
Hepatobiliary cancers and immunotherapy: where are we now and where are we heading?
Introduction
Liver cancer is a broad term encompassing primary malignancy of the liver. Metastatic tumors to the liver are managed based on the primary tumor location and stage. For the purposes of this article, we will discuss primary malignancies of the liver and adjacent biliary tract. This includes hepatocellular carcinoma (HCC), intrahepatic and extrahepatic cholangiocarcinoma (CCA), and gallbladder cancer (GBC). Due to their rarity, we will not include discussion of angiosarcoma and hemangiosarcoma. Liver and intrahepatic bile duct cancers represent the 13th most common cancer type in the US, with an estimated 42,030 new cases in 2019 (2.4% of new cancers in US in 2019), accounting for 31,780 estimated deaths in 2019 (5.2% of all cancer deaths) (1). There were an estimated 83,081 people living with liver and intrahepatic bile duct cancer in 2016 with 5-year survival of 18.4% (1). It is more common in men compared with women, across all races, with an average age of 64 years old at diagnosis (1). The incidence and rate of death have been rising since 1975, with an average increase of 2.1% annually in the rates of new diagnoses and an average of 2.4% annually in death rates over each of the last 10 years (1).
Since the first approval for ipilimumab in 2011 for the treatment of BRAF-negative metastatic melanoma there has been a steady stream of approvals for antibodies targeting of either programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) across numerous malignancies, reshaping the field of oncology (2-5). Now, more than 1,000 immunotherapy clinical trials later, we are exploring their uses in countless malignancies in first, second and later-line metastatic disease, as well as in the adjuvant setting. This review article will focus on the use of the currently studied, approved uses and the future roles of these agents in the treatment of cancers of the hepatobiliary system.
Current roles for systemic therapies in the treatment paradigm of HCC
Beyond that of resectable HCC, and locoregional therapy (chemoembolization, radioembolization…, etc.) the role for systemic therapies has been investigated both in the adjuvant and advanced, unresectable setting.
The treatment of advanced, unresectable HCC has been challenging; cytotoxic chemotherapy regimens for advanced HCC used to consist of single agent anthracyclines (namely doxorubicin) and fluoropyrimidines (such as 5-fluorouricil); however, their clinical benefit has been inconsistent. Multi-agent chemotherapy with traditional gastrointestinal malignancy regimens have been studied, namely FOLFOX. The EACH Trial, comparing FOLFOX and doxorubicin, found a benefit in progression-free survival (PFS) with a trend in improved overall survival (OS), but did not reach significance, resulting in a modest 1.5 months (mos) survival benefit (6). Since 2008, the mainstay of systemic treatment of both unresectable, locally advanced and metastatic HCC has largely been tyrosine kinase inhibitors (TKIs) and cytotoxic chemotherapy. Sorafenib became the standard of care for advanced, unresectable HCC after publication of the SHARP Trial results, showing a significant OS benefit (~2.5 mos) and time to radiologic progression (~2.7 mos) compared with placebo in patients with Child-Pugh A liver disease (7). The success of sorafenib in this setting prompted the evaluation of its use in the adjuvant setting, with disappointing results (8). Nearly a decade later, sorafenib remained the unchallenged front-line therapy. Until, in 2018, lenvatinib was approved for front-line treatment after a non-inferiority study comparing it to sorafenib (9).
Even more disappointing was the time before a viable second-line agent treatment was approved. There were limited advances or alternatives in the treatment of HCC after 2008 in targeted, or non-cytotoxic agents, until 2017, when regorafenib, an oral multi-kinase inhibitor, was approved for the second-line, after failure of sorafenib (10).
In 2019, ramucirumab, a direct VEGFR2 antagonist, gained approval in treatment of advanced, unresectable HCC who have an alpha fetoprotein of at least 400 ng/mL after failure on sorafenib (11).
Meanwhile, as immunotherapy was gaining steam in other disease states after 2011, it was not until 2017, after the publication of the initial results of the dose-escalation and dose-expansion study, CheckMate-040 (NCT01658878), that nivolumab was approved for use in HCC (12,13). El-Khoueiry et al. enrolled 262 total patients with advanced HCC and Child-Pugh A or B7 cirrhosis, regardless of Hepatitis B or C infection status, between the two phases of the trial (see Table 1) (12,13). These patients were previously treated with sorafenib, and became intolerant of treatment, or had progression of their disease (12,13). The results showed promise in these previously pre-treated patients, with an objective response rate (ORR) of 20%, complete response (CR) of 1% and disease control rate (DCR) of 64% (12,13). Median PFS (mPFS) 4.0 mos with a median duration of response (mDOR) of 9.9 mos (12,13). The study also showed treatment related toxicities similar to prior studies of nivolumab in other diseases, with grade 3 or greater treatment-related adverse effects (TRAEs) were seen in 19% of recipients (12,13). Six- and 9-mo OS rates were 83% and 74%, respectively (12,13). Median OS (mOS) was not reached at time of publication (12,13). Of the 262 patients enrolled between the two phases of this study, 75.9% of patients had received prior systemic therapy, with a large proportion receiving sorafenib previously (12,13). Three percent (3%) of patients combined between the two phases discontinued treatment due to drug toxicity (12,13).

