
Inherited iron overload disorders
Introduction
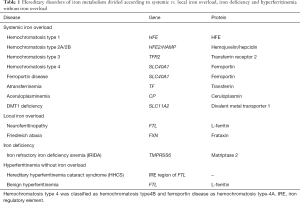
Different inherited iron metabolism defects can lead to iron overload, iron deficiency, and abnormalities of serum ferritin level (Table 1). This review is focused on those genetic defects causing systemic iron overload. Local inherited iron overload, e.g., Freidreich’s ataxia and neuroferritinopathy, leading to mitochondrial and brain iron overload, respectively, and IRIDA, leading to iron deficiency anemia are outside the scope of this review, as well as other systemic iron overload disorders caused by defects in genes not directly involved in iron regulation, e.g., thalassemia syndromes, congenital dyserythropoietic and sideroblastic anemias. Readers are referred to recent reviews (1-4). Also, inherited defects of cellular L-Ferritin synthesis and secretion (hereditary-hyperferritinemia-cataract syndrome and the so-called benign hyperferritinemia) are not included, as they do not cause iron overload (5,6).
Full table
Most of the genes causing systemic iron overload disorders codify for proteins involved in hepcidin regulation (HFE, transferrin receptor 2, hemojuvelin, hepcidin, matriptase 2) or in the interaction of hepcidin with its effector (ferroportin), in cellular iron handling [divalent metal transporter 1 (DMT1)], iron transport (transferrin), or release of iron to transferrin (ceruloplasmin). Table 1 summarizes these disorders according to causal genes and proteins.
Physiopathology of iron metabolism
Iron homeostasis is strictly regulated at cellular and systemic levels, and also in mitochondria to provide just the right amounts of iron at all times to maintain the often vital biological functions of cells and tissues avoiding the development of excess iron and the consequent iron-related toxicity (7,8). This occurs through a fine regulation of iron entry, utilization, storage, and export at mitochondrial, cellular and whole body levels through functional interconnections between different regulatory networks (9,10).
Cellular iron regulation
Although cellular iron homeostasis is under a multiple step control, the posttranscriptional regulation mediated by the binding of the iron regulatory proteins (IRP1, IRP2) to cis-regulatory mRNA iron responsive elements (IREs) has emerged as central (9). The IRE/IRP1 system regulates the expression of several mRNAs encoding proteins for iron acquisition [transferrin receptor 1 (TFR1), DMT1], storage (H- and L-ferritin), utilization [hypoxia-inducible factor 2 (HIF2a)], and export (ferroportin) (11), and additional mRNAs (12). IRE/IRP complexes formed within the 5'UTR of an mRNA (e.g., ferritin, ferroportin) inhibit translation, whereas IRP binding to IREs in the 3'UTR mRNA of TFR1 and DMT1 prevents degradation. Under iron-replete conditions, IRP1 is converted to a cytosolic aconitase preventing IRE binding while in iron deficiency IRP1 binds to IREs (9,12). The net result is the fine regulation of intracellular iron levels achieved by means of a divergent but coordinated regulation of iron-uptake, utilization, storage and export iron proteins.
Systemic iron regulation
The control of systemic iron levels occurs through the regulation of iron acquisition, recycling and storage, because there is no known regulated form of iron excretion. The most important pathway is the unidirectional recycling of iron from senescent red blood cells to the erythroid bone marrow through macrophages. The second is the cycling of iron from hepatocytes to the blood and vice versa, according to body needs. The third is the iron absorption through duodenal and upper jejunum that balances the 1–2 mg daily iron loss occurring through cellular exfoliation. Diferric transferrin (Tf-Fe2) provides iron to most cells of the body. The iron saturation of serum transferrin (TSAT) is both a major indicator and determinant of systemic iron homeostasis. TSAT is determined by the amount of iron absorbed from the intestine, recycled and released by macrophages, and utilized for erythropoiesis, the main iron consumer (7,13). In addition, Tf-Fe2 affects the expression of hepcidin that modulates intestinal iron absorption and iron release by macrophages through post-traductional regulation of the iron exporter ferroportin.
Every day, approximately 20–25 mg of iron must be supplied for hemoglobin synthesis in maturing erythroblasts. To acquire such amount of iron, erythroid cells depend on the interaction of Tf-Fe2 with TFR1 at the erythroblasts surface followed by endocytosis of the Tf-Fe2/TFR1 complex (7). This cycle is indispensable for erythroid cells at variance with other cells that may take up iron from non-transferrin-bound iron (NTBI) (7). This is demonstrated by the microcytic anemia with systemic iron overload that develop in humans lacking functional transferrin and DMT1 proteins (2,14).
Iron absorption
A common diet daily provides about 14 mg of iron as inorganic or organic (heme) iron. In the steady state in adults 1–2 mg are absorbed each day to maintain body iron balance. Iron absorption can be increased up to 25–30% of dietary iron content in response to increased iron demand (15), but this cannot always match iron requirements in children, young and pregnant women, and elder people that are more exposed to iron deficiency (16). Although still not fully clarified, heme absorption and transport at both the cellular and systemic levels involve several proteins (17,18). Non-heme iron requires to be converted in ferrous iron by the apical ferric reductase duodenal cytochrome-B to enter the cytoplasm via DMT1 (19). Iron is then released in the blood to transferrin via ferroportin.
Hepcidin-ferroportin axis
Enterocytes, macrophages, hepatocytes and placental cells, have core functions within iron metabolism by acquiring iron from different sources (diet, senescent erythrocytes, Tf-Fe2, and maternal transferrin, respectively) and delivering it according to body needs. Cellular iron release occurs through ferroportin, the only known iron exporter in mammals. FP needs copper-ferroxidases to release iron to plasma transferrin, hephaestin in enterocytes and ceruloplasmin in macrophages and hepatocytes. When defective, these proteins induce cellular iron retention in specific cell types as shown in hephaestin deficient sla mice and in humans with aceruloplasminemia (20). Ferroportin gene transcription and translation is modulated by a number of multi-layered signals (21). However, the activity of ferroportin on cell membranes is predominantly governed post-translationally by hepcidin (7,22). The liver peptide hepcidin is the master regulator of iron homeostasis, since it regulates intestinal iron absorption and iron release from storage cells by binding and blocking ferroportin either via degradation (23) or via occlusion (24), thus exerting a general inhibitory effect on iron release within the body. In physiological conditions, hepcidin production is tightly regulated in response to different signals, e.g., bone marrow iron requirements, hypoxia, TSAT, iron stores, and inflammation through different signaling pathways (7,25). Increased hepcidin expression limits iron absorption while its reduction allows greater iron absorption and macrophage iron release (26,27).
Regulation of hepcidin expression
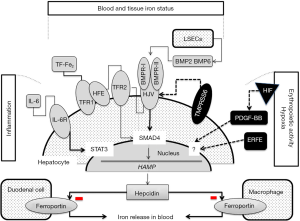
Figure 1 summarizes the regulatory pathways of hepcidin synthesis. Studies on genetic disorders of iron metabolism and corresponding animal models showed that the hemochromatosis proteins [HFE, transferrin receptor 2 (TFR2) and hemojuvelin (HJV)] are iron-dependent positive regulators of HAMP expression (22,30). Patients affected by hemochromatosis type-1, -2, -3 have a defective synthesis of hepcidin that can be absent or markedly reduced in the most severe forms of hemochromatosis or inadequate to the amount of iron overload. The higher the inhibition of hepcidin the more is the severity of iron overload and disease manifestations (22,31). HFE, TFR2 and HJV are possibly involved in the same regulatory pathway of hepcidin. Defective HFE or TFR2 prevents the formation of a functional iron sensor and signal transduction effector complex leading to reduced or inadequate hepcidin expression (7,29,30). The main activator of hepcidin in iron overload is HJV, a GPI-linked protein that activates hepcidin as a co-receptor for BMP cytokines. Hence, HJV-deficient patients and mice have undetectable levels of hepcidin. BMP2 and BMP6 and type I and type II BMP receptors are involved in this pathway (32,37). SMAD proteins act as the mediators of BMP signaling inducing hepcidin expression. Indeed, Bmp6 knock-out and Smad4 liver conditional knock-out mice develop severe iron overload and very low hepcidin expression (28,38). BMP/SMAD signaling to hepcidin is suppressed by matriptase-2, a serine protease that cleaves and generates a soluble form of HJV. Matriptase-2 mutations cause the rare form of iron refractory iron deficiency anemia (IRIDA) in mice and humans (39,40). Hepcidin is inhibited by erythropoietic expansion (7,41,42), and several lines of evidence indicate that circulating factors are involved in hepcidin suppression. Erythroferrone, a TNFα-like protein released by mature erythroblasts in condition of enhanced erythropoiesis, is a major candidate of erythropoiesis-induced hepcidin suppression, and Platelet-derived growth factor-BB is the candidate of hypoxia-induced hepcidin inhibition (33-35). Both factors could be implicated in the pathogenesis of iron overload in patients with ineffective erythropoiesis inducing persistent hepcidin suppression. Infection and inflammation markedly increase hepcidin synthesis, a mechanism largely implicated in the pathogenesis of anemia of chronic diseases (36,43).
