
Multimodality management of locally advanced gastric cancer—the timing and extent of surgery
Introduction
Locally advanced gastric adenocarcinoma (AGC) is defined as clinical T2 disease and beyond with or without confirmed nodal involvement. This is important for practitioners in that the management of these patients becomes multi-modal. Surgery is essential to the treatment strategy with curative intent along with consideration for systemic chemotherapy and radiation. The extent of lymph node dissection (LND) and the optimum number of lymph nodes evaluated have been the topic of debate over the past several decades. At this time, the decision for the optimal extent of lymph node resection is based on the international consensus in support of D2 lymphadenectomy, the significance of the number of evaluated lymph nodes, and surgeon expertise in the outcome of locally AGC patients.
There are clear differences in the management of gastric adenocarcinoma between Eastern and Western centers, related to stage of presentation, volume of cases, and treatment related outcomes. As such, national guidelines differ and no universal international standard of care for the treatment of these patients has been established. In this review, we present a comprehensive approach to the multimodal treatment of locally AGC excluding those that involve the gastroesophageal junction with an emphasis on timing and extent of surgery for these patients.
Clinical staging to determine appropriate treatment options
Guidelines for locally AGC treatment are based on best-available evidence and generalized according to patient’s clinical stage (1). The individual patient care strategies for locally AGC, however, must dissect out the key components of patient factors (age, health, expectations from treatment) and tumor characteristics (local, regional, and/or distant disease involvement) to maximize the utility of the treatment options. Therefore, appropriate work-up and proper clinical staging of gastric cancer to accurately characterize the tumor is essential for determining optimum timing of radical resection and the extent of nodal dissection.
Prior to any treatment, all patients suspected to have gastric cancer should receive a work-up that includes upper endoscopy with biopsy and contrast computed tomography (CT) scans of the chest, abdomen and pelvis, which provide the pathologic evaluation, the location of the tumor, the extent of stomach involvement, potential for nodal involvement and metastatic status. To further guide therapeutic planning, an upper endoscopic ultrasound (EUS), and diagnostic laparoscopy with or without peritoneal washings can be selectively employed. These steps differentiate between the early stage and later stage lesions (cT1 vs. cT2 or greater lesions, N0 vs. N positive disease), and also determine presence or absence of occult peritoneal metastases not evident on radiologic imaging studies, respectively.
In our practice, EUS is performed to differentiate potentially early lesions versus locally AGC when imaging studies demonstrate no evidence of distant metastases. Results of EUS can guide endoscopic resection when appropriate, and determine the extent of lymphadenectomy. Studies have reported nodal metastatic rates as high as the following for the respective cT-stage of disease: T1a (5%), T1b (24%), T2 (52%), T3 (67%), T4a (74%), and T2b (82%) with lesions least likely to have LN when they are <2 cm, well differentiated, non-ulcerated (1.7%) and more likely to have nodal metastases if the tumor size is larger tumors >4 cm, poorly differentiated, proximally located, and has lymphovascular invasion (2). Majority of the patients in the United States as in our practice are 70 year or younger, do not meet criteria for endoscopic treatment, and are medical fit to undergo surgery. For patients whose lesions fall outside the recommended guideline parameters for endoscopic mucosal resection (>2 cm) and endoscopic submucosal dissection (>1.5 cm) and/or have node positive disease, ulcerations, signet ring cell figures, or poorly differentiated disease (3,4), D2 lymphadenectomy should be considered as an integral part of the radical resection.
Diagnostic laparoscopy has become part of the staging algorithm for patients with locally AGC based on the understanding that occult peritoneal metastases is present in up to 52% of the patients without evidence of metastases on imaging studies (5). In our practice, all patients who are planned for neoadjuvant therapy undergo diagnostic laparoscopy and if gross disease is not found on the peritoneal surfaces, peritoneal washings are performed to rule out microscopic disease. Thus, the judicious use of EUS and diagnostic laparoscopy can profoundly impact locally AGC patient management.
