
Adjuvant therapy with imatinib in gastrointestinal stromal tumors (GISTs)—review and perspectives
Introduction and epidemiology
Gastrointestinal stromal tumors (GISTs) are rare cancers [<1% of primary gastrointestinal (GI) neoplasms], with an incidence close to 1.2/100,000 per year in all countries (1,2). GIST are the most common non-epithelial tumor of the gastrointestinal tract (3), with variable malignant potential. Its incidence is possibly underestimated with a higher incidence of small asymptomatic GIST and therefore undiagnosed (4-7), with evidences in two series (6,8). GISTs are highly resistant to cytotoxic chemotherapy and the role of radiotherapy is unclear (9). The identification of mutually exclusive activating mutations of 2 kinases, as early and major driving event, as well as the availability of active targeted agents make these tumors paradigmatic models of precision medicine in oncology. Imatinib is a small-molecule tyrosine-kinase inhibitor active against Breakpoint Cluster Region - Abelson (BCR-ABL), KIT proto-oncogene receptor tyrosine kinase (KIT), and platelet-derived growth factor. Since the first patient successfully treated in 2000, clinical activity was confirmed initially in an European Organization for Research and Treatment of Cancer (EORTC) phase I study (10) and in a large phase II study (11). For localized GISTs, adjuvant therapy with 3 years of the tyrosine kinase inhibitor imatinib is indicated for patients with a high (>50%) risk of relapse. This treatment has transformed the prognosis, increasing the time to GIST recurrence and prolonging survival both in metastatic and adjuvant phases.
Staging
A pretreatment staging is recommended before adjuvant therapy, assessing resectability and presence of metastases. A three-stage abdominal-spiral spiral CT with a thoracic passage is indicated (2) and magnetic resonance imaging (MRI) in case of rectal GIST. For small-size GISTs of the upper digestive tract or rectum, endoscopic ultrasound can be performed. Positron emission tomography (PET) is reserved in case of diagnostic doubt.
Anatomopathology and immunohistochemistry
GISTs arise from mesenchymal cells and originate from progenitors of the gastric pacemaker cells, the interstitial cells of Cajal (12). The diagnosis of GIST relies on morphology and immunohistochemistry. The main three several histological types are, fusiform (70%), epithelioid (20%) and mixed (5%). Ninety-five percent of GISTs are positive for the CD117 surface antigen (also known as KIT) or stem cell factor receptor including tumors with KIT wild-type (WT) genotype and most platelet derived growth factor receptor alpha (PDGFRA) mutant GISTs. KIT mutation in GIST does not cause KIT expression but modifies KIT protein function. KIT mutation affects subcellular KIT protein distribution with reports showing a trapping of the activated kinase in the endoplasmic reticulum (13).
The others key IHC markers are discovered on GIST-1 (DOG1) (>95%) and CD34 (70%, and more frequently in the esophageal and rectal locations), h-Caldesmon (80%), smooth muscle actin (40%, frequently associated with intestinal GISTs), and desmin (less than 5%). DOG1 protein expression is useful for the diagnosis of KIT negative GIST with a sensitivity close to 100% (12-14). If diagnosis remain doubtful (CD117/DOG1 negative), a mutational analysis for mutations involving KIT or PDGFRA genes may guide diagnosis. The mitotic count, that should be expressed as the number of mitoses on a total area of 5 mm2, has a prognostic value and is one of the three main prognosis factors.
Molecular alterations of GIST
Considering their predictive (sensitivity to molecular-targeted therapy) and prognostic values (15,16), the precise description of tumour KIT and PDGFRA mutations (sequence, size, codons) is recommended whenever adjuvant imatinib is considered (2).
Most GISTs are driven by mutually exclusive activating mutations in the KIT or PDGFRA receptor tyrosine kinase genes. Most of these mutations have been shown to result in constitutive (ligand-independent) kinase activation for both KIT and PDGFRA (12,17,18), and is the driver oncogenic mechanism in GISTs (19). In addition, single nucleotide variants of the KIT gene in the absence of mutation may also influence the clinical history of GIST: this has been demonstrated for an M541L variant (20).