Iron toxicity
Whatever the cause, when iron overwhelms cell storage capacity it becomes toxic. An increased cellular labile iron pool catalyzes the formation of reactive oxygen species (ROS) that overcome anti-oxidant defense and activate lipid peroxidation leading to cellular damage. Eventually, clinical complications such as liver cirrhosis and hepatocellular carcinoma, diabetes, cardiomyopathy, hypogonadism and arthropathy and osteoporosis may occur (44-47). In disorders with increased enterocyte iron absorption and macrophage iron release, too much iron enters the blood leading to oversaturation of transferrin and development of NTBI. NTBI and its component Labile Plasma Iron can enter the cells via an unregulated automatic way and disturb the delicate intracellular iron balance and contribute to ferroptosis (48-51). Parenchymal cells internalize NTBI by mechanisms that are yet to be fully characterized, but can involve the transporter SLC39A14 (ZIP14) in hepatocytes and acinar pancreatic cells (21,52), and ZIP8 and L-type calcium channels in cardiomyocytes (53,54). The liver is probably exposed to more NTBI than are other tissues because of the first-pass effect of the portal circulation, behaving in some way as a scavenger of toxic iron. There is evidence that extra-hepatic iron overload occurs after iron overwhelms hepatocyte capacity leading to reduced NTBI clearance by one hand and iron leakage from damaged hepatocytes (55-57). Hepatic damage also impairs transferrin synthesis reducing its iron scavenging capacity and increasing NTBI, further reduces hepcidin synthesis and hence favors iron accumulation (58). Although autoptic studies show diffuse tissue iron deposition in advanced iron overload, the main targets of iron toxicity are the liver, endocrine pancreas, heart, and the anterior hypophysis. Hepatocyte sideronecrosis and the local inflammatory response by Kupffer cells are the mediators of ROS-induced liver fibrogenesis by activating hepatic stellate and other mesenchymal cells to produce collagen (44,59,60). In Hfe knock-out mice pancreatic iron toxicity leads to increased b-cell apoptosis, reduced b-cell islet size and insulin content, and reduced insulin secretory capacity (46). Mitochondrial peroxidative damage may represent the most important expression of iron toxicity in the heart, which in turn may be directly responsible for the observed abnormalities in cardiac contractility and rhythmicity (48). Iron accumulation at the hypothalamic-pituitary level may impair GnRH neurons and/or pituitary gonadotroph cells leading to hypogonadotropic hypogonadism, characterized by low levels of gonadotropins (FSH and LH) and testosterone (61,62). Although joint involvement in adult and juvenile hemochromatosis is far from being minor (63,64), the role of iron in the genesis of arthropathy has never been clearly established. Synovial iron sequestration and inhibition of pyrophosphatases by iron leading to chondrocalcinosis are probable physiopathological mechanisms leading to articular damage (47). Iron may also exert direct toxicity on bone metabolism acting as an independent cause of osteoporosis (65-67).
Clinical manifestations
Table 2 summarizes the main epidemiological, genetic and patterns of presentation of the different forms of inherited disorders of iron metabolism. Whatever the underlying genetic cause leading to iron overload, clinical manifestations and organ damage depend on the amount and the rate of iron accumulation in tissues, and the different capacity for cells and tissues to cope with the iron-induced oxidative damage. The high prevalence of pituitary hypogonadism, diabetes and cardiomyopathy in juvenile hemochromatosis suggest that anterior pituitary, cardiac myocytes and pancreatic β-cells are particularly susceptible to the iron toxicity that follows the rapid iron accumulation occurring at young age (68,69). Slower rate of iron accumulation might explain milder and/or later phenotypes that characterize the adult forms of hemochromatosis, where hyperpigmentation, liver fibrosis/cirrhosis and hepatocellular carcinoma, and arthropathy represent the classical complications (70). It is to note, however, that phenotype of hemochromatosis type-1 has changed in the last decades. Patients have milder iron overload, lower prevalence of complications as compared with those diagnosed before the discovery of HFE, and have similar observed and expected survival (45). This has been ascribed to the improvement in both diagnosis and management of hemochromatosis, due to better physicians’ awareness and easier access to HFE genotyping (71).

Full table
Liver damage
Liver cirrhosis is a slow process that takes decades to develop. A threshold of liver iron concentration associated with increased risk of cirrhosis has been set at 13–15 mg of iron/g of liver tissue (dry weight) (72-74). This means that the liver can balance and compensate for iron toxicity for a long time allowing for prevention of damage if timely and adequately treated. In hemochromatosis patients a serum ferritin value of above 1000 ng/mL is a validated marker of increased risk of severe hepatic fibrosis/cirrhosis (75,76). Environmental (alcohol consumption, steatosis and coexistent viral infection) and possibly genetic factors can modify this risk (72,74,77-79). Iron depletion can improve fibrosis even in advanced stages unless cirrhosis is fully established (80). Hepatocellular carcinoma is the main cause of death in hemochromatosis type-1 and can develop on a severe fibrotic or cirrhotic substrate even after complete iron depletion (45,81).
Pancreatic damage
The pathogenesis of glucose intolerance and diabetes is likely multifactorial in hemochromatosis patients. Decreased insulin secretion is the early manifestation of islet damage (82) but glucose intolerance or diabetes can occur only when more than 70% b-cell die (83,84). Often, a concurrent genetic or acquired susceptibility to type-2 diabetes or a decreased insulin sensitivity associated to liver damage can favor the development of diabetes in hemochromatosis patients (82,85).
Cardiac damage
Cardiomyopathy most frequently occurs in the severe forms of iron overload (68,86). However, patients with adult hemochromatosis have an increased risk of cardiomyopathy compared to healthy controls (87). Independently form the underlying causes myocardial iron accumulation induces the development of restrictive cardiomyopathy with early diastolic dysfunction that may progress towards dilated cardiomyopathy and heart failure (88,89). Also, a wide variety of arrhythmias and sudden death can occur in severe iron overloaded patients (89). Iron removal can significantly improve cardiac manifestations up to normalization (88,89).
Other endocrine damage
Besides diabetes, pituitary hypogonadism is the most frequent endocrinological complication in iron overload disorders, especially in the earliest and most severe forms as juvenile hemochromatosis and transfusion-dependent thalassemia (68,90). It causes decreased libido and infertility, amenorrhea in females, and impotence in males, and participates to the development of osteoporosis. Iron removal in early stages can lead to symptoms improvement or resolution, and normalization of hormonal indices (91).
Arthropathy and osteoporosis
In some series more than 50% of the patients complain of articular symptoms that can be the revealing cause of hemochromatosis in some (47). Picture can mimic some frequent pathologies such as osteoarthritis, chondrocalcinosis or calcium pyrophosphate deposition disease (92). Symptoms can begin before 30 years of age or even earlier in the juvenile forms. Iron removal, in contrast with the visceral locations of the disease, in many cases do not have any favorable impact on symptoms (93).
Hereditary disorders of iron metabolism
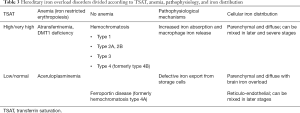
From a pathophysiological point of view inherited disorders of iron metabolism leading to iron overload can be divided two main groups: (I) those characterized by increased iron absorption and iron release from macrophages caused by absent/inadequate hepcidin transcription or abnormal hepcidin-ferroportin interaction (hepcidin resistance); (II) those characterized by inefficient iron export from storage cells due to defective expression of ferroportin on cell membrane or lack of ceruloplasmin. Different pathophysiological mechanisms lead to different manifestations, e.g., parenchymal or reticuloendothelial iron overload, iron overload with or without anemia, iron overload with high or normal/low TSAT (Table 3).
Full table
Diseases due increased iron absorption and iron release from macrophages
They include: (I) hemochromatosis type-1, -2A, -2B, and type-3 caused by mutations in 4 different genes (HFE, HFE2, HAMP, and TFR2) all involved in hepcidin signaling pathway (Figure 1); (II) hemochromatosis type-4, due to gain-of-function mutations of ferroportin causing hepcidin resistance; (III) a set of ultra-rare diseases caused by mutation in genes coding for protein involved in iron delivery to maturing erythroblasts (atransferrinemia) or erythroblast iron handling (DMT1 deficiency) causing iron restricted erythropoiesis and variable levels of anemia and erythropoiesis-induced hepcidin suppression (2,31,41). All show high TSAT and iron deposition in parenchymal cells, and except hemochromatosis type-4, manifest as autosomal recessive phenotypes. Table 2 summarizes the main epidemiological, genetic and patterns of presentation of the different forms of hemochromatosis.
