Crossref] [PubMed]
- Khandwalla HE, Graham DY, Kramer JR, et al. Barrettʼs Esophagus Suspected at Endoscopy but No Specialized Intestinal Metaplasia on Biopsy, Whatʼs Next? Am J Gastroenterol 2014;109:178-82. [Crossref] [PubMed]
- Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335-359.e2. [Crossref] [PubMed]
- Smith MS, Ikonomi E, Bhuta R, et al. Wide-area transepithelial sampling with computer-assisted 3-dimensional analysis (WATS) markedly improves detection of esophageal dysplasia and Barrett's esophagus: analysis from a prospective multicenter community-based study. Dis Esophagus 2019;32:doy099 [Crossref] [PubMed]
- Duits LC, Phoa KN, Curvers WL, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700-6. [Crossref] [PubMed]
- Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970-6. [Crossref] [PubMed]
- Phoa KN, Van Vilsteren FGI, Weusten BLAM, et al. Radiofrequency Ablation vs Endoscopic Surveillance for Patients With Barrett Esophagus and Low-Grade Dysplasia. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency Ablation in Barrett's Esophagus with Dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Prasad GA, Wang KK, Buttar NS, et al. Long-Term Survival Following Endoscopic and Surgical Treatment of High-Grade Dysplasia in Barrett’s Esophagus. Gastroenterology 2007;132:1226-33. [Crossref] [PubMed]
- Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27. [Crossref] [PubMed]
- Singh T, Sanaka MR, Thota PN. Endoscopic therapy for Barrett's esophagus and early esophageal cancer: Where do we go from here? World J Gastrointest Endosc 2018;10:165-74. [Crossref] [PubMed]
- Peters FP, Kara MA, Curvers WL, et al. Multiband mucosectomy for endoscopic resection of Barrett's esophagus: feasibility study with matched historical controls. Eur J Gastroenterol Hepatol 2007;19:311-5. [Crossref] [PubMed]
- Zhang YM, Boerwinkel DF, Qin X, et al. A randomized trial comparing multiband mucosectomy and cap-assisted endoscopic resection for endoscopic piecemeal resection of early squamous neoplasia of the esophagus. Endoscopy 2016;48:330-8. [PubMed]
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482-95.e4. [Crossref] [PubMed]
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Peter S, Monkemuller K. Ablative Endoscopic Therapies for Barrett's-Esophagus-Related Neoplasia. Gastroenterol Clin North Am 2015;44:337-53. [Crossref] [PubMed]
- Nishioka NS. Drug, light, and oxygen: a dynamic combination in the clinic. Gastroenterology 1998;114:604-6. [Crossref] [PubMed]
- Qumseya BJ, David W, Wolfsen HC. Photodynamic Therapy for Barrett's Esophagus and Esophageal Carcinoma. Clin Endosc 2013;46:30-7. [Crossref] [PubMed]
- Dunn JM, Mackenzie GD, Banks MR, et al. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett's oesophagus. Lasers Med Sci 2013;28:707-15. [Crossref] [PubMed]
- Sylantiev C, Schoenfeld N, Mamet R, et al. Acute neuropathy mimicking porphyria induced by aminolevulinic acid during photodynamic therapy. Muscle Nerve 2005;31:390-3. [Crossref] [PubMed]
- Ragunath K, Krasner N, Raman VS, et al. Endoscopic ablation of dysplastic Barrett's oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol 2005;40:750-8. [Crossref] [PubMed]
- Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [Crossref] [PubMed]
- Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc 2007;66:460-8. [Crossref] [PubMed]
- Ertan A, Zaheer I, Correa AM, et al. Photodynamic therapy vs radiofrequency ablation for Barrett's dysplasia: efficacy, safety and cost-comparison. World J Gastroenterol 2013;19:7106-13. [Crossref] [PubMed]
- Cotton CC, Wolf WA, Overholt BF, et al. Late Recurrence of Barrett’s Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681-8.e2. [Crossref] [PubMed]
- Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79-86.e1. [Crossref] [PubMed]
- Qumseya BJ, Wani S, Desai M, et al. Adverse Events After Radiofrequency Ablation in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086-95.e6. [Crossref] [PubMed]
- Parsi MA, Trindade AJ, Bhutani MS, et al. Cryotherapy in gastrointestinal endoscopy. VideoGIE 2017;2:89-95. [Crossref] [PubMed]
- Greenwald BD, Dumot JA, Horwhat JD, et al. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus 2010;23:13-9. [Crossref] [PubMed]
- Mohan BP, Krishnamoorthi R, Ponnada S, et al. Liquid Nitrogen Spray Cryotherapy in Treatment of Barrett's Esophagus, where do we stand? A Systematic Review and Meta-Analysis. Dis Esophagus 2019;32:doy130 [Crossref] [PubMed]
- Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett's esophagus (with video). Gastrointest Endosc 2018;88:438-446.e2. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Solomon SS, Kothari S, Smallfield GB, et al. Liquid Nitrogen Spray Cryotherapy is Associated With Less Postprocedural Pain Than Radiofrequency Ablation in Barrett's Esophagus: A Multicenter Prospective Study. J Clin Gastroenterol 2019;53:e84-90. [Crossref] [PubMed]
- Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett's mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006;101:1762-9. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett’s esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
Cite this article as: Ventre S, Shahid H. Endoscopic therapies for Barrett’s esophagus. Transl Gastroenterol Hepatol 2021;6:62.


