
PD-1 expression and its significance in tumour microenvironment of hepatocellular carcinoma
Hepatocellular carcinoma (HCC), the most common manifestation of primary liver cancer, is the fifth most common cancer, and the third leading cause of cancer mortality worldwide (1,2). Depending on the stage of the tumour and liver status, HCC is traditionally treated either with surgery (e.g., surgical resection; liver transplantation), localized therapy (i.e., embolization, either chemically or radiologically) or systemic treatment (e.g., tyrosine kinase inhibitor) (3). Among all these methods, only liver transplantation is considered the most effective treatment. However, due to the shortfall in cadaveric or living-donor organs, liver transplantation is limited to patients with early-stage HCC, and only after fulfilling stringent inclusion criteria, such as the Milan criteria.
With the advancement of immunotherapy, the treatment repertoire available for HCC has increased in the past decade. Immune checkpoint inhibitors (ICIs) are one such approach that has shown great potential in the field of HCC. ICI works by disrupting the immune checkpoint protein interaction between immune cells and tumour cells that exist within the tumour microenvironment (TME). Immune checkpoint protein interactions between cytotoxic T lymphocytes associated protein 4 (CTLA-4) on T cells and B7-1/B7-2 on antigen presenting cells (APCs), or the interaction between T cell expressed programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) on tumour cells, are two such examples of immune checkpoint pathways that are targeted by ICIs. ICIs are able to disrupt these immune checkpoint pathways that prevent the activation of T cells within the TME, to reinvigorate the T-cells and enhance anti-tumoural activity (4-9).
Notably, two recent early stage clinical trials reported that the immunotherapy strategy using ICIs targeting PD-1 afforded both effective and durable response in the treatment of HCC (4,5). In 2017, the CheckMate 040 phase I/II clinical trial using the anti-PD-1 mAb nivolumab, reported an overall response rate of 20% in patients with advanced HCC (n=214), regardless of hepatitis virus status and prior treatment status with the tyrosine kinase inhibitor, Sorafenib (4). Median duration of response was a striking 17 months. Similarly, in the Keynote-224 phase II clinical study of anti-PD-1 mAb pembrolizumab in patients with advanced HCC (n=104) patients previously treated with sorafenib also exhibited a comparable overall response rate of 17% (5). In early 2019, the Keynote-240 phase III double-blinded clinical study expanded the use of pembrolizumab as a second-line therapy in patients with advanced HCC (n=416) and demonstrated an objective response rate of 16.9%, consistent with the results of Keynote-224 clinical trial. Keynote-240 failed to reach primary endpoints for overall survival and progression-free survival according to the pre-specified statistical significance (10). In contrast, advanced HCC patients treated with sorafenib alone had an overall response rate of 2% (11). While these pivotal trials highlight the potential of immunotherapy in HCC, clearly immune checkpoint inhibition is not effective for every patient. Predictive biomarkers that can facilitate patient stratification, therapy selection, and tumour response to ICI are needed.
Tumoural PD-L1 expression, confirmed by via immune-histochemistry assays, remains the most common method to predict anti-PD-1/anti-PD-L1 therapeutic response across different cancers (12). Multiple studies support the correlation of tumoural PD-L1 expression with ICI responses (13,14), but findings are inconsistent in HCC (4). According to the CheckMate 040 trial, comparable proportion of patients achieved objective response to nivolumab regardless of high or low tumour PD-L1 expression level (4). The Keynote-224 trial reported that PD-L1 expressed on both the immune and tumour cells, but not tumour cells alone, had a strong association with anti-PD-1 treatment response; albeit only in a subset of patients with available data (5). The role of PD-L1, expressed either on tumour cells or immune cells, such as macrophages, remain controversial. It is interesting to note that PD-L1 expression in the immune cell compartment may play a role in predicting the success of anti-PD-1 therapy. This, however, highlights the need for an in-depth understanding of the tumour immune microenvironment of HCC and other cancers. It is crucial to identify more accurate biomarkers, beyond PD-L1, to identify HCC patients who will potentially respond to ICI therapy. In view of the potential success of combination immunotherapy strategies in HCC and other relevant cancers, biomarker identification and validation is paramount for rational and evidence-based design of future clinical trials.