Full table
The approval of nivolumab was later followed by the approval of pembrolizumab in 2018, based on the results of KEYNOTE-224 (NCT02702414) (15,16). Zhu et al. enrolled 104 patients with advanced HCC with Child-Pugh class A cirrhosis, regardless of hepatitis B or C viral status, who were previously treated with sorafenib and were intolerant to treatment, or showed progression of their disease (see Table 1) (15,16). Similar response rates to nivolumab were seen, with ORR of 17%, CR of 1%, and DCR of 62% (15,16). However, mDOR was not reached, mPFS was 4.9 mos and mOS was 12.9 mos (15,16). Pembrolizumab exhibited similar toxicity rates to other studies evaluating its use with 26% of patients experiencing grade 3 or greater TRAEs, but only 5% of patients requiring discontinuation of treatment due to TRAE (15,16).
CheckMate-040 and KEYNOTE-224, along with KEYNOTE-016, have served integral parts in establishing the role of immunotherapy in the treatment paradigm for HCC and biliary tract cancer (BTC).
Current treatment paradigm of BTCs (intrahepatic & extrahepatic CCA, GBCs)
The treatment paradigm for BTCs is quite limited despite the heterogeneous collection of malignancies in this category. First-line treatment for unresectable and metastatic disease utilizes cytotoxic chemotherapy with combined regimens, namely gemcitabine and cisplatin, although other fluoropyrimidine-based or gemcitabine-based regimens can be considered. A recent phase 2 study by Shroff et al. evaluated the addition of Nab-paclitaxel to combination cisplatin and gemcitabine in 62 patients with advanced BTCs and showed promising early results: DCR of 84%, mPFS 11.8 mos, and mOS of 19.2 mos (17). However, these patients experienced significant toxicities, including 58% with grade 3 or higher TRAEs, with 16% of patients discontinuing therapy as a result of their toxicities (17). Additionally, Lamarca et al. presented results of the Phase III ABC-06 study (NCT01926236) comparing active symptom management alone and active symptom management with mFOLFOX for locally advanced, or metastatic, BTCs in 162 patients previously treated with cisplatin and gemcitabine (18,19). Lamarca et al. report clinically significant improvements were reported in mOS (6.2 vs. 5.3 mos), 6-mo (50.6% vs. 35.5%) and 12-mo OS (25.9% vs. 11.4%) with mFOLFOX and active symptom management compared with active symptom management alone, although confidence intervals or P values were not reported (18,19). Additionally, grade 3–4 TRAEs were experienced by 59% of patients receiving mFOLFOX and 39% in those who did not, with no treatment-related deaths in either arm (18,19). Given the results of this study, and no current evidence-based second-line treatments in BTCs, the authors assert that mFOLFOX should become considered standard of care for second-line therapy in BTCs (18,19). Due to the limited effective treatment options, enrollment in clinical trials for eligible patients, or best supportive care for those who are not candidates for systemic treatment is also recommended.
In patients with resectable disease, adjuvant treatment with combinations of fluoropyrimidine-based or gemcitabine-based regimens with or without concurrent radiation therapy depending on nodal and resection status after primary resection represent the current standards of care. Clinical trial enrollment is also recommended in both settings. However, observation could also be considered with R0 resection and negative regional lymph nodes.
Rationale for use of immunotherapy in treatment in hepatobiliary cancers
Prior to 2017, the use of immunotherapy was considered experimental and was often only available as either compassionate use or if enrolled on clinical trial. However, since then, hepatobiliary cancers have seen three FDA approvals following the publication of key early phase trials in the last two years. First, pembrolizumab gained approval for either microsatellite instability-high (MSI-H) or DNA mismatch repair deficient (dMMR) unresectable, or metastatic solid tumors in May 2017 (14,20). This approval came in the wake of a study by Le et al. (see Table 1). evaluating patients with mismatch repair-deficient malignancies, after this signal was seen in colorectal cancers (14,20). ORR was seen in 53% of patients with dMMR malignancies across 12 different tumor types, including HCC and BTCs (14). Mismatch repair deficiencies are seen in between 2–3% of HCCs and BTCs (14).