Hemochromatosis types 1, 2, and 3
Since all the causing-disease proteins belong to the same pathway, the mechanism leading to iron overload depends on absent or defective hepcidin synthesis, or inability to up-regulate hepcidin production appropriately in response to increased iron stores (7,8,22,56). This in turn induces increased iron absorption and macrophage release in the plasma that exceeds the binding capacity of transferrin, causing NTBI production, iron accumulation in parenchymal tissues, and organ damage. Thus, the differences among these forms of primary iron overload are quantitative (the amount of iron overload and the severity of iron-related damage) rather than qualitative (similar alterations of serum iron indices and liver iron distribution, same targets of iron deposition).
Hemochromatosis type 1 (OMIM#235200)
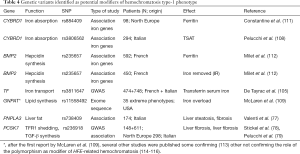
Is the most common form of hemochromatosis. More than 90% of the patients are homozygous for the p.Cys282Tyr mutation in HFE. The prevalence of the disease varies among ethnic groups from 0.000039% in Asian individuals, 0.012% in black, 0.027 in Hispanic to 0.44% in non-Hispanic individuals with a peak of 1.2% in Ireland (94,95). According to the origin of the p.Cys282Tyr mutation and its migratory pattern in Europe, there is a North-to-South decreasing gradient that is well represented in Italy with a prevalence of 0.2–0.46% in Northern Italy (96-98), 0.036% in Central, and 0.000225% in Southern Italy (99). The p.His63Asp variant is a common polymorphism observed at allele frequency of 13.6% in European countries (100). p.Cys282Tyr/p.His63Asp compound heterozygous and p.His63Asp homozygous genotypes are commonly reported in hemochromatosis patient series at low frequency (around 5% and 2%, respectively). Only a proportion of people homozygous for the p.Cys282Tyr mutation will develop symptoms of hemochromatosis, that is the clinical appearance of iron-related complications, indicating that the penetrance of this genotype is incomplete and expression variable (31). Gender, genetic background, environmental or life style factors, and coexistence with other co-factors (alcohol intake, overweight, steatosis, diabetes, beta-thalassemia trait) might significantly modify hemochromatosis phenotype (74,101-103). The role of genetic modifiers was demonstrated in Hfe-knock-out mice (104), but association studies between genetic markers and disease phenotype in p.Cys282Tyr homozygotes have given uncertain results (105-110). Table 4 shows a list of the candidate modifiers identified to date, although no polymorphisms that came up from these studies seem to exert a major effect on iron phenotype. More consistent appear the role of PCSK7 rs236918 as a genetic modifier of liver fibrosis risk (78,79). Mutations and polymorphic variants in other genes involved in iron metabolism, e.g., BMP6, TMPRSS6, FGF6, AAT (106,117-119), might contribute to generate a complex genetic background able to modulate HFE-related hemochromatosis phenotype.
Full table
Penetrance and expression of P.Cys282Tyr/p.His63Asp compound heterozygous and p.His63Asp homozygous genotypes are even much lower, and the risk to develop iron-related complications is extremely rare unless comorbid factors act synergistically in increasing iron overload and/or the risk of liver damage (103,120,121). Eventually, there are very rare forms of HFE-related HH with apparent full penetrance and severe expression, due to complete deletion of the gene (122,123) or presence of rare mutations in the homozygous (124,125) or compound heterozygous state with the p.Cys282Tyr mutation (126,127).
These very rare forms of hemochromatosis, caused by mutations of HFE2, and HAMP (128) are often referred to juvenile hemochromatosis. Excluding the p.Gly320Val mutation in exon 4 of HFE2 that was found in several unrelated patients, the other mutations in both HFE2 and HAMP are often private (129-131). Both forms of hemochromatosis are considered fully penetrant, but recent reports described patients with HFE2-related hemochromatosis presenting at an adult or old age (132,133) suggesting the existence of modifiers (still undefined) able to partially blunt mutation consequences.
Hemochromatosis type-3 (OMIM #604250)
It is caused by mutations of TFR2, whose allele prevalence was estimated between 0.0001 and 0.0004 (128). Although commonly considered an intermediate form between juvenile and adult hemochromatosis, it was shown that severe mutations could lead to juvenile-like hemochromatosis and increased iron indices in childhood (86,134,135). Mutations are most often private [reported in HGMD (130)] although the 1780-1791del (AVAQ 594-597) was found in a few patients worldwide (136,137), and a cluster of the p.Tyr250* mutation was found in Sicily (134,138).
Hemochromatosis type-4 (OMIM #606069)
Mutations of SLC40A1 gene can lead to two different disorders of iron metabolism, previously classified as hemochromatosis type-4A and -4B. Globally, allele frequency has been estimated at 0.0008–0.0009 (128). The former and most frequent type-4A is characterized by atypical manifestations that do not correspond to hemochromatosis (139,140). Owing to this, it should be considered as a distinct disorder (ferroportin disease). Type-4B shows typical serum iron indices alterations and pattern of iron overload and should be now listed as hemochromatosis type-4 (31,140). Nevertheless, it differs from the other three forms of hemochromatosis from the genetic (autosomal dominant phenotype) and physiopathological point of view (hepcidin resistance). Hemocromatosis type-4 is due to gain-of-function mutations that affect amino acids interacting with hepcidin causing complete or partial resistance to hepcidin either by impairing the binding of hepcidin to ferroportin or by altering hepcidin-induced conformational change and thereby impairing the ubiquitination required for endocytosis (24,141). The affected patients hyperabsorb iron and present with high TSAT, high serum ferritin, and tissue iron overload with evidence of toxic damage that may develop in adult age. Mutations are often private and worldwide distributed. However, recent reports suggest that the p.Tyr333His gain-of-function mutation in SLC40A1 could be the commonest genetic cause of hepatic iron overload in non‐Caucasian patients with typical hemochromatosis phenotype (142).
Diseases due to defects of iron delivery to maturing erythroblasts or erythroblast iron handling
Atransferrinemia (OMIM #209300)
It is an ultra-rare hereditary autosomal recessive disorder characterized by severe deficiency in serum transferrin (143,144). The lack of serum transferrin causes the loss of its iron scavenger and transport functions leading to severe iron deficiency anemia, NTBI formation and severe iron overload in non-hematopoietic tissues. The hypotransferrinemic (hpx/hpx) mice provide a model to understand the human disease (145,146). The severe anemia in the hpx/hpx mice and in patients with hereditary atransferrinemia indicates that very little iron enters erythroid precursors if the Tf-Fe2-TFR1 cycle pathway is unusable. By contrast, non-erythroid tissues develop massive iron overload through NTBI uptake, exacerbated by severe hepcidin inhibition and increased intestinal iron absorption (147).
DMT1 deficiency
It is a very rare cause of microcytic iron loading anemia. DMT1 has four different isoforms whose expression differ among tissue types (148). Thus, DMT1 transmembrane protein is involved in dietary non-heme iron uptake at the brush border of duodenal enterocytes and also plays a crucial role in iron utilization at the endosomal membrane of the erythroid precursors. Previous reports strengthened homologies and differences between rodent and human DMT1 deficiency disorders (149). In animal models, the reduction of DMT1 causes pure iron deficiency, while humans have a more complex phenotype characterized by congenital microcytic anemia due to defective iron transport and utilization in erythroid precursors, and biochemical and histologic features of iron overload (150,151). Irrespective of the mechanism, these findings indicate that human DMT1 has a prevalent role in erythroid cells compared with the duodenum that may still uptake iron by heme transporter.
Diseases due to inefficient iron export from storage cells
This group of iron metabolism disorders includes: (I) ferroportin disease, due to loss-of function mutations of SLC40A1 causing defective intracellular ferroportin trafficking to the plasma membrane and partial or complete loss of the iron export function of ferroportin (21,139). (II) Aceruloplasminemia, due to inactivating mutations of the ceruloplasmin gene (CP) and loss of the ferroxidase activity that facilitates iron efflux from storage cells in conjunction with ferroportin (152,153).
Ferroportin disease (OMIM #606069)
Ferroportin disease is caused by loss-of-function mutations manifesting as a dominant trait. Loss-of-function mutations limit the rate of iron export from recycling macrophage and iron accumulates in macrophages (154,155). This leads to increased production of ferritin and its release into plasma. Serum ferritin is often increased disproportionately to the iron stores while serum iron level and TSAT are normal or very rarely decreased (139). In some patient hepatocyte iron accumulation and increased TSAT may occur in the late stage of disease, and in others a mixed form of sinusoidal and hepatocellular iron overload have been reported (139). Decreased stability of some mutants, and modifiers that affect disease severity (sex, age, alcohol abuse, obesity, and metabolic syndrome) might be implicated in phenotype heterogeneity (156). Despite the inefficient iron recycling from macrophage (and intestinal iron absorption), patients with ferroportin disease do not develop iron deficiency anemia indicating that the system is still able to compensate the iron requirement from the bone marrow in the steady state. Only if phlebotomized at the same frequency of HFE-related hemochromatosis to remove iron overload, these patients disease manifest their inefficient iron absorption and recycling capacity and develop functional iron deficient anemia (139). Ferroportin disease does not appear to cause clinically important disease because of the prevalent distribution of iron in macrophages that are likely less prone to toxic iron effect than parenchymal cells. However, it can be often confused with the other common causes of hyperferritinemia, thus requiring careful diagnostic assessment.