KIT is involved (pathologic activation resulting to a gain-of-function mutations) in 75% of GISTs (12). According to subgroup analysis from the SSGXVIII/IAO trial, patients with exon 11 mutations [the most frequent one (66%) and generally sensitive to imatinib] are more likely to benefit from adjuvant imatinib [progression free survival (PFS): HR =0.35; 95% CI, 0.22–0.56] than those with the less common KIT exon 9 mutation [10%, less sensitive to imatinib; almost exclusively in the small or large bowel (21)] (PFS: HR =0.61; 95% CI, 0.22–1.68). The MetaGIST meta-analyses (combined analysis of 1,640 patients with metastatic GIST treated on two phase 3 trials) comforts this point, showing a longest PFS for patients with exon 11 mutation than those with an exon 9 mutation (22). Moreover, within KIT exon 11 mutated GISTs, those with codon 557 and/or 558 deletion have a higher risk of recurrence than others (23) but a better response rate to imatinib in advanced phase. Exon 13 or 17 can also occur (1%) (24). Exon 13 or 17 can also occur (1%) (24) with varying degree of sensitivity to imatinib (e.g., D816V exon 17 mutation is resistant to Imatinib (25).
Ten percent of GISTS carry mutations in PDGFRA (12,26). They are associated with gastric and epithelioid GISTs (27) and a less malignant course of disease. PDGFRA exon 18 mutation (Asp842Val substitution) is the most common (6%), and the second most frequent in localized phase (28). Such mutation is associated with a good prognosis, and a minimal risk of relapse, but conversely resistance to imatinib, sunitinib and regorafenib (29). The mutations of exon 12 or 14 of PDGFRA are exceptional (<1%).
A smaller proportion of GISTs do not carry such mutations and, instead, have alterations of other key signal transduction genes (12,30). Previously qualified as wild type GIST about 10% of GISTs are negative for KIT and PDGFRA mutations. This includes an heterogeneous genotypic group with mutations in HRAS, NRAS, BRAF, NF1 or the SDH complex, and several other genes (30). This subset may be considered insensitive to imatinib (25). In KIT/PDGFRA wild type (WT) GIST, immunohistochemistry for succinate dehydrogenase (SDH) iron–sulfur subunit (subunit B) (SDHB) is recommended.
Differential diagnosis
If diagnosis remains doubting, the opportunity should be taken to make a precise diagnosis, given the expected clinical benefit in term of prognosis and risk of relapse with adjuvant imatinib for GISTs.
Differential diagnoses with other abdominal tumors include other spindle cell tumors, either benign as schwannoma (mainly in the stomach, inflammatory infiltrate quite pathognomonic negative protein KIT, PS-100 expression intense and diffuse—marker of Schwann cells, melanocytes, Langerhans cells among others), leiomyoma (location on esophagus and rectum, mainly in young adult, abundant eosinophilic cytoplasm, muscle markers highly positive—smooth muscle actin, desmin, h-Caldesmon—while KIT and CD34 are negative), solitary fibrous tumor, desmoid tumors, neurofibroma; or malignant as leiomyosarcoma, dedifferentiated liposarcoma.
Prognostic and risk assessment
The current consensus is that all GISTs >2 cm (and of any size for rectal GIST) should be considered as having a potential for malignancy. Late relapses (>20 years) are possible even for small GISTs.
Independent prognostic factors for the risk of relapse are the mitotic rate, tumor size and localization (gastric GISTs have a better prognosis than small bowel or rectal GISTs). Additional key prognostic factors are tumor rupture (adverse prognostic factor and should be recorded, whether it took place before or during surgery). While mutational status has not been incorporated in any risk classification yet, genotypes with distinct pathological behavior have been described: NF1 GIST and PDGFRA D842V for instance are not considered for adjuvant treatment with imatinib.
The usual and widely used risk classifications, include therefore three main prognostic factors (size and location of the primary tumor combined with mitotic index), either alone or with tumor rupture (31,32). Molecular subtypes also guide the application of adjuvant treatment, in view of different sensitivity profiles: some mutations are associated with primary resistance (KIT mutation-negative and D842V mutation in PDGFRA gene, SDH-deficient or NF1-related GIST) or lower sensibility (KIT exon 9 mutation). This implies to, respectively, not offer adjuvant imatinib or prefer higher dose imatinib therapy in these cases.
Management of localized GIST: surgery first, and imatinib in high risk patients
The standard management of a localized tumor suspected or confirmed to be GIST or with a size ≥2 cm, is the complete surgical excision of the lesion, avoiding tumor rupture or spillage during the procedure, with no dissection of clinically negative lymph nodes. R0 excision is the goal. Choice of laparoscopic approach must take into account of the risk of tumor rupture (associated with a very high risk of relapse) and must follow the principles of oncological surgery. It must be performed by an experienced team. Adjuvant imatinib treatment is discussed in the next chapter.