Later, nivolumab and pembrolizumab were both studied in the second-line setting in advanced HCC, independent of tumor PD-L1 expression (12,13,15,16). Following CheckMate-040, nivolumab gained approval in late 2017 in the second-line setting for patients with advanced HCC and Child-Pugh A or B7 liver disease (12,13). CheckMate-040 has also gone on to study combination immunotherapy with nivolumab and Ipilimumab, which is discussed later in this review. Pembrolizumab was granted FDA approval in 2018 after publication of KEYNOTE-224 for patients with advanced HCC and Child-Pugh A liver disease (15,16). The phase 3 KEYNOTE-240 study (NCT02702401) followed these early auspicious studies to further assess the utility and safety of pembrolizumab compared with placebo in patients with advanced HCC after failing first-line treatment in 413 enrolled patients (21,22). However, pembrolizumab did not meet pre-specified endpoints for OS and PFS (see Table 2) despite a 22% reduction in the risk of death compared with placebo (21,22). This lack of significant improvement was felt to be due to the high rates of subsequent anticancer treatment in the placebo arm compared with treatment arm (47% vs. 42%) (21,22). Despite the lack of significant survival benefit, pembrolizumab did show an improved ORR compared with placebo (16.9% vs. 2.2%) with a mDOR of 13.8 mos at 13.8 mos follow-up (21,22). The safety profile was reported to be similar to prior pembrolizumab studies, namely KEYNOTE-224 (21,22).
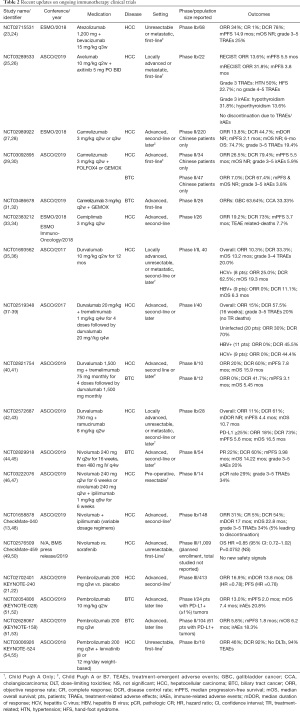
Full table
With these approvals, immunotherapy entered the treatment algorithm for HCC and BTC, including inclusion as subsequent treatment options after failure of first-line therapy. However, little was known about immunotherapy’s role in resectable disease, following locoregional therapy or first-line in advanced disease. Since the approvals above, further studies have been undertaken to answer these questions.
Future directions of immunotherapy in hepatobiliary cancers
Several established and novel immune checkpoint inhibitors (ICIs) are being evaluated for use is HCC and BTC in both the resectable and advanced setting, first-line and after failed systemic therapies. Some agents are also being assessed in combination with TKIs and/or chemotherapy. Currently, there are nine [9] different ICIs are being evaluated for efficacy and safety in HCC and BTCs. Below, we will discuss each of these agents, their recent clinical trial updates and future phase III studies on the horizon for select agents with the information summarized in Tables 2 and 3 respectively.
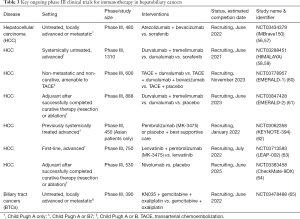
Full table
Atezolizumab
Atezolizumab has been assessed in a phase Ib study in combination with bevacizumab in the first-line setting for advanced HCC with up to Child-Pugh B7 liver disease (24). This study showed promising early findings with ORR of 34% with one CR (23,24). mPFS was 14.9 mos, while mOS and mDOR had not been reached after at least 18 weeks of follow-up (24). Furthermore, the treatment combination appeared to be well tolerated in this population, with grade 3 or higher TRAEs occurring in 25% of patients; only 6% of patients required corticosteroids for immune-related adverse events (irAEs) (24). This study was limited to patients in Asia, limiting its generalizability. However, given these findings suggesting tolerability and promising responses, the phase III IMBrave150 Trial is underway to further evaluated the use of this combination compared with sorafenib (56,57).
Avelumab
Avelumab is an anti-PD-L1 monoclonal antibody currently FDA approved for use in Merkel cell carcinoma. Its role in other advanced solid tumors is yet to be established, with ongoing clinical trials investigating its efficacy and safety in various disease states, including HCC. Kudo et al. recently presented early results from their phase Ib study evaluating the combination of first-line avelumab and axitinib at the ASCO 2019 Annual Meeting (25,26). The combination of these treatments yielded early promising responses of tumor activity (ORR 31.8% by mRECIST and 13.6% by RECIST) and toxicity profiles similar to individual medications without any discontinuations due to medication toxicities (25,26). OS data was immature with estimated study completion in June 2020 (25,26).