Expression of PD-1 on CD8+ T cell in HCC as a biomarker of ICI response
In their recent report in Gastroenterology (2018), Kim et al. proposed the use of PD-1 expression on CD8+ T cells as an alternative biomarker to identify HCC patients who will respond to anti-PD-1 therapy (15). According to their study, the HCC TME contain tumour infiltrating lymphocytes (TILs), including distinct subpopulations of CD8+ T cells with a range of PD-1 expression levels: PD-1-high, PD-1-intermediate and PD-1-negative (15). In particular, the presence of CD8+ T cells with high PD-1 expression (PD-1high CD8+) correlate with the aggressiveness of the disease, and potentially predicts for anti-PD-1 therapeutic response. Herein, in view of previous reports, the main findings from Kim et al. are discussed and future directions proposed that can advance the credibility of profiling PD-1 expression on CD8+ T cell populations as a predictive biomarker for ICI therapy in HCC.
Characteristics of PD-1high CD8+ T cells in HCC
Kim et al. observed that PD-1high CD8+ T cells were associated with exhaustion and hence poorer effector function (15). A genetic exhaustion signature was observed for PD-1high CD8+ T cells that includes significant upregulation of the LAYN cluster genes, eomesodermin, and downregulation of LEF1, CX3CR1 cluster, TCF1 and TBET (15). This translates to poorer functional T cell activity evidenced by lower cytokine (TNF-α and IFN-γ) production, and lower proliferative capacity in these cells. In addition, PD-1high CD8+ T cells also express significantly higher level of other markers of immune exhaustion such as TIM3 and LAG3 (15). These results are not observed in isolation. Using mass cytometry time-of-flight (CyTOF) immunoprofiling TILs in HCC, Chew and colleagues showed that the resident memory and effector memory CD8+ T cells (TRM and TEM, respectively) within the TME have similar increased expression of exhaustion markers, such as PD-1, TIM3 and LAG3 (7). Both studies also found that the PD-1+ CD8+ T cells exhibited lower TBET expression and cytokine production upon ex vivo immune activation. Importantly, these observations were not only limited to HCC—similar observations were also made in non-small cell lung cancer, where PD-1+ CD8+ T cells were shown to be exclusively expressing the exhaustion markers TIM3 and LAG3, while also being unable to produce substantial levels of IL2, TNF-α and IFN-γ (8).
Apart from its expression on CD8+ T cells, PD-1 has also been reported to be expressed by a variety of immune cells within the TME (16,17). In the context of HCC, PD-1 was detected on regulatory T cells (Tregs) and tumour-associated macrophages (TAMs) (7,18). According to Lim et al., PD-1+ Tregs are associated with chronic hepatitis B infection related HCC and play a role in creating an immunosuppressive milieu within the TME (18). In another study, IL10+ TAMs also expressed PD-1 within the TME of HCC patients (7). These PD-1 expressing TILs, alongside PD-1+ CD8+ T cells, as investigated in this paper (15), contributes towards an immunosuppressive TME within HCC.
PD-1high CD8+ T cells is associated with clinically more aggressive tumour phenotypes
To investigate the clinical significance of PD-1 in HCC, Kim et al. analyzed the PD-1high-related gene signature in HCC data from The Cancer Genome Atlas (TCGA) cohort (n=333). PD-1high-related gene signature was associated with shorter overall survival (15) suggesting its potential to prognosticate the survival outcome among HCC patients.
