Branched-chain amino acids in liver diseases
Introduction
The branched chain amino acids (BCAAs), valine (Val), leucine (Leu) and isoleucine (Ile), are essential amino acids for human beings and are involved in the pathophysiology of liver diseases (1). This review describes the biological properties of BCAAs and their clinical use in the management of liver cirrhosis. In addition, this review describes the role of BCAAs to suppress hepatocarcinogenesis and their potential role in the treatment of hepatocellular carcinoma (HCC).
Basic aspects of BCAAs in the liver
BCAAs and metabolism
BCAAs are involved in the metabolism of proteins, glucose and fats. BCAAs activate mammalian target of rapamycin (mTOR) signaling, stimulating the synthesis of glycogen and of proteins such as albumin (1,2). mTOR is involved in the phosphoinositide-3-kinase-protein kinase B (PI3K-Akt) signaling pathway (3) and plays central roles in cell growth (4), proliferation (5) and insulin resistance (6) (Figure 1).
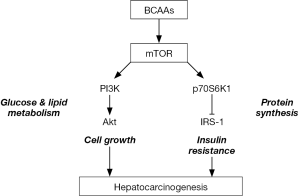
Glucose and lipid metabolism
BCAAs regulate the metabolism of glucose and lipids through the PI3K-Akt pathway. Ile was shown to mediate glucose uptake by PI3K independent of mTOR (7) and to decrease the level of plasma glucose (7). BCAAs have been shown to promote the uptake of glucose by skeletal muscle through activation of PI3K and protein kinase C, and subsequently induce glucose transporter translocation to the plasma membrane (8). Deficiencies in BCAAs reduce hepatic fatty acid synthesis, promote fatty acid β-oxidation and increase fat mobilization in white adipose tissue through the AMP-activated protein kinase (AMPK)-mTOR-FoxO1 pathway (9,10). In adipose tissue, Leu increases insulin-induced phosphorylation of mTOR and Akt, and then enhances the uptake of glucose (11). In addition, BCAAs increase peroxisome proliferator-activated receptor (PPAR)-γ and subsequently uncoupling protein 2 (UCP2) in liver and UCP3 in muscle through glucose transporter 4 (GLUT4) translocation (12), stimulating oxidation of free fatty acids and reducing triglyceride concentrations in mouse livers (13). Thus, BCAAs regulate fatty acid synthesis, transport, oxidation, lipolysis and adipokine secretion by affecting the expression of genes encoding AMPKα, mTOR, sirtuin-1 (SIRT-1) and PPAR-γ coactivator-1α (PGC-1α) (14). Furthermore Krüppel-like factor 15, a transcription factor, was shown to be involved in regulating the metabolism of glucose, lipids and amino acids (15).
Protein synthesis
BCAAs, especially Leu, contribute to protein synthesis through the mTOR pathway (16). Leu induces the phosphorylation of p70S6 kinase 1 (S6K1) and 4E-binding protein 1 (4EBP1) and the assembly of eukaryotic initiation factor 4E (eIF4E) in mTOR signaling (16), resulting in the synthesis of albumin (17-19). Leu also stimulates albumin mRNA translation through nuclear importation of polypyrimidine-tract-binding protein (20).
BCAA metabolites, such as branched-chain α-keto acids (BCKA), β-hydroxy-β-methyl butyrate (HMB) and glutamate, are also involved in regulating protein synthesis (21). BCKAs were shown to decrease protein expression in cardiomyocytes through mTORC2-Akt signaling (22). HMB was also shown to be involved in muscle protein synthesis and degradation through the mTOR signaling pathway, mainly through mTORC1 (23-25).