Timing of chemotherapy related to surgery
Patients who are found to have non-metastatic cT2 or greater disease may be considered candidates treatment with multiple modalities: either up front surgery followed by adjuvant chemotherapy or chemoradiation therapy, or peri-operative chemotherapy. Each of these options are considered in the 2018 National Comprehensive Cancer Network (NCCN) guidelines within the context of a multidisciplinary review (1).
By comparison, the European Cancer Organization states upfront surgery is typically only appropriate for patients with early stage disease (cT1a or cT1b) not suitable for endoscopic therapy (6). The Italian Research Group for Gastric cancer (7), recommends neoadjuvant treatment for T3+ disease after a multidisciplinary case review. The Japanese gastric cancer treatment guidelines recommend initial chemotherapy or chemoradiotherapy only for patients with metastatic disease, and note that treatments for non-metastatic patients other than adjuvant S1 are considered investigational (8). The diversity of recommendations is a reflection to the variations that exists within the practice changing clinical trial literature as summarized in Table 1, and are described below.
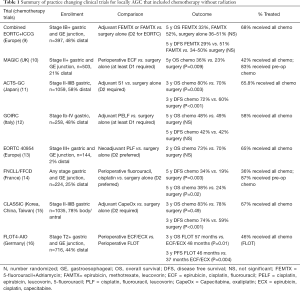
Full table
The clinical trials in locally AGC started with the recognition that recurrence rates were unacceptably high with surgery alone. The ACTS-GC study (11) acknowledged this, showing that 40% of patients with stage II-IIIB gastric cancer recurred in the first 3 years without additional therapy, and that the majority of these relapses were peritoneal. Treatment with adjuvant oral S1 (a prodrug of fluorouracil not available in the US) given for one year post-operatively significantly improved overall survival (OS) and disease free survival (DFS). The greatest benefits were derived for patients <60 years old, and those who had stage II or N1 disease. This trial was conducted in Japan at centers that perform >100 operations annually for gastric cancer. All patients underwent at least a D2 lymphadenectomy and had negative pathologic margins. Although low, the local recurrence rate was 2.8% in the surgery only arm, making it clear that even with the highest technical quality of surgery, surgery alone was insufficient for these patients.
Adjuvant chemotherapy was further studied in the CLASSIC trial (15), in which patients were treated for 6 months with capecitabine, also an oral prodrug of fluorouracil, along with oxaliplatin. These patients again all had negative margins, and were treated by surgeons who performed >50 gastrectomies a year. This study also demonstrated benefit for adjuvant chemotherapy, with the greatest effect for stage IIIB patients, whose 3-year DFS remarkably improved from 33% to 61% in the group treated with chemotherapy in the adjuvant setting. Additional large adjuvant chemotherapy studies attempted to add to fluoropyrimidine based treatment, including SAMIT (17), ITACA-S (18), and AMC0201 (19), but failed to show superiority.
While both the CLASSIC and ACTS-GC studies provided excellent rationale for using adjuvant chemotherapy, their applicability to Western centers was uncertain. This is because Western centers tend to have lower volumes, perform less extensive lymphadenectomies, and have higher rates of gastroesophageal junction/proximal tumors than Asian centers (9,20). A combined European ICCG and EORTC study compared adjuvant 5-fluorouracil + adriamycin (FAMTX) or epirubicin and methotrexate with leucovorin rescue (FEMTX) with a surgery only control arm (21). These trials included patients with gastro-esophageal junction tumors (7%) and those with positive margins (7%). They were combined due to low accrual, but remained underpowered for the anticipated 10% difference, ultimately showing no difference between any of the treatment arms. It was noted that the EORTC arm had better survival and also mandated a D2 lymphadenectomy, with a higher number of lymph nodes examined compared to the ICCG trial (20 vs. 13), but definitive claims regarding extent of lymphadenectomy could not be made. This was followed by the Italian GOIRC (12) study which examined adjuvant cisplatin, epirubicin, leucovorin, 5-fluorouracil compared to surgery alone, and also found no difference in OS or DFS between the adjuvant chemotherapy and surgery only groups. This study mandated at least a D1 lymphadenectomy, and only half of patients had more than 15 lymph nodes examined. They did show that the number of lymph nodes examined was associated with both disease free and overall survival (OS) in their multivariable analysis. As such, Western centers were unable to replicate the results of their Asian colleagues regarding adjuvant chemotherapy, but the patient populations and operative management were somewhat different.