Camrelizumab
Other novel immunotherapies have recently been studied in both the first- and later-line settings. Two phase II studies with the novel PD-1 antibody, camrelizumab (SHR-1210), both alone and in combination with combination chemotherapy in advanced HCC signaled tolerability and potential efficacy (27-30). The first is a study of single agent camrelizumab (SHR-1210) in advanced, previously treated HCC in Chinese patients (27,28). The primary end-points were 6-mo OS and confirmed ORR (27,28). Study participants were randomized to 3 mg/kg IV every 2 weeks or every 3 weeks (27,28). The study showed similar ORR response rates to nivolumab in the later-line setting, with acceptable toxicities. Interestingly, camrelizumab 3 mg/kg every 3 weeks showed a trend towards higher ORR (16.7% vs. 11.0%) and less grade 3 or higher TRAEs (6.5% vs. 12.8%), but lower 6-mo OS (73.1% vs. 76.1%), although significance was not reported (27,28).
Another phase II multicenter study of camrelizumab in combination with chemotherapy (GEMOX or FOLFOX4) in treatment naïve patients with either advanced HCC or BTC (29,30). The primary endpoints of this study were confirmed ORR and safety. This study has another ongoing study arm evaluating the combination of camrelizumab with apatinib (VEGF-2 TKI) in chemotherapy pretreated patients (30). The ORRs were 26.5% in HCC and 7% in BTC in the first-line setting and grade 3 or higher irAEs were rare, 5.9% in HCC and 3.8% in BTC (29). This study suggests tolerability and efficacy (29). Another study evaluating the combination of GEMOX and camrelizumab in first-line treatment of BTCs reported more promising results (31,32). With twelve patients (46.15%) achieving a partial response (31,32). ORRs were higher in patients with GBC than CCA, 64% vs 33% respectively, although this did not reach statistical significance (31,32). Furthermore, 19 of 26 patients had next-generation sequencing performed on tissue samples, with GBCs showing a higher median tumor mutational burden (TMB) than CCA, although this did not reach statistical significance (31,32). When assessing ORR based on TMB, those with high TMB (>8.6 mut/Mb), had significantly higher ORR (100% vs. 26%) (31,32).
Cemiplimab
Cemiplimab (REGN2810), an anti-PD-1 agent currently FDA-approved in locally advanced or metastatic cutaneous squamous cell carcinoma, was evaluated in a small, phase I dose escalation study in advanced malignancies who had failed prior systemic therapy (33,34). Results from the HCC expansion cohort were presented at two 2018 ESMO meetings (33,34). The study cohort consisted of 26 patients with median follow-up of 7.2 mos (33,34). Partial responses were seen in 19.2% of patients with stable disease in 53.8% of patients with mPFS of 3.7 mos (33,34). Two patients (7.7%) had a treatment-emergent adverse event (TEAE) resulting in death (33,34).
Durvalumab
Durvalumab (MEDI4736) is another anti-PD-L1 monoclonal antibody under investigation in HCC, BTCs, and other solid malignancies. Its only current FDA approvals are in unresectable stage III non-small cell lung cancer that has not progressed (i.e., maintenance) after concurrent platinum-based chemotherapy and radiation therapy and in locally advanced or metastatic urothelial cancer following progression on platinum-containing chemotherapy or within 12 mos of receiving platinum-containing chemotherapy perioperatively (neoadjuvant or adjuvant). Durvalumab is being evaluated as monotherapy and in combination with ramucirumab, a VEGFR2 inhibitor, in advanced HCC, and as part of combination immune checkpoint inhibition with tremelimumab, a CTLA-4 inhibitor, in both BTC and HCC (35-43).
In a phase I/II study of durvalumab monotherapy in advanced HCC patients with Child-Pugh A liver disease, ORR (10.3%) was slightly lower compared with other approved ICIs; however, in patients with hepatitis C virus (HCV) infections, ORR (25.0%) was comparable, or slightly better, with similar rates of TRAEs (see Table 2) (35,36).
Two early phase studies are investigating combination immune checkpoint inhibition with durvalumab and tremelimumab in patients with advanced HCC or BTC who received, or refused, at least one prior therapy (40,41). Amongst the 10 patients with advanced HCC, ORR was 20% with mPFS of 7.8 mos and mOS of 15.9 mos (40,41). None of the 12 patients with advanced BTC had objective responses; which was coupled with poor mPFS (3.1 mos) and mOS (5.45 mos), reflective of the grim prognosis with advanced BTC after failing first-line treatment (40,41). These results were similar to outcomes with pembrolizumab in advanced, later-line treatment of BTC, but worse when compared with nivolumab. Kelley et al. published the Phase I safety and efficacy analysis for this combination ICI in unresectable advanced HCC, including 93% with Child-Pugh A liver disease (37-39). ORR (15%) was similar to other single agent approved ICIs (37-39). However, in contrast to durvalumab monotherapy, ORR was higher amongst the 20 uninfected patients, 30%; while none of the hepatitis B virus (HBV) or HCV infected patients had confirmed ORR (see Table 2) (37-39).