Aceruloplasminemia (OMIM #604290)
It is caused by inactivating mutations of the ceruloplasmin gene whose prevalence is estimated at 1:2,000,000 in Japan (157,158). The typical manifestations make aceruloplasminemia a unique iron overload disease. In fact, it is the only one among the neurodegeneration with brain iron overload disorders, to whom aceruloplasminemia belongs, manifesting systemic iron overload, and the unique systemic iron overload disease characterized by neuropathy as a major cause of morbidity and microcytic anemia with low serum iron and TSAT as a common manifestation (153). Cp is part of the multicopper ferroxidase family that facilitate cellular iron efflux and release to transferrin (152). Cp is recognized as a serum protein secreted by the liver, but it has also been found as glycosylphosphatidylinositol (GPI)-linked protein that is relevant in regulating iron efflux from astrocytes in the brain (159,160). The lack of Cp induces cellular iron retention and progressive overload on one hand, and low cellular iron release leading to iron-restricted erythropoiesis and microcytic anemia on the other hand (Table 3). Despite the strong molecular connection between Cp and ferroportin, phenotypes of aceruloplasminemia and ferroportin disease significantly differ (Table 3), indicating that the relative function of Fpn and Cp and their interactions in different tissues requires further studies (153).
Diagnosis
Diagnosis of the different forms of hereditary disorders of iron metabolism involves a sequential strategy that combines clinical, imaging, biochemical, and genetic data (Table 3 and Figure 2).
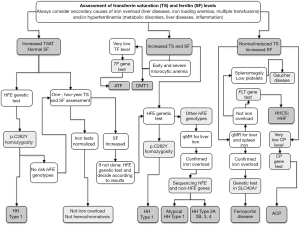
Clinical features
Diseases can manifest in the post-natal age in atransferrinemia or DMT1 deficiency, remain asymptomatic until adolescence in most of non-HFE forms of hemochromatosis and up to 50–60 years of age in hemochromatosis type-1 and -4, ferroportin disease, and aceruloplasminemia (Table 2). Commonly, symptoms can be disparate, variable, and non-specific, which explain the frequent diagnostic delay or confusion with other rare or common disorders (93,163-166).
Biochemical indices
Simple blood indices, e.g., hemoglobin and mean corpuscular volume, serum iron and transferrin to calculate TSAT [serum iron/(serum transferrin ×1.42)], and serum ferritin, are mandatory tests to guide the diagnostic work-up. High TSAT is the discriminant marker between hemochromatosis subtypes and hereditary iron loading anemias by one hand, and diseases due to inefficient iron export from storage cells, e.g., ferroportin disease and aceruloplasminemia by the other hand. TSAT is not a quantitative index of iron overload, but a high TSAT would imply a derangement of iron homeostasis characterized by increased intestinal iron absorption and iron release from macrophages and storage cells (25). The presence of anemia, usually microcytic, can help in further differentiation among diseases (Table 3). However, increased TSAT and anemia can occur in other genetic or acquired iron-loading anemias, advanced liver disease, and hemolysis that must be considered in differential diagnosis (1,2,4).
Although serum ferritin level does reflect, to some degree, the amount of iron overload of any cause, it is largely influenced by the presence of liver damage, hepatic or systemic inflammation, hyperthyroidism, alcohol intake, and alterations of the metabolic syndrome leading to frequent overestimation of the true amount of iron burden and to mistaken diagnosis. In addition, inherited defects can induce hyperferritinemia. Mutations in the IRE region of the 5'UTR L-ferritin mRNA that disrupt the iron-dependent post-transcriptional regulation of L-ferritin (hereditary-hyperferritinemia-cataract syndrome) and mutations in the first exon of L-ferritin gene that alter ferritin glycosylation and secretion in the blood (5,6). Recently, patients with unexplained hyperferritinemia not associated with iron overload and cataract and possibly inherited as a recessive trait, have been described, suggesting the existence of mutations in gene/s not directly implicated in iron metabolism that could affect ferritin secretion and turnover (167). Last, hyperferritinemia is a common manifestation of Gaucher’s disease and can be the revealing cause of the disease in some patients (168). Thus, although serum ferritin measurement is essential for the diagnosis of iron overload, evaluation of hyperferritinemia requires a careful diagnostic work including personal and family history, biochemical tests and tissue iron measurement (25,169).
Tissue iron measurement
Although liver biopsy is still considered the gold standard test for measuring liver iron concentration (170), non-invasive methodologies are increasingly recognized as valuable diagnostic tools in patients with increased serum iron indices. In this setting, quantitative magnetic resonance (qMR) represents a widely available method for non-invasive estimation of tissue iron content in the liver, heart, pancreas, and hypophysis (171,172). It also allows joint quantification of liver fat that could be necessary in the context of metabolic syndrome (173). qMR-based iron assessment in both liver and spleen can be useful in evaluating prevalent parenchymal vs. reticuloendothelial iron accumulation in different forms of hereditary iron disorders (Table 3) (31). Liver biopsy is currently limited to prognostic purposes (assessment of liver damage), whereas its use for diagnostic purposes is limited to selected situations (174). Non-invasive estimation of liver fibrosis will determine a further decline in hepatic biopsies in years to come (175).
Genetic testing
Genetic testing has two main aims: (I) to confirm the diagnosis in the proband and (II) to identify relatives who are at risk for the disease. The inherited disorders of iron metabolism represent only a portion of the heterogeneous world of iron overload and hyperferritinemic disorders. Thus, a step-by-step approach of the index case is recommended taking into account clinical and laboratory characteristics, and genetic basis of the different forms as reported in Table 3 and Figure 2 (2,5,25,139,153). Next-generation sequencing (NGS) technology could improve the molecular diagnostic approach to rare hereditary disorders of iron metabolism by implementing panels of candidate genes including phenotype modifiers to provide a more timely, reliable, and cheaper diagnosis (176,177).
Management
Assessment of organ damage
Once diagnosis is performed, a careful evaluation of complications has to be done according to disease specificity, amount of iron accumulation and targets of organ damage. A five-grade phenotypic classification has been recently proposed for hemochromatosis based on serum iron indices and quality of life evaluation (31). Besides hematological and iron indices, liver and endocrine function tests, abdominal ultrasound, qMR of different organs, echocardiography, bone densitometry and articular imaging, allow a satisfactory assessment of organ damage (Table 5).

Full table
Therapy
Since we have not mechanisms to eliminate excess iron from the body, iron must be removed artificially. Management of iron overload relies on two main therapies: blood removal and iron chelators (deferoxamine, deferiprone, and deferasirox) (178,179). They can be applied to prevention (achieve harmless levels of body iron before damage), rescue (patients with high level of body iron and/or signs of organ dysfunctions), or maintenance of harmless levels of body iron once they have been achieved. The increasing knowledge of iron overload pathology and the availability of new chelators allow physicians to personalize treatment to the patients, according to: (I) pathogenesis of iron accumulation; (II) severity of iron load; (III) presence of organ damage; (IV) clinical status; (V) goals of iron removal (prevention, rescue, maintenance). All these factors allow choosing the best therapeutic regimen: blood removal or iron chelation, monotherapy or combined therapy, frequency, dosage and duration of the therapy. From a practical point of view, the presence or absence of anemia define which patient can be treated by phlebotomy. In iron overload without anemia, blood removal is the main therapeutic option that should be tailored according to severity of iron overload and site of accumulation. In a few patients erythrocytoapheresis with or without erythropoietin stimulation can be used when clinical condition requires maintaining the isovolemic status (180,181). Generic dietary advise including low and not regular alcohol and meat intake for patients with hemochromatosis is recommended (180). Iron chelators can be used when phlebotomies are contraindicated or in combined therapy in most severe cases. Novel therapeutic options aimed to counteract hepcidin suppression have their own physiopathological rationale, but it will be difficult for them to compete with traditional therapies in the short term. Iron overload disorders with anemia require iron chelators and/or specific therapies (e.g., erythropoietin and blood transfusions in DMT1 deficiency, plasma or apotransferrin infusion in atransferrinemia) (147,150,153). Table 5 summarizes therapeutic approaches in different disorders. Early diagnosis and treatment reduce morbidity and mortality, and data indicate that adequate iron removal can reverse symptoms, significantly improve cardiomyopathy, and favor the regression of hepatic fibrosis and cirrhosis in a significant proportion of patients (80,89). It can induce partial and inconstant improvement of diabetes and hypogonadism and has variable and unpredictable effect on arthropathy (91,93,182).