Peri-operative chemotherapy arose out of an interest in treating micrometastatic disease, as well as down-staging tumors prior to surgery. The MAGIC trial (10) addressed this in patients with both gastric and gastroesophageal junction tumors, with 3 neoadjuvant and 3 adjuvant cycles of epirubicin, cisplatin, fluorouracil (ECF). While only 67% of patients enrolled in this study underwent surgery that was deemed curative, patients treated with peri-operative chemotherapy had significantly smaller tumors (3 vs. 5 cm, P<0.001) compared to the surgery only arm, with similar rates of surgical complications. Recurrence, especially as it related to distant metastases was lower in the ECF group (24% vs. 37%), and OS was also improved, with the greatest benefit derived in patients <60 years old, with gastro-esophageal tumors. These results were echoed in the French FNCLCC and FFCD multicenter study (14) that similarly examined peri-operative cisplatin and fluorouracil, and additionally showed higher rates of R0 resection in the perioperative chemotherapy group (95% vs. 74%, P=0.004). These studies were conducted in parallel with the EORTC 40954 trial (13), which was limited to stage III and greater disease, and instead compared only neoadjuvant chemotherapy to surgery alone. While patients in the neoadjuvant arm had a higher R0 resection rate (82% vs. 67%, P=0.03), and fewer positive lymph nodes (median 1 vs. 6, P=0.02), they did not differ in progression free or OS from the surgery alone cohort. Most recently, the German FLOT4 study compared the MAGIC trial protocol of ECF to docetaxel, oxaliplatin, leucovorin, fluorouracil (FLOT) given 8 weeks pre- and 8 weeks post-op (16). FLOT significantly improved disease free and OS, and was also associated with improved frequency of R0 resections (84% vs. 77%, P=0.01). It should be noted that there are no large-scale phase III randomized trials comparing neoadjuvant to adjuvant chemotherapy for locally advanced gastric cancer. A trial was attempted comparing neoadjuvant to adjuvant docetaxel, cisplatin, and fluorouracil, in which 74% of patients completed neoadjuvant therapy, but only 34% of patients completed adjuvant therapy. The study was closed early due to slow accrual, with no survival results reported (22).
Based on these studies, it is our practice to treat Stage II+ patients without compelling reasons for up front surgery with peri-operative chemotherapy (neoadjuvant followed by adjuvant). This is with the understanding that a proportion may not go on to receive all planned cycles of chemotherapy post-operatively without dose modifications or omissions, and that there are no completed randomized clinical trials that directly compare neoadjuvant to adjuvant chemotherapy. Patients who have surgery upfront and are found to be stage II-IIIB are also offered adjuvant chemotherapy. This circumstance arises in patients who are believed to be stage I by pre-operative evaluation and later found to be higher stage, and for those with a compelling clinical reason for upfront surgery, such as significant bleeding. For Western patients, this is with the caveat that studies showing benefit were performed in Eastern populations treated at significantly higher volume centers.
Use and timing of radiation—is there still a role?
Radiation for gastric cancer is typically offered with sensitizing chemotherapy, and per the NCCN guidelines, may be given neoadjuvantly (category 2B, based on lower-level evidence with consensus), or after a resection with a microscopic or macroscopic positive margin (1). Japanese guidelines conversely refer to chemoradiation in the neoadjuvant and adjuvant setting as investigational (8). Italian guidelines mention possible adjuvant chemoradiation for stage II-III disease, positive lymph nodes, or an R1 resection (7). The European guidelines recommend radiation for gastroesophageal tumors, and for gastric cancer patients with high-risk features for local recurrence (6). Again, a number of studies exist examining the role of radiation in the neoadjuvant and adjuvant setting. The practice changing clinical trials are summarized in Table 2, and are described below.