The last doublet to discuss is combination of durvalumab and the VEGF2 inhibitor, ramucirumab as part of a basket study included a cohort of advanced HCC (42,43). ORR was modest (11%) amongst all 28 enrolled patients, although slightly better (18%) in the 11 patients with “high” PD-L1 expression (greater than or equal to 25% of tumor cells and/or immune cells) (42,43). mPFS had similar results amongst all patients and those with high PD-L1 expression (4.4 and 5.6 mos, respectively), as did mOS (10.7 and 16.5 mos, respectively) (42,43).
Due to the promise of these early phase studies, there are multiple phase 3 studies evaluating the use of durvalumab in various settings (see Table 3). The first is the phase 3 HIMALAYA study (NCT03298451) evaluating durvalumab and tremelimumab compared with sorafenib as well as durvalumab monotherapy in the first-line setting in unresectable HCC is underway (see Table 3) (58,59). Two ongoing phase III studies are investigating the use of durvalumab alone, and in combination with other targeted agents in combination with other interventional procedures [transarterial chemoembolization (TACE) and surgery], EMERALD-1 (NCT03778957) and EMERALD-2 (NCT03847428) (60,61). EMERALD-1 is assessing the efficacy and safety of the combination of TACE with durvalumab alone, or in combination with bevacizumab, compared with placebo in patients with Child Pugh A or B7 liver disease and non-metastatic, non-resectable HCC (60). Meanwhile, EMERALD-2 will look at the use of immunotherapy in the adjuvant setting after successful curative therapy (surgery or ablation) for patients with HCC and Child Pugh A liver disease with durvalumab alone or in combination with tremelimumab compared with placebo (61).
KN035
The novel, subcutaneous anti-PD-L1 monoclonal antibody is currently under investigation with no current FDA approvals in HCC or BTC. It is currently being studied in patients with Child Pugh A or B liver disease and untreated, locally advanced or metastatic BTCs in combination with gemcitabine and oxaliplatin compared with chemotherapy alone in an ongoing phase 3 study (NCT03478488) with an estimated completion date of June 2022 (65).
Nivolumab
At the 2019 ASCO Annual Meeting, early data from CheckMate-040 (NCT01658878) showed significant promise with the use of combination immunotherapy in the treatment of advanced, unresectable HCC after treatment with sorafenib (see Table 2) (13,48). The combination of nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four cycles, followed by nivolumab 240 mg every 2 weeks showed the most promise (13,48). This treatment arm saw mOS of 22.8 mos, ORR 31% (compared with 14% for single agent nivolumab), 5% with CR and 26% with PR, DCR of 54%, mDOR of 17 mos with 34% grade 3 or greater TRAEs, with low rates of discontinuation for toxicity (5%) at median follow-up of 24 mos (13,48).
Nivolumab was further evaluated after failure of first-line systemic therapy for BTCs [63% intrahepatic cholangiocarcinoma (IHC), 11% extrahepatic cholangiocarcinoma (EHC), 26% GBC] in a phase II study by Kim et al. (NCT02829918) (44,45). The study enrolled 54 patients, with 45 patients evaluated for response (44,45). Patients had a median age of 65 years (44,45). The study found a DCR of 60% with 20% grade 3 or 4 TRAEs (44,45). All of the patients who responded were microsatellite stable. Furthermore, none of the patients who experienced TRAEs required discontinuation of nivolumab at median follow-up of 13.34 mos (44,45).
Some early promising data for the use of nivolumab +/− ipilimumab in the pre-operative (neoadjuvant) setting (NCT03222076) in 14 evaluable patients showing 29% pathologic CR (pCR) with nivolumab or nivolumab and Ipilimumab with 34% TRAEs (46,47). Due in part to this promise, nivolumab is currently under investigation in the phase III CheckMate-9DX study (NCT033833458) as adjuvant treatment after curative therapy (surgery or ablation) in patients with Child Pugh A liver disease and HCC compared with placebo (64).
More recently in June 2019, Bristol-Myers Squibb (BMS), the manufacturer of both nivolumab and ipilimumab, announced results from CheckMate-459 (NCT02576509) (49,50). This randomized, phase III study comparing nivolumab and sorafenib in the first-line treatment of advanced, unresectable HCC had a primary endpoint of OS with a goal enrollment of 1,009 (see Table 2) (49,50). The press release notes that the trial did not achieve statistical significance for OS based on a pre-specified analysis (49,50). The hazard ratio (HR) for OS was 0.85 (95% CI: 0.72–1.02) with P=00752 (49,50). The release does not note the total number of patients evaluated in the analysis, or details about median follow-up (49,50). It is also noted that no new safety signals were seen with nivolumab and that full study results will be presented at an future medical conference (49,50).