Future directions
The role of iron excess in human pathology is increasingly considered and the inherited iron overload disorders represent important models to understand the physiopathology of cellular and systemic iron regulation and identify new markers and possibly new therapeutic targets. Hemochromatosis is the paradigm of these clinical disorders, but many other diseases have emerged many of which are related to erythropoiesis defects leading to hepcidin downregulation.
Genetics
Previous and more recent studies in GWAS in humans, in animal models and in vitro studies in primary murine and human hepatocytes have identified several new genes and proteins that regulates hepcidin expression, e.g., Fth (encoding for H-ferritin), Serpina1 (encoding for a-antitrypsin), Bmp6, Smad4, FGF6 (119) (fibroblast growth factor-encoding gene), GNPAT (109,113,114,116), whose role in human disease is still unclear. Some of these genes have been proposed as possible modifiers of hemochromatosis, but a general vision of the complex interactions underlying iron homeostasis and related diseases is still lacking. The expanding use of NGS techniques in patients with iron overload will likely extend our knowledge identifying new rare diseases and possibly allow a better characterization of the clinical heterogeneity observed in these patients.
Physiopathology
Other open questions relate on the different phenotypes observed in patients with ferroportin and aceruloplasminemia. Both proteins are involved in the same pathway by exporting iron and facilitating its loading to transferrin. However, liver iron distribution differs in patients with classical ferroportin disease and aceruloplasminemia, being prevalent in reticuloendothelial cells in the former and in hepatocytes in the latter, and microcytic iron deficiency anemia is common in aceruloplasminemia and extremely rare in ferroportin disease (153). Although the liver is the main site of hepcidin synthesis, recent studies demonstrated the presence of small but measurable amounts of hepcidin mRNA and protein in cells and tissues other than liver in humans and animals: heart, kidney, retina, monocytes and macrophages, splenocyte and alveolar cells, adipocytes and pancreatic β-cells (22). This suggests a local role for hepcidin in regulating iron homeostasis in these organs and tissues in an autocrine and paracrine fashion, but if interactions exist with the systemic, hepcidin-based iron regulation is unknown. Last, How LSECs sense changes in body iron concentration and upregulate BMP2 and BMP6 is still unclear (37) as well as the recent observation a role for epigenetic regulation in systemic iron homeostasis (183). More studies on the pathophysiology of hemochromatosis arthropathy are needed because it is still elusive and therapies are often frustrating for patients and doctors.
Diagnosis and management
Although a substantial improvement in the diagnosis and management of hemochromatosis has been achieved there are still issues related to phenotype characterization, utilization and interpretation of the genetic tests to avoid misdiagnosis, useless test and inadequate therapies. International, National and even Regional guidelines or recommendations exist that well clarify all of these points (25,31,153,184-186). While some therapeutic approaches are very effective as phlebotomies in hemochromatosis (187), there is room for improvement: (I) extending the use of oral iron chelators in non-transfusional iron overload (currently considered an off-label treatment) in patients with hemochromatosis and coexisting anemia (188), and in the other iron loading anemias; (II) new therapeutic perspectives for aceruloplasminemia patients (ceruloplasmin infusion, targeted gene therapies); (III) drug agency approval of human transferrin for atransferrinemia patients; (IV) hepcidin-based therapies (189) for patients with hepcidin-dependent iron overload.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Cook A, Giunti P. Friedreich's ataxia: clinical features, pathogenesis and management. Br Med Bull 2017;124:19-30. [Crossref] [PubMed]
- Iolascon A, De Falco L, Beaumont C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica 2009;94:395-408. [Crossref] [PubMed]
- Levi S, Rovida E. Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiol Dis 2015;81:134-43. [Crossref] [PubMed]
- Furuyama K, Kaneko K. Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia. Int J Hematol 2018;107:44-54. [Crossref] [PubMed]
- Girelli D, Bozzini C, Zecchina G, et al. Clinical, biochemical and molecular findings in a series of families with hereditary hyperferritinaemia-cataract syndrome. Br J Haematol 2001;115:334-40. [Crossref] [PubMed]
- Kannengiesser C, Jouanolle AM, Hetet G, et al. A new missense mutation in the L ferritin coding sequence associated with elevated levels of glycosylated ferritin in serum and absence of iron overload. Haematologica 2009;94:335-9. [Crossref] [PubMed]
- Muckenthaler MU, Rivella S, Hentze MW, et al. A Red Carpet for Iron Metabolism. Cell 2017;168:344-61. [Crossref] [PubMed]
- Brissot P, Loréal O. Iron metabolism and related genetic diseases: A cleared land, keeping mysteries. J Hepatol 2016;64:505-15. [Crossref] [PubMed]
- Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 2008;28:197-213. [Crossref] [PubMed]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011;283:65-87. [Crossref] [PubMed]
- Connell GJ, Danial JS, Haastruthers CX. Evaluation of the iron regulatory protein-1 interactome. Biometals 2018;31:139-46. [Crossref] [PubMed]
- Wilkinson N, Pantopoulos K. The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol 2014;5:176. [Crossref] [PubMed]
- Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr 2017;106:1559S-66S. [Crossref] [PubMed]
- Donker AE, Raymakers RA, Vlasveld LT, et al. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood 2014;123:3873-86. [Crossref] [PubMed]
- Gergen PJ, Teach SJ, Mitchell HE, et al. Lack of a relation between serum 25-hydroxyvitamin D concentrations and asthma in adolescents. Am J Clin Nutr 2013;97:1228-34. [Crossref] [PubMed]
- Camaschella C. Iron-Deficiency Anemia. N Engl J Med 2015;373:485-6. [PubMed]
- Chiabrando D, Vinchi F, Fiorito V, et al. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol 2014;5:61. [Crossref] [PubMed]
- Staroń R, Lipiński P, Lenartowicz M, et al. Dietary hemoglobin rescues young piglets from severe iron deficiency anemia: Duodenal expression profile of genes involved in heme iron absorption. PLoS One 2017;12:e0181117. [Crossref] [PubMed]
- Morgan EH, Oates PS. Mechanisms and regulation of intestinal iron absorption. Blood Cells Mol Dis 2002;29:384-99. [Crossref] [PubMed]
- Fuqua BK, Lu Y, Darshan D, et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS One 2014;9:e98792. [Crossref] [PubMed]
- Drakesmith H, Nemeth E, Ganz T. Ironing out Ferroportin. Cell Metab 2015;22:777-87. [Crossref] [PubMed]
- Piperno A, Mariani R, Trombini P, et al. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol 2009;15:538-51. [Crossref] [PubMed]
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090-3. [Crossref] [PubMed]
- Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018;131:899-910. [Crossref] [PubMed]
- Piperno A. Molecular diagnosis of hemochromatosis. Expert Opinion on Medical Diagnostics 2013;7:161-77. [Crossref] [PubMed]
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 2001;276:7811-9. [Crossref] [PubMed]
- Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 2002;110:1037-44. [Crossref] [PubMed]
- Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2005;2:399-409. [Crossref] [PubMed]
- D'Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol 2012;57:1052-60. [Crossref] [PubMed]
- Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood 2005;106:3710-7. [Crossref] [PubMed]
- Brissot P, Pietrangelo A, Adams PC, et al. Haemochromatosis. Nat Rev Dis Primers 2018;4:18016. [Crossref] [PubMed]
- Rausa M, Pagani A, Nai A, et al. Bmp6 expression in murine liver non parenchymal cells: a mechanism to control their high iron exporter activity and protect hepatocytes from iron overload? PLoS One 2015;10:e0122696. [Crossref] [PubMed]
- Kautz L, Jung G, Valore EV, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 2014;46:678-84. [Crossref] [PubMed]
- Ravasi G, Pelucchi S, Buoli Comani G, et al. Hepcidin regulation in a mouse model of acute hypoxia. Eur J Haematol 2018;100:636-43. [Crossref] [PubMed]
