A retrospective case-controlled cohort study of inpatient drug induced liver injury: the RIDDLE study
Introduction
Drug-induced liver injury (DILI) is the most common cause of acute liver failure and indication for urgent liver transplantation in the United States and Europe (1,2). Although the most frequent cause of DILI is paracetamol, a direct and predictable hepatotoxin (3,4), non-paracetamol DILI is an important cause of morbidity and mortality that is unexpected and does not have a reversal agent.
The incidence of DILI is difficult to determine, due to inconsistent diagnostic criteria, lack of objective tests, reporting bias, and exclusion of low level/asymptomatic biochemical abnormalities. Several scoring systems have been developed to assess the likelihood that hepatic injury is the result of an individual drug. The most widely used of these are the Roussel Uclaf Causality Assessment Method (RUCAM) score (5), as well as the non-DILI specific Naranjo Score for adverse drug reactions (6). Despite issues regarding subjective score attribution, and areas of ambiguity, the RUCAM score is commonly used clinically and in research publications investigating DILI (7,8).
Antimicrobials are the most common cause of DILI, accounting for almost 50% of cases (9). This is likely due to both their intrinsic hepatotoxic potential as well as ubiquity in medicine. Of the top ten causes of DILI listed by the United States DILI registry, nine are antibiotics (10), and amoxicillin-clavulanate is the most common cause of idiosyncratic DILI worldwide (9,11,12). However, there is a paucity of Australian data.
There are inconsistencies in the literature regarding DILI risk factors likely due to unpredictable and infrequent occurrence, and reliance on retrospective series. Many series have suggested that DILI occurs more frequently in females, who also experience a more severe reaction manifest as higher rates of DILI associated liver transplant and death (3,9,12-14). This has been attributed possibly to oestrogen or interleukin 6 (15). However, other studies suggest males are at a higher risk for DILI from amoxicillin-clavulanate (16) and thiopurines (17). Increased age is a putative independent risk factor for DILI; however, this may be due to confounders including increased polypharmacy, increased disease burden, and increased health monitoring (9,18). Background chronic liver disease is a risk factor for all-cause mortality if DILI develops (10); however, baseline chronic liver disease has not been shown to increase risk of DILI occurring (19,20). Alcohol intake is a risk for paracetamol toxicity and methotrexate hepatotoxicity (21), but does not seem to play an important role in other DILI (22). It has been estimated that fatty liver increases the risk of DILI by four-fold particularly to irinotecan, MTX, tamoxifen (23-25).
DILI remains an important reason for hospitalisation and is difficult to characterise as the emergence of new medications add to a growing list of potential toxins. The aims of this study were to describe the characteristics of DILI cases at a single tertiary institution and examine possible risk factors and confounders in an inpatient population compared to propensity matched inpatient controls.
Methods
A case-control study of adult patients admitted to Eastern Health over 5.5 years between November 2011 and June 2017 was performed. Eastern Health is a one of Melbourne’s largest metropolitan health services with a catchment of over 750,000 people.
Electronic medical records were searched for patient episodes with DILI, using discharge coding diagnoses to identify cases of hepatic injury (Table S1), and case notes used to ascertain cases of DILI. Cases of paracetamol overdose and alcohol hepatotoxicity were excluded from analysis. Additional cases were identified by interrogation of data from a previous audit performed at our institution examining causes of alanine transaminase greater than 1,000 IU/L by an expert hepatologist (26). Data collected included patient demographics, history of liver disease, history of autoimmune conditions, atopy, chronic liver disease, alcohol intake, liver biochemistry, drug therapy, and outcome data. DILI specific scores including RUCAM score, Naranjo Score, and severity score were as defined by the US DILI network to grade severity of liver injury (27). The pattern of liver injury was described according to R factor (28) as hepatocellular, cholestatic, or mixed. Controls were randomly selected from all patients admitted to our institution during the study period, with inclusion criteria; over 18 years old, admitted as an inpatient, and did not have DILI during hospital stay. Randomisation of all patients was performed to yield 500 DILI-free patients. Of these 500, 178 were excluded for not meeting the inclusion criteria, leaving 322 subjects as controls. Charlson comorbidity scoring was calculated for controls and cases to ensure an equivalent comorbid burden between the groups existed. The same data fields were collected for controls as for the DILI cases.