Insulin resistance
The activation of mTORC1 promotes insulin resistance through serine phosphorylation of insulin receptor substrate (IRS)-1 and IRS-2 (3). Serum BCAA concentrations were found to be elevated in mice lacking mitochondrial BCAA aminotransferase that catalyzes BCAAs. Those mice show lower fasting blood glucose and insulin concentrations, and homeostasis model assessment scores for insulin resistance (HOMA-IR) were significantly lower as compared with those in wild-type mice (26). Furthermore, administration of Leu or Ile improved insulin sensitivity in mice fed with high-fat diets (27,28). Improvement of insulin resistance by BCAAs has been found to be achieved by several mechanisms (1). For example, BCAAs were found to stimulate liver-type glucokinase and glucose transporter (29), as well as to suppress hepatic expression of glucose-6-phosphatase. BCAAs were also reported to transiently increase plasma concentrations of insulin in healthy individuals (30). BCAAs were found to improve HOMA-IR scores and the function of beta cell in patients with chronic liver disease (31). These results strongly suggest that BCAAs can ameliorate insulin resistance (1).
BCAA and hepatocyte proliferation
BCAA has been associated with cell proliferation through activation of mTORC1 (32). In a rat model of CCl4-induced liver injury, the supplementation of BCAA was shown to suppress hepatocyte apoptosis leading to retardation of the progression of the injury (33). In contrast, BCAAs enhanced hepatocyte regeneration in a rat hepatectomy model (34) and were shown to increase the secretion of hepatocyte growth factor (35). BCAAs are also shown to suppress oxidative stress by stimulating the expression of PGC-1α or SIRT-1 (36), or by activating the genes involved in antioxidant defenses (37). Those mechanisms could also contribute to promote hepatocyte proliferation.
BCAA and immunity
Nutrition is shown to be closely associated with the maturation or activation of immunity, and BCAAs are reported to be directly involved in the proliferation of lymphocyte or the maturation of dendritic cells (DCs) (1). All of the three BCAAs are shown to be requisite for mitogen-induced lymphocyte proliferation (38), and Val has the most prominent effect for the proliferation of lymphocytes.
The importance of BCAAs for immunity has been also shown in/by vivo studies. We previously evaluated the role of BCAAs on the local immune function in the liver and spleen of rats (36). We found that supplementation of BCAAs increased the numbers of intrahepatic lymphocytes and stimulated natural killer (NK) cells and lectin-dependent cytotoxic activities in the liver. It is of note that the number of lymphocytes isolated form the liver was increased as the concentrations of Val increased in plasma and liver. Importance of valine for the stimulation of immune response is supported by a report by Kakazu et al., in which they report the critical role of Val in the maturation of DCs (37). These findings indicate that Val may have a therapeutic potential for reducing hepatocarcinogenesis in patients with cirrhosis by restoring the immune functions (1,37,39).
In patients with liver cirrhosis, BCAA supplementation has been shown to increase the numbers of intrahepatic lymphocytes and restores the phagocytic activity of neutrophils and NK activity (40,41). Furthermore, the supplementation of BCAAs was shown to increase the number of circulating lymphocytes in postsurgical patients (42,43). BCAA supplementation in patients with chronic hepatitis C can restore malnutrition-association impairments in interferon signaling through the mTOR and FoxO pathways (44). Interestingly, supplementation of Val was shown to reduce hepatitis C viral load, which could be caused by enhancing DC function or interferon signaling (45). BCAAs were also shown to increase immunoglobulin A secretion, which could enhance mucosal surface defenses (46). Thus, BCAAs could regulate both innate and adaptive immune responses.
Serum concentration of BCAAs in cirrhotic patients
In patients with advanced cirrhosis, decreased serum concentrations of BCAA and increased concentrations of aromatic amino acids (AAAs), phenylalanine and tyrosine, are often found, resulting in a decreased ratio of BCAAs to AAAs, called the Fischer ratio (47). A decreased Fischer ratio is thought to be a cause of hepatic encephalopathy (HE) (1). Fischer ratio tends to become lower with the progression of cirrhosis, which could help assess the prognosis of cirrhotic patients with or without HCC (48,49). Moreover, a simplified Fischer ratio, the BCAA to tyrosine ratio (BTR), has been shown to be useful for the prediction of albumin concentration 1 year later (50). BTR was also reported to be useful in predicting the prognosis of patients with liver cirrhosis (51). These data suggest that the imbalance of amino acid, either decreased Fischer ratio or BTR, is an indicator for progression and prognosis of cirrhosis, and that correcting either ratio may be useful not only for nutritional improvement, but also for prevention of HE, in patients with cirrhosis (1).