Pembrolizumab
Pembrolizumab has a number of ongoing studies assessing its efficacy and safety in advanced HCC and BTC. Most notably is the phase 3 KEYNOTE-240 study (NCT02702401) investigating the use of pembrolizumab’s utility and safety in patients with advanced HCC after failing first-line treatment compared to placebo, discussed in detail above (21,22). Additionally, both the KEYNOTE-028 (NCT02054806) and -158 (NCT02628067) basket studies included a cohort of patients with advanced BTCs who have failed at least one prior treatment (51-53). PD-L1 positivity (≥1%) was required in KEYNOTE-028, but not for KEYNOTE-158 (51-53). This resulted in modest ORR (13.0% vs. 5.8%), mOS (7.4 vs 6.2 mos), and PFS (2.0 vs. 1.8 mos) differences, respectively (51-53). However, the rates of irAE were fairly similar (20.8% vs. 18.3%) (51-53). These findings are in keeping with a prospective cohort study out of South Korea (66). The phase III KEYNOTE-394 study (NCT03062358) investigating the role of pembrolizumab compared with placebo and best supportive care in Asian patients with Child Pugh A liver disease and advanced, previously systemically treated HCC amongst is currently ongoing (62).
Given the non-inferiority of lenvatinib compared with sorafenib, additional first-line studies to assess the combination of lenvatinib and pembrolizumab have been undertaken. Initially, a phase Ib study, part of KEYNOTE-524, evaluated the tolerability of this combination in patients with Child-Pugh A liver disease with no dose-limiting toxicities or discontinuation of therapy amongst the 18 patients receiving this combination at time of interim analysis in 2018 (54,55). Furthermore, it showed promising early ORR (46%), prompting the development of the phase III LEAP-002 study to assess efficacy and safety compared with first-line lenvatinib monotherapy (54,55,63).
Tremelimumab
Tremelimumab (formerly ticilimumab, CP-675,206) is an anti-CTLA-4 monoclonal antibody without any FDA approvals, but has recently received orphan drug status for mesothelioma. Its role in HCC and BTC is under investigation as both monotherapy and in combination therapy. A pilot study by Sangro et al. evaluated its safety and role in patients with advanced HCC and HCV infection (67,68). Tremelimumab resulted in partial response of 17.6%, DCR of 76.4% and time to progression (TTP) of 6.48 mos with no patients requiring steroids for irAEs (67,68). Tremelimumab has since been studied in combination with the PD-L1 inhibitor, durvalumab in advanced HCC in the second-line (or later) setting (37-39). Another phase 1/2 study evaluating combination ICI with durvalumab and tremelimumab in patients with advanced HCC or BTC who received, or refused, at least one prior therapy has recently released some early results (40,41). Kelley et al. published the phase I safety and efficacy analysis for this combination. The results of this phase 1/2 study are discussed above (see section on durvalumab) (37-39).
Due to the promise of these early phase studies, the phase 3 HIMALAYA study (NCT03298451) evaluating durvalumab and tremelimumab compared with sorafenib as well as durvalumab monotherapy in the first-line setting in unresectable HCC is underway (see Table 3) (58,59). Additionally, this combination of ICIs is being studied in the phase III EMERALD-2 study (NCT03847428) for efficacy and safety of immunotherapy in the adjuvant setting after successful curative therapy (surgery or ablation) for HCC compared with placebo (61).
Conclusions
Primary liver cancer and other BTCs are a heterogenous collection of diseases with limited effective treatment options. The treatment of advanced disease has had limited advances until recent years with the discovery of immunotherapy being. The approval of pembrolizumab in both MSI-H or dMMR-deficient advanced BTC and HCC, has provided an alternative treatment option in a select number of patients. However, the addition of pembrolizumab and nivolumab in advanced HCC with limited liver disease, agnostic of PD-L1 expression, has bolstered the sparse armamentarium of treatment options in patients who have failed prior systemic therapy. However, the full extent of the benefit of immunotherapies has not yet been fully established. The early signals of efficacy with manageable toxicity profiles seen in several other agents, as well as nivolumab and pembrolizumab in various settings (perioperative, advanced disease: first-line or beyond, etc.) within HCC and BTC provide home for the future. In addition, the number of ongoing phase III studies that are nearing completion in the next 5 years provide a glimpse of what may be ahead in these devastating and difficult to treat collection of diseases. Their results will hopefully provide a vast array of options within the treatment paradigm of advanced hepatobiliary cancers.
Acknowledgments
None.