Adjuvant treatment with imatinib: the randomized trials and next questions
Duration of adjuvant therapy three phase III published trials
Three randomized phase III trials are available (33-35) [2009, 2012, 2015] to explore the question of adjuvant imatinib treatment (Table 1). All 3 demonstrated the efficacy of adjuvant therapy with imatinib in GISTs for high-risk patients: recurrence-free survival (RFS) was significantly prolonged as compared to the control arm when given for a duration from 1 to 3 years.
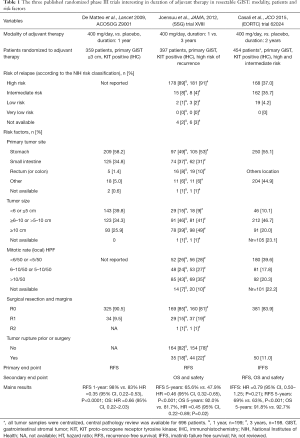
Full table
The first trial explored, 1-year imatinib compared to placebo as an adjuvant therapy with an improved recurrence free survival (HR =0.35; 95% CI, 0.22–0.53; P<0.001) (34). This trial was proposed to patients with tumors >3 cm, as the main inclusion criterion.
The second published trial randomized then 3 years of adjuvant administration of imatinib compared to 1 year, with also a significative prolonged 5-years progression free survival (HR =0.60; 95% CI, 0.44–0.81; P<0.001) and survival advantage (HR =0.60; 95% CI, 0.37–0.97; P<0.036) (16).
The largest trial phase III study was EORTC 62024 which randomized adjuvant therapy with imatinib for 2 years, versus observation in intermediate or high risk of relapse for patients with resected localized GIST. The primary end point was imatinib monotherapy failure-free survival (IFFS, defined as the time to death or starting another tyrosine kinase inhibitor than imatinib). IFFS was chosen after amending OS primary end point initially chosen (considered as requiring an excessive study duration by the study independent data monitoring committee). In 2015, the intermediate analysis on the new primary end point (IFFS) was published (35).
In this trial, from 2004 to 2008, 908 patients with localized GIST (immunostaining positive for KIT-CD117), documented as high or intermediate risk of relapse [according to the 2002 National Institutes of Health (NIH) classification, based solely on size and mitotic rate] were randomized, 2 weeks to 3 months after surgery R0 or R1 (which included intraoperative rumor rupture or intraoperative tumor spillage) between imatinib therapy 400 mg/day for 2 years or observation alone without any further therapy.
Of the 454 randomized in the adjuvant therapy arm, 336 (75%) completed 2 years of treatment. Median follow-up was 4.7 years in imatinib arm (vs. 4.6 in observed arm), and there was no significant difference of IFFS: 5-year IFFS was 87% in the imatinib arm versus 84% (HR =0.79; 98.5% CI, 0.50–1.25; P=0.21), and 5-year OS was 100% vs. 99%. Relapse occurred in 282 patients (imatinib arm, n=121; observational arm, n=161) with RFS significantly better in the adjuvant imatinib arm (84% vs. 66% at 3 years; 69% vs. 63% at 5 years; log-rank P=0.001). There was a trend toward better IFFS among the 528 patients with high-risk of relapse (according to the 2002 NIH classification, determined by central pathology review): 5-year IFFS 70% in imatinib arm versus 73% (P=0.087). This substantial improvement of RFS by adjuvant imatinib is consistent with those found in 2 other trials of adjuvant imatinib in patients with localized GIST (32,36).
In all 3 trials, the benefit in relapse free survival is however reduced after 1 to 3 years from the end of the adjuvant treatment period, with an increasing risk of relapse in this time period. However, exposure to imatinib in the adjuvant setting does not induce a detectable selection pressure toward secondary resistance in view of: (I) a similar progression free survival observed after reintroduction imatinib in the SSG XVIII trial (37); and (II) the trend for a better IFFS in the adjuvant arm. This apparent absence of selection pressure in the absence of macroscopic tumor in place is intriguing and points to a correlation between tumor cell mass and the risk of Darwinian selection of resistant clones. As such it is one of the strongest arguments to use targeted treatment in general in an adjuvant setting as recently shown in melanoma with BRAF mutation (38). In this study, half of the enrolled patients had an intermediate risk of relapse according to current risk classifications. Also, patients with tumor rupture was included in the R1 stratum (11%), and the inherent risk of relapse might be increased.