Clinical application of BCAA supplementation in liver cirrhosis
BCAAs for liver cirrhosis
Since the liver is a central organ for nutrient metabolism, cirrhotic patients may develop various metabolic complications (52). Patients with cirrhosis frequently show protein and energy deficiencies. Protein deficiencies could lead to hypoalbuminemia, resulting in ascites retention and hepatic edema, whereas energy deficiencies could reduce fat and muscle mass and cause muscle weakness, both of which may significantly reduce their quality of life (QOL) (53). Significant improvement of QOL and prognosis in patients with cirrhosis can be achieved by the supplementation of BCAA. Two randomized trials showed a significant improvement of Short Form-36 scores of general health perception by BCAA supplementation (54,55). Another randomized study showed that BCAA supplementation improved weakness and fatigue (56). The supplementation of BCAA was also reported to improve sleep disturbance (57).
A large-scale post marketing study in Japan showed that oral BCAA administration significantly reduced the incidence of complications such as liver failure, esophageal varices rupture, HCC, and death (55). Furthermore, the supplementation of BCAA in cirrhotic patients showed the improvement of glucose metabolism and an increase in serum albumin concentration (58), and the effect of BCAA on maintenance or an increase in serum albumin concentrations in patients with both compensated and decompensated cirrhosis was confirmed by a randomized study (59). The effect of BCAA supplementation on serum albumin concentration may be more effective in the early stages of cirrhosis than in the advanced stage, which could enhance total hepatic parenchymal volume (60-62).
Accelerated fat oxidation and a catabolic state after fasting are frequently observed in cirrhotic patients, which can be presented by a decrease in respiratory quotient (RQ) (63). A late evening snack containing BCAA was shown to improve RQ, nutritional state and glucose intolerance (63,64). Since BCAAs have a higher energy efficiency than glucose or fatty acids, BCAAs may be the preferred nutrients for cirrhotic patients (65).
The guidelines of the European Society for Clinical Nutrition and Metabolism and the Study Group for the Standardization of Treatment of Viral Hepatitis Including Cirrhosis of the Ministry of Health, Labour and Welfare of Japan recommend BCAA supplementation in the treatment of advanced cirrhosis due to those preferential effects of BCAAs (1,66,67).
BCAA for HE
HE is a major and serious complication of advanced cirrhosis associated with decreased QOL in those patients, and often shows recurrence. Increased blood ammonia is usually found in HE patients, and ammonia is a pathogenic factor for HE development (68). BCAAs are used for the treatment of HE especially in cirrhotic patients, with preferential effects in most cases. The effects of BCAAs are not due to decrease in blood ammonia levels, but is thought to be due to the correction of a decreased Fischer ratio in patients with HE. HE frequently occurs in advanced cirrhotic patients after gastrointestinal bleeding, which is possibly associated with an absence of Ile and an abundance of Leu in hemoglobin molecules. The blood proteins with imbalanced BCAAs are absorbed from the gut lumen, leading to HE by way of BCAA antagonism (69).
A systematic review showed that BCAAs appeared to be beneficial on HE without adverse events (70,71). In contrast, two randomized studies showed that BCAAs did not clearly prevent HE in advanced cirrhotic patients (54,55). Furthermore, postoperative BCAA supplementation did not prevent the development of postoperative HE (72). Recently, a randomized, double-blind, multicenter study found that the supplementation of BCAAs did not reduce the recurrence of HE, although minimal HE can be prevented and muscle mass reduction can be recovered (73). Moreover, a systematic review showed that oral but not intravenous supplementation of BCAAs improved HE development (74). Non-absorbable disaccharides, which is much cheaper than BCAAs, have been shown to improve the development of HE and prevent overt HE, suggesting that non-absorbable disaccharides should be used for HE first, with BCAAs considered as the second line treatment (74). A meta-analysis showed that oral supplementation of BCAAs in patients with cirrhosis inhibited manifestations of HE especially in patients with overt HE rather than in those with minimal HE (66). Thus, oral administration of BCAAs, especially in combination with non-absorbable disaccharides, should be the treatment of choice in cirrhotic patients with HE (1).