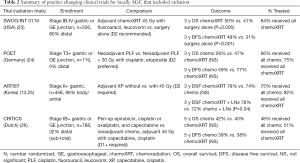
Full table
The initial studies examining radiation for locally AGC were performed in the adjuvant setting. The SWOG/INT 0116 study (23) demonstrated improved disease free and OS with 45 Gy of adjuvant chemoradiation compared to surgery alone. Patients had fewer local, regional, and also distant recurrences in the chemoradiation arm despite radiation primarily being a locoregional treatment. Serious criticisms of this study include that 54% of patients did not receive any kind of operative lymphadenectomy, and furthermore that 17% of patients were not able to complete post-operative radiation therapy. The ARTIST trial (27) evaluated adjuvant chemotherapy with and without radiation. While there was no significant difference in DFS for all comers, sub-group analysis showed a benefit with radiation for the 86% of patients who had positive lymph nodes (3-year DFS 78% vs. 72%, P=0.04). These findings were echoed in other randomized studies (28), and also persisted with 7 years of follow-up (25). Results of the ARTIST trial prompted a subsequent analysis at Samsung Medical Center, Seoul, South Korea, examining patients treated with D2 lymphadenectomy who had positive lymph nodes confirmed on pathology, and were then treated with adjuvant chemotherapy or chemoradiotherapy according to the INT 0116, ARTIST, ACTS-GC, or CLASSIC trials (29). This showed significantly higher recurrence free survival in the chemoradiotherapy group and improved OS for patients with N3 disease, when compared to adjuvant chemotherapy alone. A further study comparing ECF to fluorouracil and leucovorin given with adjuvant radiation in the CALGB80101 study (30) did not yield positive results. Post-operative chemoradiation was added to peri-operative chemotherapy in the recently published CRITICS trial (26); this also did not show a difference in OS or DFS, without any identified subgroups that derived significant benefit.
Neoadjuvant radiation therapy has also been extensively studied. The German POET trial (24) compared patients treated with neoadjuvant chemotherapy to a total neoadjuvant therapy approach. While OS and DFS did not achieve statistical significance, the trend toward statistical significance (OS P=0.07, and DFS P=0.06) and the magnitude of potential clinical benefits of higher pathological complete response rates favoring chemoradiotherapy generated great interest. In a similar study, the global TOPGEAR trial (31) is currently enrolling to compare the MAGIC protocol to 2 cycles of neoadjuvant ECF followed by 45 Gy over 5 weeks with concurrent 5-fluorouracil. Both groups go on surgery with at least a D1+ lymphadenectomy, followed by adjuvant ECF. Their interim report demonstrated improved tolerability compared to the SWOG/INT 0116 study, with 98% of patients receiving all neoadjuvant treatment, and similar post-operative complication rates. Due to issues with tolerability of post-operative therapy, the CRITICS-II trial is currently enrolling to randomize three different neoadjuvant only regimens—docetaxel, oxaliplatin, and capecitabine (DOC) for 12 weeks vs. DOC for 6 weeks, followed by 45 Gy radiation therapy with concurrent paclitaxel and carboplatin vs. only neoadjuvant chemoradiotherapy (32).
There is no convincing data at this time to give neoadjuvant or adjuvant chemoradiation. This may change, however, with the pending data from the TOPGEAR and CRITICS-II trials. Aside from its potential role in the curative treatment of locally AGC, radiation can also be used to treat symptomatic bleeding from gastric tumors, with an efficacy of 74% in pooled analysis (33), and has also been shown to prolong survival in non-metastatic gastric cancer patients who are not able to undergo curative resection (34).