Full table
The pattern of liver injury was described according to R factor (28) as hepatocellular, cholestatic, or mixed. Severity of DILI was in accordance with definitions published by the US DILI network (27). DILI was defined as previously described (9). Severe biochemical abnormality was defined as either bilirubin >200 µmol/L, Alanine transferase >1,000 IU/L, or Alkaline phosphatase >500 IU/L. Levels of alcohol intake were defined as abstinent, low risk, risky, or high risk according to definitions set out by the National Health and Medical Research Council (29). Causality was scored according to the Naranjo probability index and RUCAM scores (Table S1).
Baseline characteristics were assessed with descriptive statistics as appropriate. Univariate and multivariate logistic regressions were used to evaluate the association between potential risk factors and DILI. To minimise the effect of confounding by differences in key characteristics between cases and controls, we constructed an inverse probability weighted multivariate logistic regression models as sensitivity analyses. Variables with P<0.05 in the multivariate model were considered to be independent associations. All analysis was performed using STATA 15 (StataCorp, College Station, TX).
This retrospective review was approved by the Office of Research and Ethics, Eastern Health, Melbourne QA43-2017.
Results
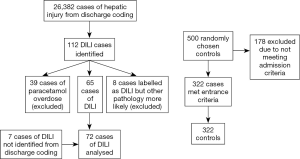
One hundred and nineteen cases of DILI were identified [112 from medical records (Table S1), seven from an audit of patients with raised alanine transaminases]. Forty-seven cases were excluded (39 due to paracetamol, 8 misdiagnosis), as shown in Figure 1. There were no instances of recurrent DILI in the same individual during this study period.
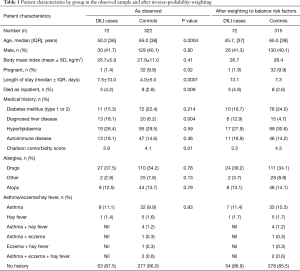
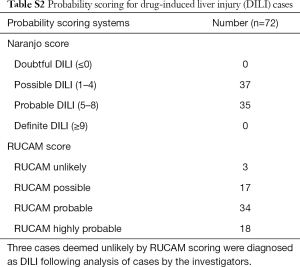
The final cohort of 72 DILI cases consisted of 42 (58.3%) females, with a median age at admission 50 (range, 18–89) years. Pre-existing liver disease was present in 13 (18.1%) DILI cases; diabetes (type 1 or 2) in 11 (15.3%); autoimmune disease in 13 (18.1%); and atopic conditions in 9 (12.5%) cases (Table 1). RUCAM scoring determined DILI to be unlikely in 3 (4.2%) cases; however, DILI was deemed the most likely diagnosis after review by an expert hepatologist, with low RUCAM score due to incomplete data. All other cases were possible: 17/72 (23.6%), probable: 34/72 (47.2%), or highly probable: 18/72 (25.0%) (Table S2).
Full table
Full table
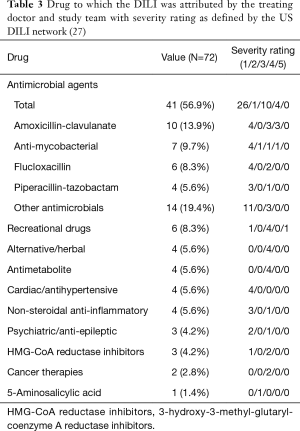
Antimicrobials were the most common causative agents, identified in 41 cases (56.9%). The single agent most commonly associated with DILI was amoxicillin-clavulanate: 10 cases (13.9%), followed by flucloxacillin: 6 cases (8.3%) (Table S3).