Footnote
Conflicts of Interest: K Almhanna, MD, MPH receives consulting fees from Merck. A Zayac, MD has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer: National Cancer Institute (NCI); 2019. Available online: https://seer.cancer.gov/statfacts/html/livibd.html
- Ipilimumab (Yervoy): US Food & Drug Administration; 2011 [updated May 8, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03695952?term=pembrolizumab&cond=Hepatobiliary+Cancer&rank=1
- Nivolumab (Opdivo): US Food & Drug Administration; 2014 [updated May 2, 2019. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125554
- Pembrolizumab (Keytruda): 2014; 2014 [updated April 19, 2019]. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
- Atezolizumab (Tecentriq): US Food & Drug Administration; 2016 [updated May 6, 2019. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761034
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-8. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Bang Y, Doi T, Kondo S, et al. Updated results from a phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-Beta, in patients with pretreated recurrent or refractory gastric cancer. Ann Oncol 2018;29:viii205-70.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- FDA grants accelerated approval to pembrolizumab for advanced gastric cancer: US Food & Drug Administration; 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-advanced-gastric-cancer
- Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:824-30. [Crossref] [PubMed]
- Lamarca A, Palmer DH, Wasan HS, et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 2019;37:abstr 4003.
- Active Symptom Control Alone or with mFOLFOX Chemotherapy for Locally Advanced/ Metastatic Biliary Tract Cancers. Available online: https://ClinicalTrials.gov/show/NCT01926236
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019;37:abstr 4004.
- Study of Pembrolizumab (MK-3475) vs. Best Supportive Care in Participants with Previously Systemically Treated Advanced Hepatocellular Carcinoma (MK-3475-240/KEYNOTE-240). Available online: https://ClinicalTrials.gov/show/NCT02702401
- A Study of the Safety and Efficacy of Atezolizumab Administered in Combination With Bevacizumab and/or Other Treatments in Participants With Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02715531
- Pishvaian MJ, Lee MS, Ryoo BY, et al. Updated safety and clinical activity results from a Phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). ESMO 2018 Congress; October 21, 2018.
- Kudo M, Motomura K, Wada Y, et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). J Clin Oncol 2019;37:abstr 4072.
- A Study Of Avelumab In Combination With Axitinib In Advanced HCC (VEGF Liver 100). Available online: https://ClinicalTrials.gov/show/NCT03289533
- A Study to Evaluate SHR-1210 in Subjects With Advanced HCC. Available online: https://ClinicalTrials.gov/show/NCT02989922
- Qin SK, Ren ZG, Meng ZQ, et al. A Randomized Multicentered Phase 2 Study to Evaluate SHR-1210 (PD-1 Antibody) in Subjects with Advanced Hepatocellular Carcinoma (HCC) who Failed or Intolerant to Prior Systemic Treatment. ESMO 2018 Congress; 2018.
- Qin S, Chen Z, Liu Y, et al. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol 2019;37:abstr 4074.
- A Study of SHR-1210 in Combination with Apatinib or Chemotherapy in Subjects With Advanced PLC or BTC. Available online: https://ClinicalTrials.gov/show/NCT03092895
- Chen X, Wu X, Wu H, et al. SHR-1210 plus GEMOX as first line treatment in biliary tract cancer: Results from a single-arm exploratory study. J Clin Oncol 2019;37:abstr 4092.
- SHR-1210 in Combination with GEMOX in Patients With Advanced BTC. Available online: https://ClinicalTrials.gov/show/NCT03486678
- Pishvaian MJ, Weiss GJ, Falchook GS, et al. Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): Data from an expansion cohort in a Phase I Study. Ann Oncol 2018;29:viii400-41.
- He AR, Weiss GJ, Falchook G, et al. Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): Data from an expansion cohort (EC) in a phase I study. Ann Oncol 2018;29:x24-38. [Crossref]
- Albertsson M, Johansson B, Friesland S, et al. Phase II studies on docetaxel alone every third week, or weekly in combination with gemcitabine in patients with primary locally advanced, metastatic, or recurrent esophageal cancer. Med Oncol 2007;24:407-12. [Crossref] [PubMed]
- Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2017;35:abstr 4071.
- Abou-Alfa GK, Sangro B, Morse M, et al. Phase 1/2 study of durvalumab and tremelimumab as monotherapy and in combination in patients with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2016;34:abstr TPS3103.
- Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol 2017;35:abstr 4073.
- Kim GM, Jeung HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 2012;48:518-26. [Crossref] [PubMed]
- A Pilot Study of Combined Immune Checkpoint Inhibition in Combination With Ablative Therapies in Subjects With Hepatocellular Carcinoma (HCC) or Biliary Tract Carcinomas (BTC). Available online: https://ClinicalTrials.gov/show/NCT02821754
- Floudas CS, Xie C, Brar G, et al. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC). J Clin Oncol 2019;37:abstr 336.
- Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667-73. [Crossref] [PubMed]
- Bang YJ, Golan T, Lin CC, et al. Ramucirumab (Ram) and durvalumab (Durva) treatment of metastatic non-small cell lung cancer (NSCLC), gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, and hepatocellular carcinoma (HCC) following progression on systemic treatment(s). J Clin Oncol 2019;37:abstr 2528.