In further segregating HCC patient isolated CD8+ TILs into two distinct PD-1-high and PD-1-low expression groups, Kim et al. revealed an additional prognostic value of PD-1 as a biomarker of clinical tumour aggressiveness (15). Etiological factors (i.e., viral or non-viral hepatitis), and most liver function parameters (e.g., total bilirubin, total albumin, alanine aminotransferase activity) were generally the same between these two groups. However, tumours classified by PD-1high CD8+ TILs exhibited features associated with more aggressive tumour phenotypes: higher alpha-fetoprotein serum levels, larger tumour size, association with microvascular invasion, and advanced histological Edmondson-Steiner grade (15).
The views towards the utility of PD-1 expression in CD8+ TILs as a prognostic marker of tumour aggressiveness and survival benefit remains divided both for HCC and other cancer types. Long et al. reported that HCC patients with PD-1+ TILs do not correlate to postoperative survival, clinical staging or cancer severity (19). In contrast, Zeng et al. reported that HCC patients with high-PD-1 expressing peripheral blood mononuclear cells (PBMCs) population had a significantly higher rate of tumour recurrence and progression as compared to the low-PD-1 expressing PBMCs cohort (20). Such polarity in evidence for the use of PD-1 as a prognostic marker is also present in the other cancer types such as clear-cell renal cell carcinoma (ccRCC) (21,22) and head and neck cancer (9,23). In ccRCC, Kang MJ et al. reported a correlation between tumoural infiltration of PD-1+ cells with early distant metastatic relapse, relapse-free survival and overall survival (21), whereas Kim et al. observe no correlation between PD-1+ TILs to disease recurrence or to decreased survival benefit (22). Therefore, in order to better evaluate the role of PD-1 as a prognostic marker, a prospective cohort study will need to be carried out.
HCCs with a distinctive subpopulation of PD-1high CD8+ TILs are significantly more responsive to anti-PD-1 therapy
Kim et al., alternatively suggests that PD-1 expression could be used as a predictive biomarker of response to anti-PD-1 therapy. As mentioned prior, the authors segregated the HCC patients into high-PD-1 expressers or low-PD-1 expressers according to PD-1 expression level on CD8+ TILs (15). Genomic and functional assessments established that CD8+ TILs within the high-PD-1 expressers had an enhanced immune exhaustion profile. The authors then introduced anti-PD-1 ICI therapeutic antibodies and measured the proliferation and cytokine production capacity of the treated CD8+ TILs. Indeed, this treatment reinvigorates the previously exhausted CD8+ TILs from high-PD-1 expressers more than the low-PD-1 expressers, as there was a significantly greater proliferative potential and cytokine production observed upon single anti-PD-1 ICI treatment. This difference was even more prominent when a combination of immune checkpoint blockade agents was used (e.g., anti-TIM3 and anti-LAG3 (15).
As depicted in Figure 1, these results propose that PD-1high CD8+ TILs could be the key target for anti-PD-1 ICI and thus serve as the predictive biomarker for anti-PD-1 therapy in HCC patients. This finding corresponds to similar studies performed in HCC (7), non-small cell lung cancer (8) and head and neck cancer (9). For example, Chew et al. demonstrated that exhausted PD-1+ TEM and TRM from HCC could be reinvigorated with ex vivo treatment of anti-PD-1 ICI therapy (7). In short, these independent studies further substantiate Kim et al. claim and support the potential of PD-1+ CD8+ TILs as a predictive biomarker for anti-PD-1 ICI therapy.