Results of mutational analysis (by centralized tumor samples) in the EORTC 62024 trial are missing currently and will be the subject of a separate report. These data, will allow to discuss about benefit of imatinib in non-exon 11 KIT mutated GIST, and of adjuvant imatinib in imatinib-insensitive mutations and in wild-type GIST.
Joensuu and collaborators published an important exploratory analysis looking at benefits of 3- vs. 1-year imatinib in molecular subsets of GIST. In this study, 3 years treatment was particularly beneficial for patients with KIT exon 11 mutations with deletions of codon 557 and/or 558, and inpatients with high mitotic rate. In the very small subgroup KIT exon 9 mutated GIST, the benefits of longer treatment with 400 mg/day was not significant (33).
Clinical practice guidelines recommendations
Adjuvant therapy with imatinib given at a dose of 400 mg/day for 3 years is the standard treatment of patients with a high-risk of relapse, based in a randomized trial with a relapse free survival and OS advantages (33).
Actually, imatinib 400 mg/day for 3 years is recommended possible treatment option for patient with high or intermediate risk of relapse (based on the NIH classification).
For those with intermediate-risk, the decision of treatment is debated. A randomized trial is exploring the application of 3-year imatinib in patients with an intermediate risk tumor, but with a high risk of relapse according to the genomic index reported by Chibon et al. (39) (Trial GIGIST, NCT02576080). Adjuvant therapy should not be considered when the risk is low (2).
Mutational analysis is essential to make an adapted decision about adjuvant therapy. Less sensitive or resistant genotypes such as PDGFRA D842V-mutated or KIT exon 17 (D816V) mutations, both resistant to imatinib should not be recommended for imatinib adjuvant therapy. There is also a consensus not to recommend adjuvant treatment in neurofibromatosis 1-related GISTs which are insensitive to imatinib in the advanced setting (25).
The use of higher dose of imatinib (800 mg daily) in the case of an exon 9 KIT mutation has never been tested formally in a clinical trial in an adjuvant setting, but is proposed by some experts in view of its increased efficacy in metastatic phase over standard 400 mg/day dose. For wild-type GIST, a case-by-case analysis to decide for treatment with imatinib since there is no consensus regarding adjuvant treatment).
In case of tumor rupture at the time of surgery, patients should be considered for imatinib therapy due to a very high risk of peritoneal relapse (40). The duration of treatment is debated, from 3 years to lifelong treatment since these patients could be considered metastatic.
Neo-adjuvant therapy with imatinib can be considered if R0 surgery can be more safely reached after tumor cytoreduction (less mutilating/function-sparing surgery) (2,41,42). A 6–12 months duration of neoadjuvant imatinib could be recommended to reach maximum response to treatment (although optimal duration is not clearly defined), assessed by PET-CT one month after beginning (to confirm sensitivity to imatinib, with absence of progression).
Management after 3-year completion: continue or discontinue adjuvant therapy?
Could a prolonged adjuvant treatment with imatinib beyond 3 years further reduce the risk or recurrence and improve overall survival in subsets of high-risk gist patients? The initial results of the single arm phase II trial PERSIST, testing 5 years of imatinib adjuvant therapy (GIST with high risk of relapse), show RFS rates (5 and 8 years, estimated) were 90% (95% CI, 80–95%) and 81% (95% CI, 62–91%) but is not conclusive on this question in the absence of a control arm (43). The utility of prolonged treatment needs actually to be established in a randomized controlled study vs. the standard 3 years treatment.
Two randomized clinical studies are ongoing to test longer durations of adjuvant therapy in GISTs (Table 2).

Full table
IMADGIST is a French open-label randomized multicenter phase III study aiming to evaluate the clinical impact of maintaining imatinib treatment beyond 3 years in the adjuvant setting for patients with resected GISTs at high risk of recurrence according to the National Comprehensive Cancer Network Task Force on GIST (NCCN) risk classification. After a 3-year-completion of imatinib adjuvant therapy, patients are randomized for either the continuation of this treatment or the discontinuation and eventually the re-introduction at relapse. Primary outcome is disease free survival (NCT02260505).
Likewise, the Scandinavian Sarcoma Group start the sSGXXII phase III study, which hypothesizes that adjuvant imatinib given for a total of 5 years may prevent some of the GISTs to recur as compared to patients who receive adjuvant imatinib for 3 years, and there may be a difference in the rate of GIST recurrence between the two groups. Patients with operable GIST and a high risk for recurrence are randomized for three or five years of adjuvant imatinib as treatment (NCT02413736).
For these two studies, inclusions are still open, and estimated study completion dates are projected respectively for 2020 and 2028.