Extent of lymphadenectomy during curative resection
The primary goal of radical gastrectomy is to remove all known disease from the patient for both long-term survival benefit and improvement in the quality of life. In an effort to remove all disease, a lymphadenectomy is recommended as part of curative surgery. The extent of lymphadenectomy performed during gastrectomy for locally AGC, however, has been the subject of debate in the past, and the center of much research. Lymph node stations used to describe the extent of lymphadenectomy were first defined by the Japanese Research Society for Gastric Cancer in 1982 (35). In general, peri-gastric lesser curvature and greater curvature lymph nodes (stations 1–6) are grouped into a D1 lymphadenectomy, additional lymph nodes along the left gastric artery, common hepatic artery, celiac artery, and splenic artery (stations 7–11) are grouped into a D2 lymphadenectomy (36), and additional hepatoduodenal ligament, retropancreatic, and superior mesenteric vein lymph nodes are grouped into a D3 lymphadenectomy (stations 12–14) (37). Other lymph node stations along the aorta, as well as paraesophageal lymph nodes have been defined (35).
NCCN guidelines recommend a D2 lymphadenectomy, with a goal of examining >15 lymph nodes (1). Japanese guidelines recommend a D1+ lymphadenectomy for T1b tumors, and D2 lymphadenectomy for T2+ or N1+ tumors (8). Italian guidelines recommend a D2 resection for all gastrectomies with curative intent, excluding patients who are very high risk, and early tumors not treatable by endoscopy (7). This is based on the fact that an adequate lymphadenectomy leads to more accurate staging, which seems to be most important for stage II and III patients. The practice changing clinical trials are summarized in Table 3, and are described below.
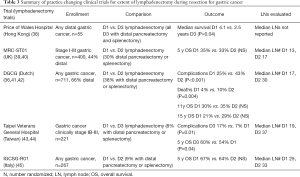
Full table
Historically, more extensive lymphadenectomies were favored in the East, whereas less extensive lymphadenectomies were more often performed in the West. Evidence of this can be found in the literature reviewed above; no lymphadenectomy was performed at the time of resection for more than half of US patients enrolled in the SWOG/INT 0116 study despite specifications for it (23), compared to only 1 out of 1,059 patients not having a D2 or greater lymphadenectomy in the Japanese ACTS-GC trial (11). A review of more than 10,000 gastrectomies at Yonsei University in South Korea from 1987–2007 found that only 3.2% of patients had fewer than 15 lymph nodes examined (46). Reasons for this are undoubtedly related to the pronounced discrepancies between the patient populations and the volume of surgically treated gastric cancers at Eastern vs. Western centers.
The Dutch Gastric Cancer Group has been instrumental in producing long-term high quality data regarding lymphadenectomy for Western patients with gastric cancer. In their study comparing D1 to D2 lymphadenectomy that enrolled from 1983-1993, patients with proximal tumors that invaded beyond the muscularis propria (T3 or T4 disease) also underwent distal pancreatectomy and splenectomy (36). This is because at the time of enrollment, it was felt that a complete D2 lymphadenectomy for these patients required distal pancreatectomy and splenectomy to obtain stations 10 and 11. These particular patients had significantly higher mortality rates (36). Additional risk factors for post-operative morbidity and mortality were age >70, male gender, and total vs. distal gastrectomy procedure (41). At 11 and 15 year follow ups, this study did not show significant differences for OS or DFS between the D1 and D2 groups (41,42), however, at 15 years, there was a significant difference in deaths caused from gastric cancer (D1 48% vs. D2 37%, P=0.01), as well as for D1 vs. D2 lymphadenectomies excluding those who underwent distal pancreatectomy and/or splenectomy (15 year OS D2 35% vs. D1 22%, P=0.006). The apparent benefit was greatest for patients who were stage II–III.
The MRC-ST01 UK trial also randomized D1 to D2 lymphadenectomy, and similarly did not find a significant difference in OS between the groups, but also reported increased complications and poorer survival in patients who had a distal pancreatectomy and splenectomy (P=0.01) (39,40). After adjusting for distal pancreatectomy and splenectomy, the complication rate between the D1 and D2 groups were similar. The IGCSG-R01 study randomized D1 to D2 lymphadenectomy, but had fewer distal pancreatectomies and splenectomies in the D2 group (45). While OS was similar between groups, the disease specific survival for patients with pT1 tumors was longer in the D1 group (5-year survival 98% vs. 83%, P=0.02). Conversely, patients with T2+ tumors trended towards longer 5-year disease specific survival with a D2 dissection (59% vs. 38%, P=0.06). It should be noted that in this study, the average lymph node yield for the patients who had a D1 lymphadenectomy was 28.