Full table
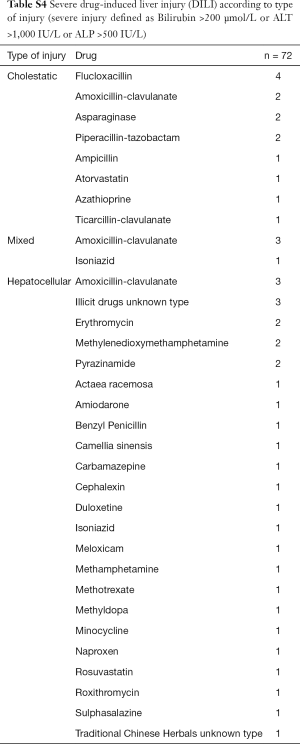
The most common pattern of liver injury was hepatocellular, accounting for 39 cases (54.2%). Severe biochemical abnormality was seen in 48 cases (66.7%); and was associated with 30/39 (76.9%) hepatocellular DILI cases, 14/22 (63.6%) cholestatic cases, and 4/11 (36.4%) mixed cases (Table 2). Overall, antimicrobials were the most common causative agents (56.9%) in severe DILI cases [defined as grade 4 or 5 in the DILI network severity scale (27)], largely attributed by amoxicillin-clavulanate (13.9%) and flucloxacillin (8.3%) (Table 3, Table S4). There was no difference of rates of severe DILI between males: 20/30 (66.7%) and females: 28/42 (66.7%).
Full table
Full table
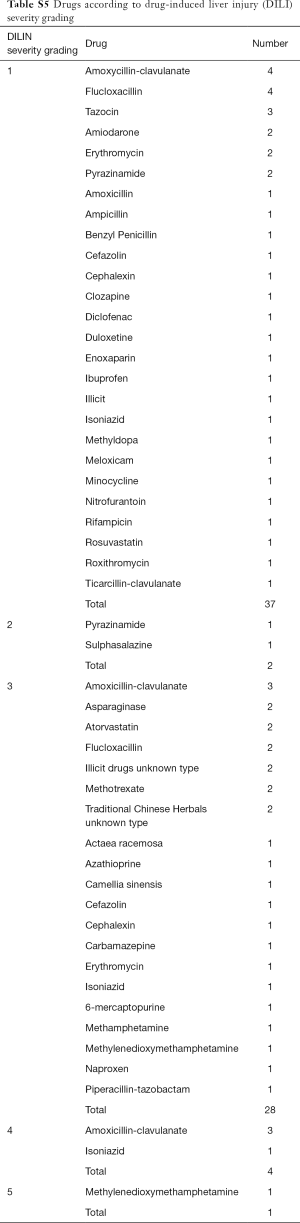
Three deaths occurred within the cohort but none were deemed to be as a direct consequence of DILI. One patient required liver transplantation following DILI secondary to methylenedioxymethamphetamine (MDMA) (Table S5).
Full table
When both groups were compared, younger age (50.2 vs. 65.0 years, P<0.001), presence of underlying liver disease (18.1% vs. 6.2%, P=0.004), average length of stay (7.5 vs. 4.0 days, P=0.0001) and in-hospital death (4.2% vs. 2.8%, P=0.009) were associated with DILI cases (Table 1). Pregnancy was more common in controls (1.4% vs. 9.9%, P=0.02) compared with DILI cases; however, the low number of pregnant cases included is likely to have influenced this result.
Alcohol drinking risk was higher in the control group compared to the DILI group, however, this was excluded from analysis due to high levels of missingness (approximately 50% in both cases and controls).