Sarcopenia
Sarcopenia is an age-related progressive loss of skeletal muscle volume that leads to muscle weakness (75), and is associated with poor prognosis in patients with cirrhosis (76-78). Sarcopenia may be due to reduced muscle protein synthesis (79,80), and deficiencies in essential amino acids including BCAAs are thought to contribute to these processes (81,82). For example, Leu can stimulate mTOR signaling including S6K1 and 4E-BP1, leading to translation of mRNAs encoding ribosomal proteins (83,84), as well as in older rats (85). A recent clinical study showed that mTOR signaling is impaired and autophagy is increased in cirrhotic patients, and that these alterations can be reversed by supplementation with Leu-enriched BCAAs (86).
In clinical settings, supplementation with BCAAs, especially Leu, may increase skeletal muscle loss by activating mTOR signaling (86,87). However, the improvements in muscle volume were limited only to patients who showed improvements in metabolic parameters (87). Nutritional intervention with high-protein diets in elderly individuals, however, showed negative results (79,88). A recent study suggested that the effect of BCAA supplementation may be strengthened by light exercise, which upregulates amino acid transporter in skeletal muscle (89), and a prospective study also showed that BCAA supplementation and exercise had beneficial effects in cirrhotic patients (90). BCAA supplementation for sarcopenia in cirrhotic patients should therefore be combined with adequate exercise.
Insulin resistance
Insulin resistance is often observed in patients with chronic hepatitis C virus (HCV) infection, which are thought to be associated with various complications, such as steatosis, disturbances in glucose metabolism, and hepatocarcinogenesis (91). BCAAs, especially Leu and Ile, were shown to have beneficial effects on glucose metabolism (92). It has been revealed that BCAAs directly act on insulin target organs, such as adipose tissue, skeletal muscles, and the liver (93). Intravenous administration of BCAAs was shown to decrease plasma glucose levels in advanced cirrhotic patients (94), and oral BCAA supplementation was shown to reduce both blood glucose levels (95,96) and insulin resistance in male patients with cirrhosis (31,97). Furthermore, long-term BCAA administration was reported to improve glucose tolerance in patients with nonalcoholic steatohepatitis (NASH)-related cirrhosis, suggesting that long-term BCAA may be an effective treatment for NASH (98). A randomized study showed that BCAA treatment decreased HbA1c concentrations and improved insulin resistance in patients with chronic hepatitis C (99).
BCAA in the prevention and treatment of HCC
Prevention of HCC by BCAA
HCC usually develops in patients with chronic liver diseases, especially liver cirrhosis. Chronic inflammation and fibrosis are thought to be the major mechanisms for the development of HCC, with chronic hepatitis B virus (HBV) or HCV infection being the leading cause of HCC. Although viral eradication is likely the most effective strategy for preventing the development of HCC in patients with chronic HCV infection (100), elderly patients and patients with advanced liver fibrosis were found to develop HCC even after complete eradication of HCV by direct-acting antivirals (101). Nucleos(t)ide analogs have been found to strongly suppress viral replication in patients with chronic HBV infection, significantly inhibiting the development of HCC. However, hepatocarcinogenesis can still be observed in patients with undetectable HBV-DNA but high levels of serum HBV surface antigen (102). Therefore, other supportive therapies are needed to suppress HCC development in those patients.
