
The “obese liver” and gastrointestinal cancer risk
Background, definitions and history
From an evolutionary point of view the accumulation of nutrients under the form of fatty substrates offers a protection during periods of famine (1). While the adipose tissue is the physiological reservoir of energetic fatty substrates, the liver is not (2). The normal liver is indeed virtually devoid of any fat content (3). The accumulation of fatty substrates (predominantly triglycerides) in more than 5% of the hepatocytes (often associated with mild inflammatory changes) is named steatosis; steatosis associated with hepatocyte ballooning, inflammatory changes and a variable proportion of hepatic fibrosis defines nonalcoholic steatohepatitis (NASH) (4). Simple steatosis, NASH as well as cirrhosis and hepatocellular carcinoma which may eventually occur in a proportion of patients with steatosis and NASH are collectively alluded to as nonalcoholic fatty liver disease (NAFLD) (4). A variety of extra-hepatic manifestations and complications attests to the systemic nature of NAFLD. These include the metabolic syndrome (type 2 diabetes, visceral obesity, atherogenic dyslipidemia, arterial hypertension, hyperuricemia); cardiovascular disease (atherosclerosis, aortic valve sclerosis, mitral annulus calcification, cardiomyopathy, heart failure, arrhythmias, chronic kidney disease); endocrine derangements (hypothyroidism, PCOS, hypogonadism, deficiency of growth hormone, hyperadrenalism); disorders of the respiratory system (obstructive sleep apnea syndrome, chronic obstructive pulmonary disease); conditions affecting the musculoskeletal system (osteoporosis, osteopenia); the skin (psoriasis); and various types of tumours (colorectal adenoma and carcinoma, carcinoma of pancreas, prostate, and breast) (2).
NAFLD is mutually and bi-directionally connected with the Metabolic Syndrome (4). Interestingly, so strictly are metabolism and immunity linked to each other under the evolutionary and physiological perspective that the excess of energetic substrates may be viewed by the innate immune system similar to an infectious state and this accounts for obesity resulting in a systemic sub-clinical inflammatory state (5,6).
Obesity is commonly defined by two anthropometric indices: body mass index (BMI) and waist circumference. BMI simply relates body weight to squared height (kg/m2) and does not identify the distribution of body fat nor does it differentiate lean from fat mass. Waist circumference is an index of accumulation of body fat in the visceral compartment and this is relevant given that visceral fat is deemed to be harmful from a metabolic point of view and subcutaneous fat is protective (7). It is waist circumference, not BMI, that is included in the definition of the Metabolic Syndrome based on ethnicity-specific cut-offs which take into account the fact that Asians, compared to other ethnicities, tend to manifest earlier metabolic complications for any given amount of body fat accumulation.
Obesity is a global epidemic accounting for a substantial financial burden worldwide and a phenotypically heterogeneous condition whose health risks span from easy fatigability to cancer, osteoarthritis, cardio-nephro-metabolic diseases (type 2 diabetes, dyslipidemia, NAFLD, arterial hypertension, coronary artery disease arrhythmias, sudden cardiac death, chronic kidney disease, stroke), asthma, sleep apnea, thrombophlebitis, gallstones, urinary incontinence, depression, male sexual dysfunction, low quality of life, loss of global and healthy years of life.
Obesity & cancer: a dangerous association
Many years ago, in her seminal study, Doctor Calle and Colleagues, by following-up for 16 years 900,000 individuals who were free of cancer at enrollment in 1982 demonstrated that the more severely obese members of their cohort (BMI ≥40) had combined mortality rates owing to all cancer types that were 52% to 62% higher (for men and women, respectively) than those of non-obese controls. The most affected organ sites were the gastrointestinal (GI) tract (esophagus, colon and rectum, liver, gallbladder, pancreas), kidney, and blood (non-Hodgkin’s lymphoma and multiple myeloma). Moreover, higher BMI values were also associated with significant trends of increasing risk of mortality owing to cancers of the stomach and prostate (men) and of the genital system (breast, uterus, cervix, and ovary) in women (8). In subsequent years, studies have universally confirmed the finding that obesity is strongly associated with GI cancer and, in particular, colonic and rectal cancer. For example, in their more recent prospective study, Levi and coworkers followed for 40 years adolescents who had stratified them based on BMI at 17 years, and found that the cumulative incidence of colonic and rectal cancer was strictly associated with BMI (9). Along the same line, Renehan et al. conducted a systematic review and meta-analysis aimed at assessing the strength of associations between BMI and different sites of cancer as well as to disclose how sex and ethnicity modulate such BMI-cancer associations (10). By analyzing 141 articles, including 282,137 incident cases these authors found that an increase in BMI of 5 kg/m2 was strongly associated in men, with cancers of esophagus, thyroid, colon, and kidney; in women, with cancers of endometrium, gallbladder, esophagus, and kidney. Increased BMI was more weakly associated positively with rectal cancer in men; postmenopausal breast, pancreatic, and colon cancers in women. Associations were stronger in men than in women for colon and did not generally vary across the various continental regions evaluated (North America, Europe and Australia, and the Asia-Pacific region) (10).