Asian studies examined the validity of a D1 compared to D3 lymphadenectomies. Three surgeons at Taipei Veterans General Hospital performed a randomized study comparing D1 to D3 lymphadenectomy, in which only 8% of patients had a distal pancreatectomy or splenectomy, and found that while morbidity was higher in the D3 group (longer operative times, higher blood loss, more need for transfusions) (43), survival outcomes were actually superior for the D3 group (44). The authors concluded that D3 lymphadenectomies should be done by experienced surgeons in hospitals that treat many patients with gastric cancer. By comparison, a trial of distal gastrectomy out of Hong Kong randomizing D1 vs. D3 lymphadenectomy that mandated a distal pancreatectomy and splenectomy for the D3 group found that survival was superior for the D1 group, along with shorter operative time, lower blood loss, and shorter hospitalization (38). Additional Eastern (47,48) studies that compared D2 lymphadenectomy to more extensive dissection found no difference in OS or recurrence free survival.
It should be acknowledged that none of the patients in any of the above lymphadenectomy trials received neoadjuvant or adjuvant chemotherapy. D2 lymphadenectomy has, however, been incorporated into many trials related to neoadjuvant and adjuvant care. Extent of lymphadenectomy is described for each of the summarized trials in Tables 1 and 2.
An additional focus related to the extent of lymphadenectomy has been on the number of lymph nodes examined. A large study from Korea demonstrated that evaluating <15 lymph nodes correlated with shorter survival for patients with T1 tumors, and for those without positive lymph nodes (46). This effect was strongest on male patients >60 years old. There was not an association with OS for the entire cohort, however, 42% of patients in this study had T1 tumors. Conversely, a study out of China found that survival was only significantly improved for patients with T2–T4 disease who had >15 lymph nodes evaluated, and that this was less important for T1 tumors, given that 5-year survival was 97% in the T1 group (49). Another large study out of China determined that >22 lymph nodes was most important specifically for survival in patients with N3+ disease, reporting 5-year OS of 30% vs. 22% in N3a disease, and 13% vs. 0% in N3b disease (50). Recovery of >15 lymph nodes were adequate for all other nodal stages in this report. The largest analysis on this topic combined a Korean and US database, and found that survival was significantly improved for all stages when >29 lymph nodes were examined (37). This was due to stage migration, wherein patients who had fewer lymph nodes examined exhibited outcomes similar to the next highest stage who had an adequate lymphadenectomy (37).
Given the heterogeneity of the results, it is difficult to derive firm conclusions regarding the number of lymph nodes required for optimal lymphadenectomy. It is our practice, as recommended now almost universally, to perform a D2 lymphadenectomy in locoregionally advanced patients undergoing gastrectomy with curative intent. Cadaver studies in patients without gastric cancer demonstrated that 17–44 lymph nodes are expected for a D2 lymphadenectomy (51). Studies have shown that lymph node recovery varies by hospital, surgeon, and pathology technician (52), and that lymph node yield increases when the surgeon performs an ex-vivo dissection (as opposed to sending lymph nodes en bloc with the specimen) (53). For this reason, we separate lymph nodes from the gastrectomy specimen prior to submitting them to pathology, and ask our pathologists to evaluate for as many lymph nodes as possible, with a goal of 29 or more.