Inhibition of HCC cell proliferation by BCAA in vitro
Early in vitro studies showed that increased BCAA/AA ratios (103) in the medium inhibited the proliferation of HepG2 liver tumor cells. BCAAs were found to decrease the expression of insulin-like growth factor-1 (IGF-1) receptor on HepG2 cells, leading to a decrease in insulin-mediated proliferation of HepG2 cells (104). In the presence of high insulin concentrations, BCAAs were also found to reduce the expression of vascular endothelial growth factor (VEGF) by HepG2 cells, an effect mediated by shortening of VEGF mRNA (105). Although these findings suggest that BCAAs directly inhibit the growth of HCC cells, studies are needed to show whether BCAAs can clinically inhibit tumor growth inhibition.
BCAAs were shown to reduce the expression of a cancer stem cell marker, epithelial cell adhesion molecule, via the mTOR pathway, thereby increasing tumor sensitivity to 5-flourouracil (106). Clinical trials showed that BCAA supplementation synergized with acyclic retinoid (107) or sorafenib (108) in tumor treatment, suggesting that combination of BCAAs and anticancer drugs can potentiate the antitumor effects of the latter.
Prevention of HCC development in animal models
Obese diabetic rats spontaneously develop liver tumors. BCAA supplementation was shown to inhibit the development of preneoplastic lesions in these rats, an effect mediated by the suppression of VEGF expression (109). BCAAs showed similar antitumor effects in obese diabetic mice (110).
BCAAs in clinical trials
A report from Japan revealed that BCAA supplementation reduced the development of HCC in obese [body mass index (BMI) >25 kg/m2] cirrhotic men and in those with alpha-fetoprotein levels >20 ng/mL (111). Another Japanese study also showed that BCAA supplementation (for longer than 6 months) significantly reduced HCC occurrence while enhancing event-free survival rates in Child-Pugh grade A cirrhotic patients (112). Those results are coincided with the preferential antitumor effects observed in animal models. Although there are no other data showing that BCAAs suppress hepatocarcinogenesis in cirrhotic patients, BCAA supplementation is thought to have beneficial effects on the suppression of the liver cancer development, an effect that may be associated with improvements in insulin resistance and/or the suppression of VEGF expression.
BCAA administration as supportive therapy during treatment of HCC
BCAA supplementation in patients undergoing liver resection for HCC resulted in a shorter hospital stay and rapid improvements in liver function after surgery (113). In addition, BCAA supplementation was found to increase protein metabolism and suppress progression to liver cirrhosis after hepatectomy for HCC (114). A recent randomized controlled trial showed that preoperative administration of BCAAs before liver resection for HCC resulted in a lower frequency of ascites and higher serum albumin concentrations postoperatively (111). BCAA supplementation also suppressed early recurrence after liver resection for HCC (115).
In contrast to these findings, one report showed that pre-, peri-, and post-postoperative supplementation with BCAAs did not reduce tumor recurrence rate or improve overall survival after liver resection (116). Despite these findings, the results of most studies indicate that BCAA supplementation has beneficial effects following liver resection.
Recent studies have assessed the effects of BCAA supplementation as supportive therapy during treatment for HCC. Patients with HCC are frequently treated by radiofrequency ablation (RFA) or transcatheter arterial chemoembolization (TACE). BCAA supplementation in patients undergoing RFA has been shown to maintain patient QOL (general health, physical functioning and social functioning) (117), liver function and serum albumin levels (118), and to improve overall survival and recurrence-free survival (119). BCAA supplementation showed similar results in HCC patients undergoing TACE (120) or radiotherapy (121).
Collectively, BCAA administration has various benefits, such as preventing the development of HCC in patients with liver cirrhosis, improving or maintaining liver reserve functions during invasive treatments for HCC, and enhancing overall survival after curative treatment for HCC. However, BCAA formulations are expensive and most reports to date have been from Japan. It will be necessary to analyze the cost effectiveness of BCAA supplementation in patients with liver cirrhosis or HCC, as well as to assess the effects of BCAA supplementation in non-Japanese patient populations.