- Kim RD, Kim DW, Alese OB, et al. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers (BTC). J Clin Oncol 2019;37:abstr 4097.
- Ilson DH, Wadleigh RG, Leichman LP, et al. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol 2007;18:898-902. [Crossref] [PubMed]
- Kaseb AO, Pestana RC, Vence LM, et al. Randomized, open-label, perioperative phase II study evaluating nivolumab alone or nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol 2019;37:abstr 4098.
- Lin C, Doi T, Muro K, et al. Phase 1 study results from an esophageal squamous cell carcinoma (ESCC) cohort treated with M7824 (MSB0011359C), a bifunctional fusion protein targeting transforming growth factor-beta (TGF-Beta) and-PD-L1. Ann Oncol 2018;29:viii205-70.
- Yau T, Kang YK, Kim TY, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol 2019;37:abstr 4012.
- An Investigational Immuno-therapy Study of Nivolumab Compared to Sorafenib as a First Treatment in Patients With Advanced Hepatocellular Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT02576509
- Bristol-Myers Squibb Announces Results from CheckMate-459 Study Evaluating Opdivo (nivolumab) as a First-Line Treatment for Patients with Unresectable Hepatocellular Carcinoma Princeton, NJ: Bristol-Myers Squibb; 2019 [updated June 24, 2019]. Available online: https://news.bms.com/press-release/bmy/bristol-myers-squibb-announces-results-checkmate-459-study-evaluating-opdivo-nivol
- Bang YJ, Ueno M, Malka D, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol 2019;37:abstr 4079.
- FDA Approves New Monotherapy Indication for Merck’s KEYTRUDA® (pembrolizumab) [press release]. Kenilworth, NJ: Merck, July 31, 2019.
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Ikeda M, Sung MW, Kudo M, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 2018;36:abstr 4076.
- A Study of Atezolizumab in Combination With Bevacizumab Compared With Sorafenib in Patients With Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma IMbrave150. Available online: https://ClinicalTrials.gov/show/NCT03434379
- Ducreux MP, Cheng A, Qin S, et al. Atezolizumab + bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma: the randomised Phase III study IMbrave150. Ann Oncol 2018;29:viii205-70.
- Doi T, Iwasa S, Muro K, et al. Avelumab (anti–PD-L1) in Japanese patients with advanced gastric or gastroesophageal junction cancer (GC/GEJC): updated results from the phase 1b JAVELIN Solid Tumor JPN trial. Ann Oncol 2018;29:viii205-70.
- Abou-Alfa GK, Chan SL, Furuse J, et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J Clin Oncol 2018;36:abstr TPS4144.
- A Global Study to Evaluate Transarterial Chemoembolization (TACE) in Combination with Durvalumab and Bevacizumab Therapy in Patients with Locoregional Hepatocellular Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03778957
- Assess Efficacy and Safety of Durvalumab Alone or Combined with Bevacizumab in High Risk of Recurrence HCC Patients After Curative Treatment. Available online: https://ClinicalTrials.gov/show/NCT03847428
- Study of Pembrolizumab (MK-3475) or Placebo Given With Best Supportive Care in Asian Participants With Previously Treated Advanced Hepatocellular Carcinoma (MK-3475-394/KEYNOTE-394). Available online: https://ClinicalTrials.gov/show/NCT03062358
- Safety and Efficacy of Lenvatinib (E7080/MK-7902) in Combination with Pembrolizumab (MK-3475) Versus Lenvatinib as First-line Therapy in Participants With Advanced Hepatocellular Carcinoma (MK-7902-002/E7080-G000-311/LEAP-002). Available online: https://ClinicalTrials.gov/show/NCT03713593
- A Study of Nivolumab in Patients with Hepatocellular Carcinoma Who Are at High Risk of Recurrence After Curative Hepatic Resection or Ablation. Available online: https://ClinicalTrials.gov/show/NCT03383458
- Programmed Death Ligand (PD-L1) Combined with Chemotherapy for Patients With BTC. Available online: https://ClinicalTrials.gov/show/NCT03478488
- Kang J, Yoo C, Jeong JH, et al. Efficacy and safety of pembrolizumab in patients with PD-L1 positive advanced biliary tract cancer (BTC): A prospective cohort study. J Clin Oncol 2019;37:abstr 4082.
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- Kelly RJ, Lee J, Bang YJ, et al. Safety and efficacy of durvalumab in combination with tremelimumab, durvalumab monotherapy, and tremelimumab monotherapy in patients with advanced gastric cancer. J Clin Oncol 2018;36:abstr 4031.
Cite this article as: Zayac A, Almhanna K. Hepatobiliary cancers and immunotherapy: where are we now and where are we heading? Transl Gastroenterol Hepatol 2020;5:8.