What is next?—immunotherapies and targeted therapies
The next frontier for the treatment of locally advanced gastric cancer is with agents that are targeted to specific proteins expressed by tumors, and agents that can modulate the body’s own immune system to target and kill cancer cells. Recent studies have shown that microsatellite instability within tumors is associated with immune checkpoint proteins enabling these tumors to survive (54). Further, drugs that block immune checkpoint proteins have shown positive effects in patients with high microsatellite instability tumors (55). A secondary post-hoc analysis of the MAGIC trial examined patients for microsatellite instability and mismatch repair protein deficiency, and found that 8.5% of patients with gastric cancer had high microsatellite instability, and 5.2% had a mismatch repair protein deficiency. These patients had significantly inferior responses to chemotherapy, and actually had longer survival when treated with surgery alone (56). The CLASSIC trial also had similar results, showing no survival benefit to adjuvant chemotherapy in the high microsatellite instability group (57). Based on these findings, there are now a number of active clinical trials examining the role of immunotherapeutic drugs in the peri-operative and adjuvant treatment of locally AGC, including the Checkmate 577, KEYNOTE 585, and ATTRACTION-05 trials.
It is also known that ~20% of gastric cancers will have over-expression of the HER2 receptor, which can be targeted using anti-HER2 antibodies, including trastuzumab and pertuzumab (58). The ToGA trial evaluated the effect of trastuzumab in addition to chemotherapy (capecitabine plus cisplatin or fluorouracil plus cisplatin) on patients with advanced gastric cancer and tumors that stained strongly for HER2 or who were FISH positive (59). Addition of trastuzumab significantly increased OS, with similar side effects compared to the control group. Following this study, trastuzumab was tested with FLOT for locally advanced esophagogastric cancer on a small scale, and was shown to be safe (60). There PETRARCA clinical trial is also evaluating the combination of pertuzumab and trastuzumab with FLOT for peri-operative treatment in locally advanced patients. Results of these trials will further clarify the optimal multimodal treatment for these patients.
Conclusions
The management of patients with locally AGC is multimodal and complex. The timing and extent of surgery for locoregional control of AGC is becoming ever more critical in optimizing patient outcome with improving effectiveness of systemic treatments. As clinical trials continue to clarify and guide the roles of chemotherapy, radiation therapy, targeted molecular agents, and immunotherapies, we expect that timing and extent of curative resection to evolve. While interpretation of data and implementation of standardized gastric cancer care are complicated by geographic differences in patient factors, tumor characteristics, hospital volume, and practice patterns; the longstanding international collaborations amongst the gastric cancer care physician and surgeon communities promise to narrow the gap in the disparate outcomes in gastric cancer In the meantime, a locally AGC patient is likely to benefit most from an individualized multimodal treatment plan derived from studies that include similar patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 2. 2018. Available online: https://www.nccn.org/
- Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel) 2011;3:2141-59. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3-15. [Crossref] [PubMed]
- Fujishiro M, Yoshida S, Matsuda R, et al. Updated evidence on endoscopic resection of early gastric cancer from Japan. Gastric Cancer 2017;20:39-44. [Crossref] [PubMed]
- Juhl H, Stritzel M, Wroblewski A, et al. Immunocytological detection of micrometastatic cells:comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal and pancreatic cancer patients. Int J Cancer 1994;57:330-5. [Crossref] [PubMed]
- Allum W, Lordick F, Alsina M, et al. ECCO essential requirements for quality cancer care: Oesophageal and gastric cancer. Crit Rev Oncol Hematol 2018;122:179-93. [Crossref] [PubMed]
- De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment:2015. Gastric Cancer 2017;20:20-30. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer 2017;20:1-19. [Crossref] [PubMed]
- Woo Y, Goldner B, Son T, et al. Western validation of a novel gastric cancer prognosis prediction model in US gastric cancer patients. J Am Coll Surg 2018;226:252-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Di Costanzo F, Gasperoni S, Manzione L, et al. Adjuvant chemotherapy in completely resected gastric cancer:a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst 2008;100:388-98. [Crossref] [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia:European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC):a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Al-Batran SE, Pauligk C, Homann N, et al. Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) for resectable esophagogastric cancer:updated results from multicenter, randomized phase 3 FLOT4- AIO trial. Ann Oncol 2017;28:v605-9. [Crossref]