Conclusions
BCAAs have been shown to have various biological effects, including the promotion of protein synthesis and hepatocyte proliferation, simulation of immune systems, improvement of insulin resistance, inhibition of liver cancer cell proliferation and neovascularization. All of these findings indicate that BCAAs may have beneficial effects on the management of patients with chronic liver diseases with/without HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol 2013;19:7620-9. [Crossref] [PubMed]
- Nishitani S, Ijichi C, Takehana K, et al. Pharmacological activities of branched-chain amino acids: specificity of tissue and signal transduction. Biochem Biophys Res Commun 2004;313:387-9. [Crossref] [PubMed]
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723-36. [Crossref] [PubMed]
- Zhenyukh O, Civantos E, Ruiz-Ortega M, et al. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 2017;104:165-77. [Crossref] [PubMed]
- Liu KA, Lashinger LM, Rasmussen AJ, et al. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab 2014;2:6. [Crossref] [PubMed]
- Jang C, Oh SF, Wada S, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 2016;22:421-6. [Crossref] [PubMed]
- Doi M, Yamaoka I, Fukunaga T, et al. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun 2003;312:1111-7. [Crossref] [PubMed]
- Nishitani S, Matsumura T, Fujitani S, et al. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem Biophys Res Commun 2002;299:693-6. [Crossref] [PubMed]
- Du Y, Meng Q, Zhang Q, et al. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012;43:725-34. [Crossref] [PubMed]
- Bai J, Greene E, Li W, et al. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol Nutr Food Res 2015;59:1171-81. [Crossref] [PubMed]
- Hinault C, Mothe-Satney I, Gautier N, et al. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. Faseb J 2004;18:1894-6. [Crossref] [PubMed]
- Duan Y, Li F, Wang W, et al. Alteration of muscle fiber characteristics and the AMPK-SIRT1-PGC-1alpha axis in skeletal muscle of growing pigs fed low-protein diets with varying branched-chain amino acid ratios. Oncotarget 2017;8:107011-21. [Crossref] [PubMed]
- Arakawa M, Masaki T, Nishimura J, et al. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J 58:161-70. [Crossref] [PubMed]
- Ma X, Han M, Li D, et al. L-Arginine promotes protein synthesis and cell growth in brown adipocyte precursor cells via the mTOR signal pathway. Amino Acids 2017;49:957-64. [Crossref] [PubMed]
- Fan L, Hsieh PN, Sweet DR, et al. Kruppel-like factor 15: Regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol Res 2018;130:123-6. [Crossref] [PubMed]
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 2006;83:500S-7S. [Crossref] [PubMed]
- Okuno M, Moriwaki H, Kato M, et al. Changes in the ratio of branched-chain to aromatic amino acids affect the secretion of albumin in cultured rat hepatocytes. Biochem Biophys Res Commun 1995;214:1045-50. [Crossref] [PubMed]
- Ijichi C, Matsumura T, Tsuji T, et al. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun 2003;303:59-64. [Crossref] [PubMed]
- Montoya A, Gomez-Lechon MJ, Castell JV. Influence of branched-chain amino acid composition of culture media on the synthesis of plasma proteins by serum-free cultured rat hepatocytes. In Vitro Cell Dev Biol 1989;25:358-64. [Crossref] [PubMed]
- Kuwahata M, Yoshimura T, Sawai Y, et al. Localization of polypyrimidine-tract-binding protein is involved in the regulation of albumin synthesis by branched-chain amino acids in HepG2 cells. J Nutr Biochem 2008;19:438-47. [Crossref] [PubMed]
- Nie C, He T, Zhang W, et al. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int J Mol Sci 2018;19. [Crossref] [PubMed]
- Guo X, Huang C, Lian K, et al. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem Biophys Res Commun 2016;480:106-13. [Crossref] [PubMed]