- Sonnweber T, Nachbaur D, Schroll A, et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 2014;63:1951-9. [Crossref] [PubMed]
- Verga Falzacappa MV, Vujic Spasic M, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007;109:353-8. [Crossref] [PubMed]
- Silvestri L, Nai A, Dulja A, et al. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam Horm 2019;110:71-99. [Crossref] [PubMed]
- Meynard D, Kautz L, Darnaud V, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 2009;41:478-81. [Crossref] [PubMed]
- Rausa M, Ghitti M, Pagani A, et al. Identification of TMPRSS6 cleavage sites of hemojuvelin. J Cell Mol Med 2015;19:879-88. [Crossref] [PubMed]
- Zhang AS, Anderson SA, Wang J, et al. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood 2011;117:1687-99. [Crossref] [PubMed]
- Camaschella C, Pagani A, Nai A, et al. The mutual control of iron and erythropoiesis. Int J Lab Hematol 2016;38 Suppl 1:20-6. [Crossref] [PubMed]
- Pak M, Lopez MA, Gabayan V, et al. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006;108:3730-5. [Crossref] [PubMed]
- Weiss G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin Hematol 2015;52:313-20. [Crossref] [PubMed]
- Deugnier YM, Loréal O, Turlin B, et al. Liver pathology in genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology 1992;102:2050-9. [Crossref] [PubMed]
- Fracanzani AL, Piperno A, Valenti L, et al. Hemochromatosis in Italy in the last 30 years: role of genetic and acquired factors. Hepatology 2010;51:501-10. [Crossref] [PubMed]
- Cooksey RC, Jouihan HA, Ajioka RS, et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004;145:5305-12. [Crossref] [PubMed]
- Guggenbuhl P, Brissot P, Loréal O. Miscellaneous non-inflammatory musculoskeletal conditions. Haemochromatosis: the bone and the joint. Best Pract Res Clin Rheumatol 2011;25:649-64. [Crossref] [PubMed]
- Link G, Konijn AM, Hershko C. Cardioprotective effect of alpha-tocopherol, ascorbate, deferoxamine, and deferiprone: mitochondrial function in cultured, iron-loaded heart cells. J Lab Clin Med 1999;133:179-88. [Crossref] [PubMed]
- Porter JB, Walter PB, Neumayr LD, et al. Mechanisms of plasma non-transferrin bound iron generation: insights from comparing transfused diamond blackfan anaemia with sickle cell and thalassaemia patients. Br J Haematol 2014;167:692-6. [Crossref] [PubMed]
- Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol 2005;18:277-87. [Crossref] [PubMed]
- Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ 2016;23:369-79. [Crossref] [PubMed]
- Liuzzi JP, Aydemir F, Nam H, et al. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A 2006;103:13612-7. [Crossref] [PubMed]
- Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 2003;9:1187-94. [Crossref] [PubMed]
- Paterek A, Mackiewicz U, Mączewski M. Iron and the heart: A paradigm shift from systemic to cardiomyocyte abnormalities. J Cell Physiol 2019;234:21613-29. [Crossref] [PubMed]
- Subramaniam VN, McDonald CJ, Ostini L, et al. Hepatic iron deposition does not predict extrahepatic iron loading in mouse models of hereditary hemochromatosis. Am J Pathol 2012;181:1173-9. [Crossref] [PubMed]
- Delima RD, Chua AC, Tirnitz-Parker JE, et al. Disruption of hemochromatosis protein and transferrin receptor 2 causes iron-induced liver injury in mice. Hepatology 2012;56:585-93. [Crossref] [PubMed]
- Mandelli C, Cesarini L, Piperno A, et al. Saturability of hepatic iron deposits in genetic hemochromatosis. Hepatology 1992;16:956-9. [Crossref] [PubMed]
- Détivaud L, Nemeth E, Boudjema K, et al. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood 2005;106:746-8. [Crossref] [PubMed]
- Pietrangelo A. Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis 1996;16:13-30. [Crossref] [PubMed]
- Bacon BR, Britton RS. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology 1990;11:127-37. [Crossref] [PubMed]
- Piperno A, Rivolta MR, D'Alba R, et al. Preclinical hypogonadism in genetic hemochromatosis in the early stage of the disease: evidence of hypothalamic dysfunction. J Endocrinol Invest 1992;15:423-8. [Crossref] [PubMed]
- Walton C, Kelly WF, Laing I, et al. Endocrine abnormalities in idiopathic haemochromatosis. Q J Med 1983;52:99-110. [PubMed]
- Richette P, Ottaviani S, Vicaut E, et al. Musculoskeletal complications of hereditary hemochromatosis: a case-control study. J Rheumatol 2010;37:2145-50. [Crossref] [PubMed]
- Sahinbegovic E, Dallos T, Aigner E, et al. Hereditary hemochromatosis as a risk factor for joint replacement surgery. Am J Med 2010;123:659-62. [Crossref] [PubMed]
- Diamond T, Nery L, Posen S. Spinal and peripheral bone mineral densities in acromegaly: the effects of excess growth hormone and hypogonadism. Ann Intern Med 1989;111:567-73. [Crossref] [PubMed]
- Valenti L, Varenna M, Fracanzani AL, et al. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 2009;20:549-55. [Crossref] [PubMed]
- Sinigaglia L, Fargion S, Fracanzani AL, et al. Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 1997;24:1809-13. [PubMed]
- De Gobbi M, Roetto A, Piperno A, et al. Natural history of juvenile haemochromatosis. Br J Haematol 2002;117:973-9. [Crossref] [PubMed]
- Yang G, Liu R, Peng P, et al. How early can myocardial iron overload occur in beta thalassemia major? PLoS One 2014;9:e85379. [Crossref] [PubMed]
- Deugnier Y, Brissot P, Loréal O. Iron and the liver: update 2008. J Hepatol 2008;48 Suppl 1:S113-23. [Crossref] [PubMed]
- Hover AR, McDonnell SM, Burke W. Changing the clinical management of hereditary hemochromatosis: translating screening and early case detection strategies into clinical practice. Arch Intern Med 2004;164:957-61. [Crossref] [PubMed]
- Adams PC. Is there a threshold of hepatic iron concentration that leads to cirrhosis in C282Y hemochromatosis? Am J Gastroenterol 2001;96:567-9. [Crossref] [PubMed]
- Angelucci E, Muretto P, Nicolucci A, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood 2002;100:17-21. [Crossref] [PubMed]
- Powell EE, Ali A, Clouston AD, et al. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology 2005;129:1937-43. [Crossref] [PubMed]
- Allen KJ, Bertalli NA, Osborne NJ, et al. HFE Cys282Tyr homozygotes with serum ferritin concentrations below 1000 microg/L are at low risk of hemochromatosis. Hepatology 2010;52:925-33. [Crossref] [PubMed]
- Guyader D, Jacquelinet C, Moirand R, et al. Noninvasive prediction of fibrosis in C282Y homozygous hemochromatosis. Gastroenterology 1998;115:929-36. [Crossref] [PubMed]
- Valenti L, Maggioni P, Piperno A, et al. Patatin-like phospholipase domain containing-3 gene I148M polymorphism, steatosis, and liver damage in hereditary hemochromatosis. World J Gastroenterol 2012;18:2813-20. [Crossref] [PubMed]
- Stickel F, Buch S, Zoller H, et al. Evaluation of genome-wide loci of iron metabolism in hereditary hemochromatosis identifies PCSK7 as a host risk factor of liver cirrhosis. Hum Mol Genet 2014;23:3883-90. [Crossref] [PubMed]
- Pelucchi S, Galimberti S, Greni F, et al. Proprotein convertase 7 rs236918 associated with liver fibrosis in Italian patients with HFE-related hemochromatosis. J Gastroenterol Hepatol 2016;31:1342-8. [Crossref] [PubMed]
- Falize L, Guillygomarc'h A, Perrin M, et al. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology 2006;44:472-7. [Crossref] [PubMed]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004;127:S79-86. [Crossref] [PubMed]
- McClain DA, Abraham D, Rogers J, et al. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 2006;49:1661-9. [Crossref] [PubMed]
- Yasugi H, Mizumoto R, Sakurai H, et al. Changes in carbohydrate metabolism and endocrine function of remnant pancreas after major pancreatic resection. Am J Surg 1976;132:577-80. [Crossref] [PubMed]
- Robertson RP, Lanz KJ, Sutherland DE, et al. Relationship between diabetes and obesity 9 to 18 years after hemipancreatectomy and transplantation in donors and recipients. Transplantation 2002;73:736-41. [Crossref] [PubMed]
- Niederau C, Berger M, Stremmel W, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia 1984;26:441-4. [Crossref] [PubMed]
- Ravasi G, Rausa M, Pelucchi S, et al. Transferrin receptor 2 mutations in patients with juvenile hemochromatosis phenotype. Am J Hematol 2015;90:E226-7. [Crossref] [PubMed]