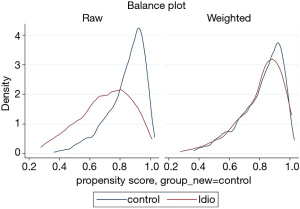
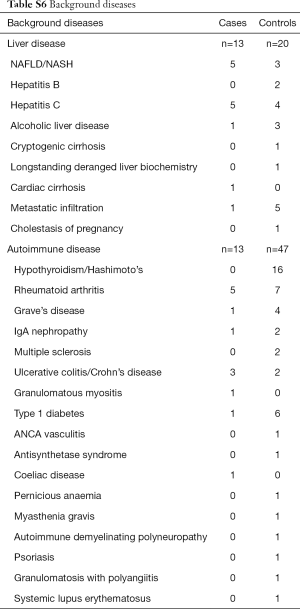
The prevalence of pre-existing liver disease (Table S6) were significantly higher in DILI cases versus controls for fatty liver (6.9% vs. 0.9%, P=0.001) and hepatitis C (6.9% vs. 1.2%, P=0.003), but not alcohol related liver disease (1.4% vs. 0.9%, P=0.726) and metastatic disease to the liver (1.4% vs. 1.6%, P=0.918). No significant difference in prevalence of autoimmune diseases were observed between cases and controls (18.1% vs. 14.6%, P=0.46); individually, significant differences were seen between groups for inflammatory bowel disease (4.2% cases vs. 0.6% controls, P=0.015), and rheumatoid arthritis (6.9% cases vs. 2.2% controls, P=0.033) but not for autoimmune thyroid disease (1.4% cases vs. 6.2% controls, P=0.100) (Table S6). Prevalence of allergy and of atopic conditions was not different between the two groups. A plot of covariate balance is shown in Figure 2.
Full table
Risk factors for DILI
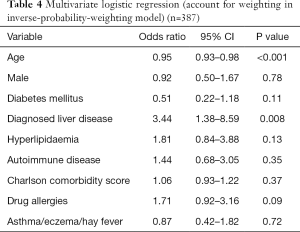
On multivariate analysis, younger age (OR, 0.95; 95% CI, 0.93–0.98; P<0.001) and the presence of pre-existing liver disease (OR, 3.44; 95% CI, 1.38–8.59; P=0.008) were associated with DILI. Male gender (OR, 0.92; 95% CI, 0.50–1.67; P=0.78), presence of diabetes (OR, 0.51; 95% CI, 0.22–1.18; P=0.11), hyperlipidaemia (OR, 1.81; 95% CI, 0.84–3.88; P=0.13), concomitant autoimmune disease (OR, 1.44; 95% CI, 0.68–3.05; P=0.35), other drug allergies (OR, 1.71; 95% CI, 0.92–3.16; P=0.09), or presence of atopic conditions (OR, 0.87; 95% CI, 0.42–1.82; P=0.72) were not found to be associated with DILI. Charlson comorbidity scores were not significantly different between groups (OR, 1.06; 95% CI, 0.93–1.22; P=0.37), consistent with a similar comorbid disease burden (Table 4).
Full table
Discussion
This retrospective case control study has characterised patients with DILI admitted to an Australian Hospital and compared them to a similar inpatient control group. It did not identify any risk factors for the development of DILI. The only associations were age, which was likely confounded as the elderly are more frequently exposed to pharmacologic therapy, both as inpatients and in the community, than younger patients; and pre-existing liver disease, which is more likely to be identified and noted in patients presenting with abnormal liver biochemistry. The previously held conviction that female gender, older age, higher BMI, and history of allergies were more common in patients with DILI was not demonstrated in this cohort. It does not appear from our study that older patients were at greater risk of hospital admission from DILI. The presence of autoimmune disease was not more common despite current theories on the importance of the adaptive and innate immune system in the development of DILI (30,31). This is despite the fact that some DILI, such as with amoxicillin-clavulanate, have classic autoimmune/allergy features of eosinophilia, rash, and fever. Inflammatory bowel disease and rheumatoid arthritis were more commonly observed in the DILI cohort, however, this may reflect increased exposure to potential hepatotoxic drugs.
A large number of inpatient encounters were identified using search terms for liver toxicity (Table S1) resulting in only 72 likely cases on detailed case review. This is still likely an underestimation as many cases of DILI are not diagnosed or may not be recorded in patient case files correctly. Using our estimated catchment area population of 750,000 people this equates to a rate of 1.7 cases per 100,000 person years; less than other quoted estimates of DILI incidence in community settings, which range from 2.4–19 cases per 100,000 person years (11-13,32-34). This study did identify DILI as a significant cause of morbidity; average length of stay is longer in the DILI group compared to controls (7.5 vs. 4.0 days, P=0.0001), and patients in the DILI group were more likely to die (4.2% vs. 2.8%, P=0.009). This rate is not dissimilar to that of other series, in which 5–10% of patients admitted with idiosyncratic DILI undergo liver transplant or die within 6 months (9,35).