Conclusion and perspectives
Imatinib is approved worldwide for use in adjuvant therapy for GISTs and demonstrated in all trials a substantial improvement of PFS, and in one trial a substantial improvement of overall survival of a duration of 3 years in high risk patients. Two years duration of adjuvant imatinib does not induce a detectable selection pressure of resistant clones. It is recommended as adjuvant therapy for all localized GISTs with high risk of relapse according to the NIH classification. For intermediate risk patients according to current classification, further refinements of the risk groups are needed.
Longer duration of adjuvant treatment is under investigation, since relapses occurring in the 2 years following adjuvant interruption are frequently observed. Molecular subsets of GIST benefiting the most from long term adjuvant treatment need also to be investigated in these trials.
Acknowledgements
The authors would like to thank the teams and leaders of the French National Cancer Institute (INCa) for the continuous support to the project.
Funding: Supported by NetSARC (INCA) and RREPS (INCA), Association DAM’s, Ensemble contre Le GIST, Eurosarc (FP7-278742), la Fondation ARC, Infosarcome, InterSARC (INCA), LabEx DEvweCAN (ANR-10-LABX-0061), Ligue de L’Ain contre le Cancer, INCa_INSERM_DGOS_12563and EURACAN (EU project 739521).
Footnote
Conflicts of Interest: JY Blay, M Brahmi, A Dufresne, I Ray-Coquard received grants and research support from Novartis, Bayer, Pfizer, GSK.
References
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-26. [Crossref] [PubMed]
- Casali PG, Abecassis N, Bauer S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv68-iv78. [Crossref] [PubMed]
- Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110-8. [Crossref] [PubMed]
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 2005;41:2868-72. [Crossref] [PubMed]
- Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006;37:1527-35. [Crossref] [PubMed]
- Agaimy A, Wünsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 2007;31:113-20. [Crossref] [PubMed]
- Agaimy A, Wünsch PH, Dirnhofer S, et al. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol 2008;32:867-73. [Crossref] [PubMed]
- Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006-11. [Crossref] [PubMed]
- van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421-3. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011;11:865-78. [Crossref] [PubMed]
- Tabone-Eglinger S, Subra F, El Sayadi H, et al. KIT mutations induce intracellular retention and activation of an immature form of the KIT protein in gastrointestinal stromal tumors. Clin Cancer Res 2008;14:2285-94. [Crossref] [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [Crossref] [PubMed]
- Martín-Broto J, Rubio L, Alemany R, et al. Clinical implications of KIT and PDGFRA genotyping in GIST. Clin Transl Oncol 2010;12:670-6. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 2004;22:3813-25. [Crossref] [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Brahmi M, Alberti L, Dufresne A, et al. KIT exon 10 variant (c.1621 A > C) single nucleotide polymorphism as predictor of GIST patient outcome. BMC Cancer 2015;15:780. [Crossref] [PubMed]
- Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res 2003;9:3329-37. [PubMed]
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer 2003;106:887-95. [Crossref] [PubMed]
- Bachet J-B, Landi B, Laurent-Puig P, et al. Diagnosis, prognosis and treatment of patients with gastrointestinal stromal tumour (GIST) and germline mutation of KIT exon 13. Eur J Cancer 2013;49:2531-41. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008;53:245-66. [Crossref] [PubMed]
- Cassier PA, Ducimetière F, Lurkin A, et al. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhône Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer 2010;103:165-70. [Crossref] [PubMed]
- Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 2012;18:4458-64. [Crossref] [PubMed]
- Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am 2009;23:15-34. vii. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276-83. [Crossref] [PubMed]
- Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Reichardt P, Hartmann JT, Hall KS, et al. Response to Imatinib Rechallenge of GIST That Recurs Following Completion of Adjuvant Imatinib Treatment - the First Analysis in the SSGXVIII/AIO Trial Patient Population. Eur J Cancer 2011;47:15. [Crossref]
- Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med 2017;377:1813-23. [Crossref] [PubMed]
- Chibon F, Lagarde P, Salas S, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med 2010;16:781-7. [Crossref] [PubMed]
- Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;97:1854-9. [Crossref] [PubMed]
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Raut CP, Espat NJ, Maki RG, et al. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol 2017;35:11009. [Crossref]
Cite this article as: Laurent M, Brahmi M, Dufresne A, Meeus P, Karanian M, Ray-Coquard I, Blay JY. Adjuvant therapy with imatinib in gastrointestinal stromal tumors (GISTs)—review and perspectives. Transl Gastroenterol Hepatol 2019;4:24.