- Elmberg M, Hultcrantz R, Simard JF, et al. Risk of ischaemic heart disease and cardiomyopathy in patients with haemochromatosis and in their first-degree relatives: a nationwide, population-based study. J Intern Med 2012;272:45-54. [Crossref] [PubMed]
- Cecchetti G, Binda A, Piperno A, et al. Cardiac alterations in 36 consecutive patients with idiopathic haemochromatosis: polygraphic and echocardiographic evaluation. Eur Heart J 1991;12:224-30. [Crossref] [PubMed]
- Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail 2010;16:888-900. [Crossref] [PubMed]
- Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004;89:1187-93. [PubMed]
- Siemons LJ, Mahler CH. Hypogonadotropic hypogonadism in hemochromatosis: recovery of reproductive function after iron depletion. J Clin Endocrinol Metab 1987;65:585-7. [Crossref] [PubMed]
- Carroll GJ, Breidahl WH, Bulsara MK, et al. Hereditary hemochromatosis is characterized by a clinically definable arthropathy that correlates with iron load. Arthritis Rheum 2011;63:286-94. [Crossref] [PubMed]
- Sahinbegovic E, Dallos T, Aigner E, et al. Musculoskeletal disease burden of hereditary hemochromatosis. Arthritis Rheum 2010;62:3792-8. [Crossref] [PubMed]
- Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005;352:1769-78. [Crossref] [PubMed]
- Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am J Epidemiol 2001;154:193-206. [Crossref] [PubMed]
- Salvioni A, Mariani R, Oberkanins C, et al. Prevalence of C282Y and E168X HFE mutations in an Italian population of Northern European ancestry. Haematologica 2003;88:250-5. [PubMed]
- De Gobbi M, D'Antico S, Castagno F, et al. Screening selected blood donors with biochemical iron overload for hemochromatosis: a regional experience. Haematologica 2004;89:1161-7. [PubMed]
- Distante S, Robson KJ, Graham-Campbell J, et al. The origin and spread of the HFE-C282Y haemochromatosis mutation. Hum Genet 2004;115:269-79. [Crossref] [PubMed]
- Floreani A, Rosa Rizzotto E, Basso D, et al. An open population screening study for HFE gene major mutations proves the low prevalence of C282Y mutation in Central Italy. Aliment Pharmacol Ther 2007;26:577-86. [Crossref] [PubMed]
- Merryweather-Clarke AT, Pointon JJ, Shearman JD, et al. Global prevalence of putative haemochromatosis mutations. J Med Genet 1997;34:275-8. [Crossref] [PubMed]
- Adams PC, Agnew S. Alcoholism in hereditary hemochromatosis revisited: prevalence and clinical consequences among homozygous siblings. Hepatology 1996;23:724-7. [Crossref] [PubMed]
- Lainé F, Jouannolle AM, Morcet J, et al. Phenotypic expression in detected C282Y homozygous women depends on body mass index. J Hepatol 2005;43:1055-9. [Crossref] [PubMed]
- Piperno A, Mariani R, Arosio C, et al. Haemochromatosis in patients with beta-thalassaemia trait. Br J Haematol 2000;111:908-14. [PubMed]
- Fleming RE, Feng Q, Britton RS. Knockout mouse models of iron homeostasis. Annu Rev Nutr 2011;31:117-37. [Crossref] [PubMed]
- de Tayrac M, Roth MP, Jouanolle AM, et al. Genome-wide association study identifies TF as a significant modifier gene of iron metabolism in HFE hemochromatosis. J Hepatol 2015;62:664-72. [Crossref] [PubMed]
- Tanaka T, Roy CN, Yao W, et al. A genome-wide association analysis of serum iron concentrations. Blood 2010;115:94-6. [Crossref] [PubMed]
- Benyamin B, Esko T, Ried JS, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun 2014;5:4926. [Crossref] [PubMed]
- Pelucchi S, Mariani R, Calza S, et al. CYBRD1 as a modifier gene that modulates iron phenotype in HFE p.C282Y homozygous patients. Haematologica 2012;97:1818-25. [Crossref] [PubMed]
- McLaren CE, Emond MJ, Subramaniam VN, et al. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology 2015;62:429-39. [Crossref] [PubMed]
- Chambers JC, Zhang W, Li Y, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet 2009;41:1170-2. [Crossref] [PubMed]
- Constantine CC, Anderson GJ, Vulpe CD, et al. A novel association between a SNP in CYBRD1 and serum ferritin levels in a cohort study of HFE hereditary haemochromatosis. Br J Haematol 2009;147:140-9. [Crossref] [PubMed]
- Milet J, Le Gac G, Scotet V, et al. A common SNP near BMP2 is associated with severity of the iron burden in HFE p.C282Y homozygous patients: a follow-up study. Blood Cells Mol Dis 2010;44:34-7. [Crossref] [PubMed]
- Barton JC, Chen WP, Emond MJ, et al. GNPAT p.D519G is independently associated with markedly increased iron stores in HFE p.C282Y homozygotes. Blood Cells Mol Dis 2017;63:15-20. [Crossref] [PubMed]
- Greni F, Valenti L, Mariani R, et al. GNPAT rs11558492 is not a Major Modifier of Iron Status: Study of Italian Hemochromatosis Patients and Blood Donors. Ann Hepatol 2017;16:451-6. [Crossref]
- Bardou-Jacquet E, Ben Ali Z, Beaumont-Epinette MP, et al. Non-HFE hemochromatosis: pathophysiological and diagnostic aspects. Clin Res Hepatol Gastroenterol 2014;38:143-54. [Crossref] [PubMed]
- Ryan E, Russell J, Ryan JD, et al. GNPAT variant is not associated with severe iron overload in Irish C282Y homozygotes. Hepatology 2016;63:2055-6. [Crossref] [PubMed]
- Daher R, Kannengiesser C, Houamel D, et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology 2016;150:672-83.e4. [Crossref] [PubMed]
- Schaefer B, Haschka D, Finkenstedt A, et al. Impaired hepcidin expression in alpha-1-antitrypsin deficiency associated with iron overload and progressive liver disease. Hum Mol Genet 2015;24:6254-63. [Crossref] [PubMed]
- Guo S, Jiang S, Epperla N, et al. A gene-based recessive diplotype exome scan discovers. Blood 2019;133:1888-98. [Crossref] [PubMed]
- Gurrin LC, Bertalli NA, Dalton GW, et al. HFE C282Y/H63D compound heterozygotes are at low risk of hemochromatosis-related morbidity. Hepatology 2009;50:94-101. [Crossref] [PubMed]
- Walsh A, Dixon JL, Ramm GA, et al. The clinical relevance of compound heterozygosity for the C282Y and H63D substitutions in hemochromatosis. Clin Gastroenterol Hepatol 2006;4:1403-10. [Crossref] [PubMed]
- Le Gac G, Congiu R, Gourlaouen I, et al. Homozygous deletion of HFE is the common cause of hemochromatosis in Sardinia. Haematologica 2010;95:685-7. [Crossref] [PubMed]
- Pelucchi S, Mariani R, Bertola F, et al. Homozygous deletion of HFE: the Sardinian hemochromatosis? Blood 2009;113:3886. [Crossref] [PubMed]
- Steiner M, Leiendecker-Foster C, McLaren GD, et al. Hemochromatosis (HFE) gene splice site mutation IVS5+1 G/A in North American Vietnamese with and without phenotypic evidence of iron overload. Transl Res 2007;149:92-5. [Crossref] [PubMed]
- Takano A, Niimi H, Atarashi Y, et al. A novel Y231del mutation of HFE in hereditary haemochromatosis provides in vivo evidence that the Huh-7 is a human haemochromatotic cell line. Liver Int 2011;31:1593-7. [Crossref] [PubMed]
- Mariani R, Pelucchi S, Arosio C, et al. Genetic and metabolic factors are associated with increased hepatic iron stores in a selected population of p.Cys282Tyr heterozygotes. Blood Cells Mol Dis 2010;44:159-63. [Crossref] [PubMed]
- Piperno A, Arosio C, Fossati L, et al. Two novel nonsense mutations of HFE gene in five unrelated italian patients with hemochromatosis. Gastroenterology 2000;119:441-5. [Crossref] [PubMed]
- Wallace DF, Subramaniam VN. The global prevalence of HFE and non-HFE hemochromatosis estimated from analysis of next-generation sequencing data. Genet Med 2016;18:618-26. [Crossref] [PubMed]
- Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet 2004;36:77-82. [Crossref] [PubMed]
- Stenson PD, Ball EV, Mort M, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 2003;21:577-81. [Crossref] [PubMed]
- Kong X, Xie L, Zhu H, et al. Genotypic and phenotypic spectra of hemojuvelin mutations in primary hemochromatosis patients: a systematic review. Orphanet J Rare Dis 2019;14:171. [Crossref] [PubMed]
- Koyama C, Hayashi H, Wakusawa S, et al. Three patients with middle-age-onset hemochromatosis caused by novel mutations in the hemojuvelin gene. J Hepatol 2005;43:740-2. [Crossref] [PubMed]
- Ravasi G, Pelucchi S, Mariani R, et al. A severe hemojuvelin mutation leading to late onset of HFE2-hemochromatosis. Dig Liver Dis 2018;50:859-62. [Crossref] [PubMed]
- Piperno A, Roetto A, Mariani R, et al. Homozygosity for transferrin receptor-2 Y250X mutation induces early iron overload. Haematologica 2004;89:359-60. [PubMed]
- Le Gac G, Mons F, Jacolot S, et al. Early onset hereditary hemochromatosis resulting from a novel TFR2 gene nonsense mutation (R105X) in two siblings of north French descent. Br J Haematol 2004;125:674-8. [Crossref] [PubMed]