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT):a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [Crossref] [PubMed]
- Bajetta E, Floriani I, Di Bartolomeo M, et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann Oncol 2014;25:1373-8. [Crossref] [PubMed]
- Kang YK, Chang HM, Yook JH, et al. Adjuvant chemotherapy for gastric cancer:a randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201) Br J Cancer 2013;108:1245-51. [Crossref] [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [Crossref] [PubMed]
- Nitti D, Wils J, Dos Santos JG, et al. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol 2006;17:262-9. [Crossref] [PubMed]
- Biffi R, Fazio N, Luca F, et al. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol 2010;16:868-74. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS):an international, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:616-28. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 2012;104:361-6. [Crossref] [PubMed]
- Yu JI, Lim DH, Lee J, et al. Necessity of adjuvant concurrent chemo-radiotherapy in D2-resected LN-positive gastric cancer. Radiother Oncol 2018;129:306-12. [Crossref] [PubMed]
- Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer:Results From CALGB 80101 (Alliance). J Clin Oncol 2017;35:3671-7. [Crossref] [PubMed]
- Leong T, Smithers BM, Haustermans K, et al. TOPGEAR:A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017;24:2252-8. [Crossref] [PubMed]
- Slagter AE, Jansen E, van Laarhoven H, et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer 2018;18:877. [Crossref] [PubMed]
- Tey J, Soon YY, Koh WY, et al. Palliative radiotherapy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017;8:25797-805. [Crossref] [PubMed]
- Li R, Hou WH, Chao J, et al. Chemoradiation Improves Survival Compared With Chemotherapy Alone in Unresected Nonmetastatic Gastric Cancer. J Natl Compr Canc Netw 2018;16:950-8. [Crossref] [PubMed]
- Rosa F, Costamagna G, Doglietto GB, et al. Classification of nodal stations in gastric cancer. Transl Gastroenterol Hepatol 2017;2:2. [Crossref] [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [Crossref] [PubMed]
- Woo Y, Goldner B, Ituarte P, et al. Lymphadenectomy with Optimum of 29 Lymph Nodes Retrieved Associated with Improved Survival in Advanced Gastric Cancer:A 25,000-Patient International Database Study. J Am Coll Surg 2017;224:546-55. [Crossref] [PubMed]
- Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg 1994;220:176-82. [Crossref] [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer:15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer:who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 2004;91:283-7. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer:a randomised controlled trial. Lancet Oncol 2006;7:309-15. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012;118:4687-93. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Hu JK, Yang K, Zhang B, et al. D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today 2009;39:207-13. [Crossref] [PubMed]
- Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS One 2012;7:e38681.
- Zheng G, Feng F, Guo M, et al. Harvest of at Least 23 Lymph Nodes is Indispensable for Stage N3 Gastric Cancer Patients. Ann Surg Oncol 2017;24:998-1002. [Crossref] [PubMed]
- Wagner PK, Ramaswamy A, Rüschoff J, et al. Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg 1991;78:825-7. [Crossref] [PubMed]
- Schoenleber SJ, Schnelldorfer T, Wood CM, et al. Factors influencing lymph node recovery from the operative specimen after gastrectomy for gastric adenocarcinoma. J Gastrointest Surg 2009;13:1233-7. [Crossref] [PubMed]
- Afaneh C, Levy A, Selby L, et al. Ex Vivo Lymphadenectomy During Gastrectomy for Adenocarcinoma Optimizes Lymph Node Yield. J Gastrointest Surg 2016;20:165-71. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Choi YY, Kim H, Shin SJ, et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled study. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Hofheinz R, Hegewisch-Becker S, Thuss-Patience PC, et al. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the AIO Gastric Cancer Study Group. J Clin Oncol 2014;32:4073. [Crossref]
Cite this article as: Stewart C, Chao J, Chen YJ, Lin J, Sullivan MJ, Melstrom L, Hyung WJ, Fong Y, Paz IB, Woo Y. Multimodality management of locally advanced gastric cancer—the timing and extent of surgery. Transl Gastroenterol Hepatol 2019;4:42.