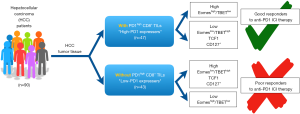
Similar to other studies on PD-L1 tumour biomarker, establishing the utility of PD-1+ CD8+ TILs requires tumour tissue typically obtained via invasive procedures, such as biopsy or surgery. To overcome this potential limitation, the authors of the study collected peripheral blood by less invasive means and similarly evaluated if PD-1 expression status in circulating cells is reflective of HCC TME (15). Indeed, high-PD-1 expressers and low-PD-1 expressers can be similarly segregated by the percentage of PD-1+ CD8+ T cells within the peripheral blood. Separately, Zeng et al. also similarly observed the correlation between PD-1/PD-L1 expression in PBMCs with tumoural PD-1/PD-L1 expression thus supporting the possibility of using peripheral blood as a surrogate to reflect the PD-1 expression status within the TME (20). This demarcation was even more prominent when the percentage of PD-1+ tumour associated antigen (TAA: NY-ESO-1157 and AFP41) specific CD8+ T cells in peripheral blood was compared (15). Moreover, this correlation is also not affected by viral status (Hepatitis B/C or non-viral) of the HCC patient thus further supporting the use of this predictive biomarker in HCC patients regardless of aetiologies.
Conclusions
To conclude, the findings from Kim et al. (Gastroenterology, 2018) demonstrate the potential of using a distinct subpopulation of PD-1high CD8+ TILs as a prognostic and predictive biomarker of anti-PD-1 therapy in HCC patients. More studies need to be carried out to validate this biomarker in HCC, culminating in a prospective clinical trial. Firstly, this trial will have to address if PD-1+ CD8+ TILs, as a predictive biomarker, is superior to other currently available predictive biomarkers. Secondly, this trial will also need to determine the threshold value at which the highest sensitivity and specificity of the biomarker could be achieved before its value is made clear for future routine clinical use.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2018;4:1553-68. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20:4115-27. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99. [Crossref] [PubMed]
- Chew V, Lai L, Pan L, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci U S A 2017;114:E5900-9. [Crossref] [PubMed]
- Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018;24:994-1004. [Crossref] [PubMed]
- Kansy BA, Concha-Benavente F, Srivastava RM, et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res 2017;77:6353. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019;37:abstr 4004.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018;17:129. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018;33:843-52.e4. [Crossref] [PubMed]
- Kim HD, Song GW, Park S, et al. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8+ T Cells and Features of Hepatocellular Carcinoma. Gastroenterology 2018;155:1936-50.e17. [Crossref] [PubMed]
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434-52. [Crossref] [PubMed]
- Gibbons Johnson RM, Dong H. Functional Expression of Programmed Death-Ligand 1 (B7-H1) by Immune Cells and Tumor Cells. Front Immunol 2017;8:961. [Crossref] [PubMed]
- Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019;68:916-27. [Crossref] [PubMed]
- Long J, Qu T, Pan X, et al. Expression of programmed death ligand-1 and programmed death 1 in hepatocellular carcinoma and its clinical significance. J Cancer Res Ther 2018;14:S1188-92. [Crossref] [PubMed]
- Zeng Z, Shi F, Zhou L, et al. Upregulation of Circulating PD-L1/PD-1 Is Associated with Poor Post-Cryoablation Prognosis in Patients with HBV-Related Hepatocellular Carcinoma. PLoS One 2011;6:e23621. [Crossref] [PubMed]
- Kang MJ, Kim KM, Bae JS, et al. Tumor-infiltrating PD1-Positive Lymphocytes and FoxP3-Positive Regulatory T Cells Predict Distant Metastatic Relapse and Survival of Clear Cell Renal Cell Carcinoma. Transl Oncol 2013;6:282-9. [Crossref] [PubMed]
- Kim KS, Sekar RR, Patil D, et al. Evaluation of programmed cell death protein 1 (PD-1) expression as a prognostic biomarker in patients with clear cell renal cell carcinoma. Oncoimmunology 2018;7:e1413519. [Crossref] [PubMed]
- Oliva M, Spreafico A, Taberna M, et al. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol 2019;30:57-67. [Crossref] [PubMed]
Cite this article as: Tan KW, Chacko AM, Chew V. PD-1 expression and its significance in tumour microenvironment of hepatocellular carcinoma. Transl Gastroenterol Hepatol 2019;4:51.