- Girelli D, Bozzini C, Roetto A, et al. Clinical and pathologic findings in hemochromatosis type 3 due to a novel mutation in transferrin receptor 2 gene. Gastroenterology 2002;122:1295-302. [Crossref] [PubMed]
- Hattori A, Wakusawa S, Hayashi H, et al. AVAQ 594-597 deletion of the TfR2 gene in a Japanese family with hemochromatosis. Hepatol Res 2003;26:154-6. [Crossref] [PubMed]
- Camaschella C, Roetto A, Calì A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet 2000;25:14-5. [Crossref] [PubMed]
- Pietrangelo A. Ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica 2017;102:1972-84. [Crossref] [PubMed]
- Viveiros A, Schaefer B, Tilg H, et al. Iron Matryoshka-Haemochromatosis nested in Ferroportin Disease? Liver Int 2019;39:1014-5. [Crossref] [PubMed]
- Drakesmith H, Schimanski LM, Ormerod E, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood 2005;106:1092-7. [Crossref] [PubMed]
- Zhang W, Xu A, Li Y, et al. A novel SLC40A1 p.Y333H mutation with gain of function of ferroportin: A recurrent cause of haemochromatosis in China. Liver Int 2019;39:1120-7. [Crossref] [PubMed]
- Hayashi A, Wada Y, Suzuki T, et al. Studies on familial hypotransferrinemia: unique clinical course and molecular pathology. Am J Hum Genet 1993;53:201-13. [PubMed]
- Beutler E, Gelbart T, Lee P, et al. Molecular characterization of a case of atransferrinemia. Blood 2000;96:4071-4. [Crossref] [PubMed]
- Trenor CC, Campagna DR, Sellers VM, et al. The molecular defect in hypotransferrinemic mice. Blood 2000;96:1113-8. [Crossref] [PubMed]
- Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med 1987;110:690-705. [PubMed]
- Trombini P, Coliva T, Nemeth E, et al. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica 2007;92:1407-10. [Crossref] [PubMed]
- Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med 2019;133:55-63. [Crossref] [PubMed]
- Camaschella C. DMT1 mutations: mice and humans are not alike. Blood 2005;105:916-17. [Crossref]
- Iolascon A, De Falco L. Mutations in the gene encoding DMT1: clinical presentation and treatment. Semin Hematol 2009;46:358-70. [Crossref] [PubMed]
- Fleming MD, Romano MA, Su MA, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A 1998;95:1148-53. [Crossref] [PubMed]
- Vashchenko G, MacGillivray RT. Multi-copper oxidases and human iron metabolism. Nutrients 2013;5:2289-313. [Crossref] [PubMed]
- Piperno A, Alessio M. Aceruloplasminemia: Waiting for an Efficient Therapy. Front Neurosci 2018;12:903. [Crossref] [PubMed]
- Rice AE, Mendez MJ, Hokanson CA, et al. Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol 2009;386:717-32. [Crossref] [PubMed]
- Pelucchi S, Mariani R, Salvioni A, et al. Novel mutations of the ferroportin gene (SLC40A1): analysis of 56 consecutive patients with unexplained iron overload. Clin Genet 2008;73:171-8. [Crossref] [PubMed]
- Le Lan C, Mosser A, Ropert M, et al. Sex and acquired cofactors determine phenotypes of ferroportin disease. Gastroenterology 2011;140:1199-207.e1-2.
- Miyajima H, Nishimura Y, Mizoguchi K, et al. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 1987;37:761-7. [Crossref] [PubMed]
- Miyajima H, Kohno S, Takahashi Y, et al. Estimation of the gene frequency of aceruloplasminemia in Japan. Neurology 1999;53:617-9. [Crossref] [PubMed]
- Musci G, Polticelli F, Bonaccorsi di Patti MC. Ceruloplasmin-ferroportin system of iron traffic in vertebrates. World J Biol Chem 2014;5:204-15. [PubMed]
- Jeong SY, David S. Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J Neurosci 2006;26:9810-9. [Crossref] [PubMed]
- Milman N, Byg KE, Ovesen L. Iron status in Danes 1994. II: Prevalence of iron deficiency and iron overload in 1319 Danish women aged 40-70 years. Influence of blood donation, alcohol intake and iron supplementation. Ann Hematol 2000;79:612-21. [Crossref] [PubMed]
- Milman N, Byg KE, Ovesen L, et al. Iron status in Danish men 1984-94: a cohort comparison of changes in iron stores and the prevalence of iron deficiency and iron overload. Eur J Haematol 2002;68:332-40. [Crossref] [PubMed]
- Pelucchi S, Mariani R, Ravasi G, et al. Phenotypic heterogeneity in seven Italian cases of aceruloplasminemia. Parkinsonism Relat Disord 2018;51:36-42. [Crossref] [PubMed]
- Brissot P, Bardou-Jacquet E, Jouanolle AM, et al. Iron disorders of genetic origin: a changing world. Trends Mol Med 2011;17:707-13. [Crossref] [PubMed]
- Goldwurm S, Casati C, Venturi N, et al. Biochemical and genetic defects underlying human congenital hypotransferrinemia. Hematol J 2000;1:390-8. [Crossref] [PubMed]
- Riva A, Trombini P, Mariani R, et al. Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J Gastroenterol 2008;14:4745-52. [Crossref] [PubMed]
- Ravasi G, Pelucchi S, Mariani R, et al. Unexplained isolated hyperferritinemia without iron overload. Am J Hematol 2017;92:338-43. [Crossref] [PubMed]
- Regenboog M, van Kuilenburg AB, Verheij J, et al. Hyperferritinemia and iron metabolism in Gaucher disease: Potential pathophysiological implications. Blood Rev 2016;30:431-7. [Crossref] [PubMed]
- Aguilar-Martinez P, Schved JF, Brissot P. The evaluation of hyperferritinemia: an updated strategy based on advances in detecting genetic abnormalities. Am J Gastroenterol 2005;100:1185-94. [Crossref] [PubMed]
- Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 2000;343:327-31. [Crossref] [PubMed]
- Wood JC. Estimating tissue iron burden: current status and future prospects. Br J Haematol 2015;170:15-28. [Crossref] [PubMed]
- Henninger B, Alustiza J, Garbowski M, et al. Practical guide to quantification of hepatic iron with MRI. Eur Radiol 2020;30:383-93. [PubMed]
- Galimberti S, Trombini P, Bernasconi DP, et al. Simultaneous liver iron and fat measures by magnetic resonance imaging in patients with hyperferritinemia. Scand J Gastroenterol 2015;50:429-38. [Crossref] [PubMed]
- Bassett ML, Hickman PE, Dahlstrom JE. The changing role of liver biopsy in diagnosis and management of haemochromatosis. Pathology 2011;43:433-9. [Crossref] [PubMed]
- Fu F, Li X, Chen C, et al. Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom Radiol (NY) 2020;45:73-82. [PubMed]
- McDonald CJ, Ostini L, Wallace DF, et al. Next-generation sequencing: Application of a novel platform to analyze atypical iron disorders. J Hepatol 2015;63:1288-93. [Crossref] [PubMed]
- Badar S, Busti F, Ferrarini A, et al. Identification of novel mutations in hemochromatosis genes by targeted next generation sequencing in Italian patients with unexplained iron overload. Am J Hematol 2016;91:420-5. [Crossref] [PubMed]
- Adams PC, Barton JC. A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation. J Hepatol 2011;55:453-8. [Crossref] [PubMed]
- Sheth S. Iron chelation: an update. Curr Opin Hematol 2014;21:179-85. [Crossref] [PubMed]
- Adams PC, Barton JC. How I treat hemochromatosis. Blood 2010;116:317-25. [Crossref] [PubMed]
- Mariani R, Pelucchi S, Perseghin P, et al. Erythrocytapheresis plus erythropoietin: an alternative therapy for selected patients with hemochromatosis and severe organ damage. Haematologica 2005;90:717-8. [PubMed]
- Creighton Mitchell T, McClain DA. Diabetes and hemochromatosis. Curr Diab Rep 2014;14:488. [Crossref] [PubMed]
- Pasricha SR, Lim PJ, Duarte TL, et al. Hepcidin is regulated by promoter-associated histone acetylation and HDAC3. Nat Commun 2017;8:403. [Crossref] [PubMed]
- Porto G, Brissot P, Swinkels DW, et al. EMQN best practice guidelines for the molecular genetic diagnosis of hereditary hemochromatosis (HH). Eur J Hum Genet 2016;24:479-95. [Crossref] [PubMed]
- Liver EAFTSOT. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53:3-22. [Crossref] [PubMed]
- Pantopoulos K. Inherited Disorders of Iron Overload. Front Nutr 2018;5:103. [Crossref] [PubMed]
- Adams P, Altes A, Brissot P, et al. Therapeutic recommendations in HFE hemochromatosis for p.Cys282Tyr (C282Y/C282Y) homozygous genotype. Hepatol Int 2018;12:83-6. [Crossref] [PubMed]
- Phatak P, Brissot P, Wurster M, et al. A phase 1/2, dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis. Hepatology 2010;52:1671-779. [Crossref] [PubMed]
- Liu J, Sun B, Yin H, et al. Hepcidin: A Promising Therapeutic Target for Iron Disorders: A Systematic Review. Medicine (Baltimore) 2016;95:e3150. [Crossref] [PubMed]
Cite this article as: Piperno A, Pelucchi S, Mariani R. Inherited iron overload disorders. Transl Gastroenterol Hepatol 2020;5:25.