Given that their findings have been universally confirmed by subsequent studies, it may be interesting to discover how Dr. Calle and Colleagues, in their pioneering study, mechanistically explained the excess of cancer risk observed at various organ sites. The potential biologic mechanisms that were advocated by these Authors included increased levels of endogenous hormones such as sex steroids, insulin, and insulin-like growth factor I, and abdominal obesity contributing to gastroesophageal reflux (8). At that time, probably none had thought that the (obese) liver could be the culprit accounting for the association of obesity with cancer.
NAFLD and GI cancer
A recent line of research has specifically focused on the excess risk of colorectal tumors in asymptomatic individuals with NAFLD undergoing screening colonoscopy (11). Doctor Mantovani and Colleagues, by meta-analyzing 8 cross-sectional and 3 longitudinal observational studies collectively enrolling data of 91,124 asymptomatic adults, approximately one third of whom had NAFLD, and who totaled 14,911 colorectal adenomas and 1,684 colon cancers found that NAFLD diagnosed with either imaging studies or liver biopsy was independently associated with a moderately increased prevalence and incidence of colorectal adenomas and cancer independent of confounding factors such as age, sex, smoking, BMI, diabetes or metabolic syndrome. Prudently, these Authors highlight that the original studies evaluated were based mostly on individuals of Asian descent; that the observational design of the studies which they have meta-analyzed does not allow for proving causality, and also that they cannot rule out the possibility of residual confounding by some unmeasured factors. Based on such limitations, they conclude that additional prospective studies, particularly in European and American individuals, and further research is required to better define the pathobiological mechanisms underlying the association of NAFLD with colonic carcinogenesis (11).
The study by Dr. Allen and Colleagues
Bringing our understanding of such a topic further, Doctors Allen, Hicks, Mara, Larson and Therneau, from the Mayo Clinic Mayo Clinic, Rochester, Minnesota, USA compared all incident cases of NAFLD occurring in adults from 1997 to 2016 in a US population-based cohort to age and sex-matched controls from the same population. In their study population, these Authors evaluated the incidence of cancer (12). A total of 4,722 individuals with NAFLD (age 54, 46% male) and 14,441 age- and sex- matched controls were followed for a median of 8 (range, 1–21) years, during which 2,224 incident cancers occurred. After this longitudinal follow-up, NAFLD turned out to be associated with a nearly 2-fold increased risk of developing cancers [IRR =1.9 (95% CI, 1.3–2.7)]. The most often affected organs were the liver, the GI tract and the uterus. Revolutionizing previous disease paradigms, data of this study have shown that the increased risk of cancer was more strongly associated with NAFLD than with obesity. Indeed, in this study obesity alone was not associated with incident cancer (IRR =1.0; 95% CI, 0.8–1.4) though obesity was evaluated only with BMI (12).
Comments
How can the presence of NAFLD affect the risk of developing extra-hepatic cancers? The answer to this question is now more complex than that given at the times when the paper by Dr Calle was published (i.e., increased levels of endogenous hormones such as sex steroids, insulin, and insulin-like growth factor I, and abdominal obesity) (8). For example the role of the (fatty) liver now cannot be ignored. How would NAFLD increase the risk of cancer? (Figure 1). NAFLD is strongly associated with the development of a sub-clinical systemic pro-inflammatory state and insulin resistance (13) therefore promoting carcinogenesis in several organs and tissues (14,15). In the setting of the dysmetabolic-systemic inflammatory milieu, adipokines may play a role given that they are involved both in steatogenesis lipid accumulation in the liver as well as in colorectal carcinogenesis (16,17).
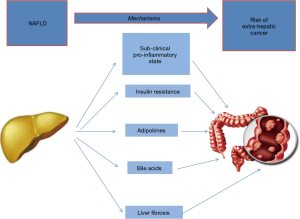
Adiponectin is an insulin-sensitizing, anti-steatotic and anti-inflammatory adipokine whose concentrations are typically reduced in NAFLD and this has been postulated to link NAFLD with colorectal cancer (11).
Moreover, NAFLD is also associated with a deranged bile acid metabolism (18), in particular their flow on the GI tract is enhanced, producing local inflammation and focal defects on the intestinal mucosa, thus possibly driving the carcinogenic process. This mechanism may be important, in particular, in localizing carcinogenesis in the large bowel (19). Irrespective of the mechanisms involved, the more advanced NAFLD forms are expected to be associated with an increased risk. For example a retrospective study conducted in 6,332 Koreans found that the subjects with NAFLD and advanced fibrosis had a significantly higher risk for colorectal adenoma, advanced adenoma, and multiple adenomas than those with NAFLD without advanced fibrosis (20).