- Wilkinson DJ, Hossain T, Limb MC, et al. Impact of the calcium form of beta-hydroxy-beta-methylbutyrate upon human skeletal muscle protein metabolism. Clin Nutr 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Giron MD, Vilchez JD, Salto R, et al. Conversion of leucine to beta-hydroxy-beta-methylbutyrate by alpha-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J Cachexia Sarcopenia Muscle 2016;7:68-78. [Crossref] [PubMed]
- Kao M, Columbus DA, Suryawan A, et al. Enteral beta-hydroxy-beta-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 2016;310:E1072-84. [Crossref] [PubMed]
- She P, Reid TM, Bronson SK, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 2007;6:181-94. [Crossref] [PubMed]
- Ikehara O, Kawasaki N, Maezono K, et al. Acute and chronic treatment of L-isoleucine ameliorates glucose metabolism in glucose-intolerant and diabetic mice. Biol Pharm Bull 2008;31:469-72. [Crossref] [PubMed]
- Zhang Y, Guo K, LeBlanc RE, et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007;56:1647-54. [Crossref] [PubMed]
- Higuchi N, Kato M, Miyazaki M, et al. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem 2011;112:30-8. [Crossref] [PubMed]
- Zhang Y, Kobayashi H, Mawatari K, et al. Effects of branched-chain amino acid supplementation on plasma concentrations of free amino acids, insulin, and energy substrates in young men. J Nutr Sci Vitaminol (Tokyo) 2011;57:114-7. [Crossref] [PubMed]
- Kawaguchi T, Nagao Y, Matsuoka H, et al. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med 2008;22:105-12. [PubMed]
- Schieke SM, Phillips D, McCoy JP Jr, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem 2006;281:27643-52. [Crossref] [PubMed]
- Kuwahata M, Kubota H, Kanouchi H, et al. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res 2012;32:522-9. [Crossref] [PubMed]
- Kim SJ, Kim DG, Lee MD. Effects of branched-chain amino acid infusions on liver regeneration and plasma amino acid patterns in partially hepatectomized rats. Hepatogastroenterology 2011;58:1280-5. [Crossref] [PubMed]
- Tomiya T, Omata M, Fujiwara K. Significance of branched chain amino acids as possible stimulators of hepatocyte growth factor. Biochem Biophys Res Commun 2004;313:411-6. [Crossref] [PubMed]
- Kakazu E, Kanno N, Ueno Y, et al. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol 2007;179:7137-46. [Crossref] [PubMed]
- Chuang JC, Yu CL, Wang SR. Modulation of human lymphocyte proliferation by amino acids. Clin Exp Immunol 1990;81:173-6. [Crossref] [PubMed]
- Tsukishiro T, Shimizu Y, Higuchi K, et al. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J Gastroenterol Hepatol 2000;15:849-59. [Crossref] [PubMed]
- Kakazu E, Ueno Y, Kondo Y, et al. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology 2009;50:1936-45. [Crossref] [PubMed]
- Nakamura I, Ochiai K, Imai Y, et al. Restoration of innate host defense responses by oral supplementation of branched-chain amino acids in decompensated cirrhotic patients. Hepatol Res 2007;37:1062-7. [Crossref] [PubMed]
- Nakamura I. Impairment of innate immune responses in cirrhotic patients and treatment by branched-chain amino acids. World J Gastroenterol 2014;20:7298-305. [Crossref] [PubMed]
- Nuwer N, Cerra FB, Shronts EP, et al. Does modified amino acid total parenteral nutrition alter immune-response in high level surgical stress. JPEN J Parenter Enteral Nutr 1983;7:521-4. [Crossref] [PubMed]
- Cerra FB, Mazuski JE, Chute E, et al. Branched chain metabolic support. A prospective, randomized, double-blind trial in surgical stress. Ann Surg 1984;199:286-91. [Crossref] [PubMed]
- Honda M, Takehana K, Sakai A, et al. Malnutrition impairs interferon signaling through mTOR and FoxO pathways in patients with chronic hepatitis C. Gastroenterology 2011;141:128-40, 40.e1-2.