Compared to matched controls, DILI was statistically significantly more common in younger patients (P<0.001), however the difference was of minimal clinical significance (OR 0.95). This is despite the fact that older patients are more likely to be on a greater number of medications and undergo more frequent health monitoring. It is plausible that increasing age is associated with less effective drug metabolism and may result in greater drug toxicity, flucloxacillin has been shown to cause DILI more frequently in the elderly and in those on protracted courses (36). All patients, in both the DILI and control groups, were exposed to pharmacologic therapy and thus at risk for DILI. Although data was not collected it is plausible that DILI patients, who were older than the control group, were on more medications than the controls and hence at increased risk of DILI.
Chronic liver disease was more common in the DILI group compared to controls (OR =3.44, P=0.008), which is in contrast to other work which suggests no such relationship exists (19,20). Rather than a true association it is likely this represents confounding. Patients admitted with DILI were reviewed by a specialty gastroenterology service and extensively investigated for causes of abnormal liver biochemistry, increasing the chance of detecting background disease.
Alcohol use was higher in the control group compared to the DILI group. As with background liver disease, this is likely secondary to more accurate history taking in the DILI group due to their abnormal liver biochemistry. Control patients were more likely to have alcohol intake recorded only if deemed clinically relevant, usually if intake was to excess. Patients without recorded alcohol intake history had this covariate excluded from analysis. In contrast, DILI patients were more likely to have an alcohol history recorded (65% recorded vs. 44% recorded in control).
The single agent responsible for the greatest number of episodes of DILI in our cohort was the amoxicillin-clavulanate combined formulation, followed by flucloxacillin. This is consistent with previous series in which amoxicillin-clavulanate was the commonest cause of DILI, and in Australia this was followed by flucloxacillin (12,37,38). Some series have suggested DILI to amoxicillin-clavulanate to occur more frequently in males (16). Our study showed antibiotics to cause 46% of DILI cases, which is similar to published rates (9). In addition, according to R factor (28), 54.2% of patients had hepatocellular injury, 30.6% had cholestatic, and 15.3% mixed. These rates are similar to those quoted by a large prospective series, which found 57% of cases hepatocellular, 23% cholestatic, and 20% mixed (9).
Three deaths occurred in patients suffering DILI. DILI was not thought to be the primary cause of death in any of these cases. Death in these cases was due to neutropenic sepsis following an allogenic stem cell transplant, Escherichia coli sepsis, and exacerbation of chronic obstructive pulmonary disease, respectively. One patient required liver transplantation following MDMA induced DILI.
A strength of this study was that it used a control group of propensity matched inpatient controls to try to characterise the DILI group. Charlson comorbidity scores were well matched between the DILI patients and controls confirming that their overall health and prognosis from underlying disease were similar. The limitations of this study are that it was retrospective and some data such as alcohol consumption and body mass index were incomplete. Also, for a study looking for characteristics and predictive factors for DILI, it is clear that very large patient samples are required as events are relatively rare.
Putative risk factors for DILI such as female gender, and history of other drug allergies and autoimmunity are not reflected in inpatient cohorts such as this. It is likely that clinical features of the host will not guide future research in DILI and other features such as genotypes and specific biomarkers may be more useful in advancing the science of this problem.
Acknowledgments
Health information staff, A/Prof John Lubel, Dr Danny Con.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective review was approved by the Office of Research and Ethics, Eastern Health, Melbourne QA43-2017.
References
- Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010;52:2065-76. [Crossref] [PubMed]
- Wei G, Bergquist A, Broome U, Lindgren S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med 2007;262:393-401. [Crossref] [PubMed]
- Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947-54. [Crossref] [PubMed]
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364-72. [Crossref] [PubMed]
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-30. [Crossref] [PubMed]
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. [Crossref] [PubMed]
- Lin J, Moore D, Hockey B, et al. Drug-induced hepatotoxicity: incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int 2014;34:576-82. [Crossref] [PubMed]
- Nicoll A, Moore D, Njoku D, et al. Repeated exposure to modern volatile anaesthetics may cause chronic hepatitis as well as acute liver injury. BMJ Case Rep 2012;2012. doi: 10.1136/bcr-2012-006543. [Crossref]
- Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924-34, 1934.e1-4.
- Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015;148:1340-52.e7. [Crossref] [PubMed]
- Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005;129:512-21. [Crossref] [PubMed]
- Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419-25, 1425.e1-3; quiz e19-20.
- Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 2002;36:451-5. [Crossref] [PubMed]
- Lucena MI, Andrade RJ, Kaplowitz N, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology 2009;49:2001-9. [Crossref] [PubMed]
- Sutti S, Tacke F. Liver inflammation and regeneration in drug-induced liver injury: sex matters! Clin Sci (Lond) 2018;132:609-13. [Crossref] [PubMed]
- Gresser U. Amoxicillin-clavulanic acid therapy may be associated with severe side effects -- review of the literature. Eur J Med Res 2001;6:139-49. [PubMed]
- Bastida G, Nos P, Aguas M, et al. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2005;22:775-82. [Crossref] [PubMed]
- De Valle MB, Av Klinteberg V, Alem N, et al. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther 2006;24:1187-95. [Crossref] [PubMed]
- Chalasani N, Regev A. Drug-Induced Liver Injury in Patients With Preexisting Chronic Liver Disease in Drug Development: How to Identify and Manage? Gastroenterology 2016;151:1046-51. [Crossref] [PubMed]
- Gupta NK, Lewis JH. Review article: the use of potentially hepatotoxic drugs in patients with liver disease. Aliment Pharmacol Ther 2008;28:1021-41. [Crossref] [PubMed]
- Schmidt LE, Dalhoff K, Poulsen HE. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology 2002;35:876-82. [Crossref] [PubMed]
- Chalasani N, Björnsson E. Risk Factors for Idiosyncratic Drug-Induced Liver Injury. Gastroenterology 2010;138:2246-59. [Crossref] [PubMed]
- Langman G, Hall PM, Todd G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol 2001;16:1395-401. [Crossref] [PubMed]
- Bruno S, Maisonneuve P, Castellana P, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ 2005;330:932. [Crossref] [PubMed]
- Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 2005;200:845-53. [Crossref] [PubMed]
- Con D, Buckle A, Nicoll A, et al. Severe liver injury in a large tertiary center cohort: Establishing etiology, outcomes, and prognostic factors Journal of Gastroenterology and Hepatology 2017;32:91. (abstract).
- Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) Prospective Study: Rationale, Design and Conduct. Drug Saf 2009;32:55-68. [Crossref] [PubMed]
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11:272-6. [Crossref] [PubMed]
- NHMRC. Australian Alcohol Guidelines: Health Risks and Benefits | National Health and Medical Research Council. In: NHMRC, 2001.
- Adams DH, Ju C, Ramaiah SK, et al. Mechanisms of immune-mediated liver injury. Toxicol Sci 2010;115:307-21. [Crossref] [PubMed]
- Ju C, Reilly T. Role of immune reactions in drug-induced liver injury (DILI). Drug Metab Rev 2012;44:107-15. [Crossref] [PubMed]
- Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis 2002;22:145-55. [Crossref] [PubMed]
- de Abajo FJ, Montero D, Madurga M, et al. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol 2004;58:71-80. [Crossref] [PubMed]
- Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015;148:1353-61.e3. [Crossref] [PubMed]
- Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 2014;147:96-108.e4. [Crossref] [PubMed]
- Fairley CK, McNeil JJ, Desmond P, et al. Risk factors for development of flucloxacillin associated jaundice. BMJ 1993;306:233-5. [Crossref] [PubMed]
- deLemos AS, Ghabril M, Rockey DC, et al. Amoxicillin-Clavulanate-Induced Liver Injury. Dig Dis Sci 2016;61:2406-16. [Crossref] [PubMed]
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther 2013;38:115-20. [Crossref] [PubMed]
Cite this article as: Worland T, Chin KL, Rodrigues B, Nicoll A. A retrospective case-controlled cohort study of inpatient drug induced liver injury: the RIDDLE study. Transl Gastroenterol Hepatol 2020;5:33.