Research agenda
The finding of NAFLD (rather than obesity) being associated with GI cancer independent of confounders is a major breakthrough. Additional, large, prospective, well conducted studies should better differentiate the risk of colon as opposed to rectal cancer, given that the pathogenesis behind these two cancer sites may be significantly different (21). In addition, the impact of genetics and epigenetics of NAFLD and NASH (22) on the risk of GI cancer should carefully be evaluated given that data suggest some differences, for example, in insulin resistance and cardiovascular risk (23). Finally, waist circumference is a good surrogate marker of abdominal adiposity and is the most clinically accessible measure of adiposopathy and dysfunctional adipose tissue (24). Interestingly, waist circumference is also the strongest predictor of liver fibrosis in patients with NAFLD, irrespective of BMI (25). Therefore, in the future, it will also be important to better stratify patients based on the amount of visceral adipose tissue evaluated with imaging techniques. Finally, should data on the association of colorectal adenoma and carcinoma with the more advanced forms of NAFLD be confirmed, (20) this notion could be exploited to modulate colonoscopic surveillance based on liver status.
In conclusion, the mechanisms underlying the association of NAFLD with colorectal cancer are incompletely understood. While subclinical pro-inflammatory state and insulin resistance are systemic mechanism which can increase the risk of cancer in any organ of the body, adipokines (e.g., adiponectin) and bile acids may localize this risk to the colonic (and rectal) mucosa. Finally, the mechanism(s) mediating the risk in those with fibrosing liver disease remain to be elucidated although, in principle, may be reconducted to one of those named above.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Reddon H, Patel Y, Turcotte M, et al. Revisiting the evolutionary origins of obesity: lazy versus peppy-thrifty genotype hypothesis. Obes Rev 2018;19:1525-43. [Crossref] [PubMed]
- Ballestri S, Mantovani A, Nascimbeni F, et al. Extra-hepatic manifestations and complications of nonalcoholic fatty liver disease. Future Med Chem 2019;11:2171-92. [Crossref] [PubMed]
- Petäjä EM, Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int J Mol Sci 2016. [Crossref] [PubMed]
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis 2017;49:471-83. [Crossref] [PubMed]
- Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol 2013;132:287-94. [Crossref] [PubMed]
- Gonzalez LL, Garrie K, Turner MD. Type 2 diabetes - An autoinflammatory disease driven by metabolic stress. Biochim Biophys Acta Mol Basis Dis 2018;1864:3805-23.
- Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 2019;129:4022-31. [Crossref] [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-38. [Crossref] [PubMed]
- Levi Z, Kark JD, Katz LH, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer 2017;123:4022-30. [Crossref] [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [Crossref] [PubMed]
- Mantovani A, Dauriz M, Byrne CD, et al. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metabolism 2018;87:1-12. [Crossref] [PubMed]
- Allen AM, Hicks SB, Mara KC, et al. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J Hepatol 2019;71:1229-36. [Crossref] [PubMed]
- Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol 2018;68:335-52. [Crossref] [PubMed]
- Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol 2014;20:9716-31. [Crossref] [PubMed]
- Roncucci L, Mora E, Mariani F, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2008;17:2291-7. [Crossref] [PubMed]
- Adolph TE, Grander C, Grabherr F, et al. Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions. Int J Mol Sci 2017. [Crossref] [PubMed]
- Tae CH, Kim SE, Jung SA, et al. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 2014;14:811. [Crossref] [PubMed]
- Jiao N, Baker SS, Chapa-Rodriguez A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018;67:1881-91. [Crossref] [PubMed]
- Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 2000;9:1271-9. [PubMed]
- Kim MC, Park JG, Jang BI, et al. Liver fibrosis is associated with risk for colorectal adenoma in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2019;98:e14139. [Crossref] [PubMed]
- Paschke S, Jafarov S, Staib L, et al. Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci 2018. [Crossref] [PubMed]
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018;68:268-79. [Crossref] [PubMed]
- Lonardo A, Ballestri S, Targher G. "Not all forms of NAFLD were created equal". Do metabolic syndrome-related NAFLD and PNPLA3-related NAFLD exert a variable impact on the risk of early carotid atherosclerosis? Atherosclerosis 2017;257:253-55. [Crossref] [PubMed]
- Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes 2014;21:345-51. [Crossref] [PubMed]
- Dogan T, Ozturk K, Celikkanat S, et al. Comparison of anthropometric measurements for prediction of the atherosclerosis and liver histology in young adults with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2019;31:1460-66. [PubMed]
Cite this article as: Lonardo A, Roncucci L. The “obese liver” and gastrointestinal cancer risk. Transl Gastroenterol Hepatol 2020;5:44.