- Kawaguchi T, Torimura T, Takata A, et al. Valine, a branched-chain amino Acid, reduced HCV viral load and led to eradication of HCV by interferon therapy in a decompensated cirrhotic patient. Case Rep Gastroenterol 2012;6:660-7. [Crossref] [PubMed]
- Ma N, Guo P, Zhang J, et al. Nutrients Mediate Intestinal Bacteria-Mucosal Immune Crosstalk. Front Immunol 2018;9:5. [Crossref] [PubMed]
- Campollo O, Sprengers D, McIntyre N. The BCAA/AAA ratio of plasma amino acids in three different groups of cirrhotics. Rev Invest Clin 1992;44:513-8. [PubMed]
- Steigmann F, Szanto PB, Poulos A, et al. Significance of serum aminograms in diagnosis and prognosis of liver diseases. J Clin Gastroenterol 1984;6:453-60. [Crossref] [PubMed]
- Watanabe A, Higashi T, Sakata T, et al. Serum amino acid levels in patients with hepatocellular carcinoma. Cancer 1984;54:1875-82. [Crossref] [PubMed]
- Suzuki K, Suzuki K, Koizumi K, et al. Measurement of serum branched-chain amino acids to tyrosine ratio level is useful in a prediction of a change of serum albumin level in chronic liver disease. Hepatol Res 2008;38:267-72. [Crossref] [PubMed]
- Ishikawa T, Imai M, Ko M, et al. Evaluation of the branched-chain amino acid-to-tyrosine ratio prior to treatment as a prognostic predictor in patients with liver cirrhosis. Oncotarget 2017;8:79480-90. [Crossref] [PubMed]
- Charlton MR. Protein metabolism and liver disease. Baillieres Clin Endocrinol Metab 1996;10:617-35. [Crossref] [PubMed]
- Miwa Y, Moriwaki H. Nocturnal energy and BCAA supplementation in patients with liver cirrhosis. Hepatol Res 2004;30S:63-6. [Crossref] [PubMed]
- Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 2003;124:1792-801. [Crossref] [PubMed]
- Muto Y, Sato S, Watanabe A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 2005;3:705-13. [Crossref] [PubMed]
- Nakaya Y, Okita K, Suzuki K, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007;23:113-20. [Crossref] [PubMed]
- Ichikawa T, Naota T, Miyaaki H, et al. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Hepatol Res 2010;40:971-8. [Crossref] [PubMed]
- Sato S, Watanabe A, Muto Y, et al. Clinical comparison of branched-chain amino acid (l-Leucine, l-Isoleucine, l-Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV-EN study). Hepatol Res 2005;31:232-40. [Crossref] [PubMed]
- Habu D, Nishiguchi S, Nakatani S, et al. Comparison of the effect of BCAA granules on between decompensated and compensated cirrhosis. Hepatogastroenterology 2009;56:1719-23. [PubMed]
- Koreeda C, Seki T, Okazaki K, et al. Effects of late evening snack including branched-chain amino acid on the function of hepatic parenchymal cells in patients with liver cirrhosis. Hepatol Res 2011;41:417-22. [Crossref] [PubMed]
- Habu D, Nishiguchi S, Nakatani S, et al. Effect of oral supplementation with branched-chain amino acid granules on serum albumin level in the early stage of cirrhosis: a randomized pilot trial. Hepatol Res 2003;25:312-8. [Crossref] [PubMed]
- Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res 2004;30S:36-41. [Crossref] [PubMed]
- Nakaya Y, Harada N, Kakui S, et al. Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched-chain amino-acid-enriched nutrient mixture. J Gastroenterol 2002;37:531-6. [Crossref] [PubMed]
- Tsuchiya M, Sakaida I, Okamoto M, et al. The effect of a late evening snack in patients with liver cirrhosis. Hepatol Res 2005;31:95-103. [Crossref] [PubMed]
- Kato M, Miwa Y, Tajika M, et al. Preferential use of branched-chain amino acids as an energy substrate in patients with liver cirrhosis. Intern Med 1998;37:429-34. [Crossref] [PubMed]
- Plauth M, Cabre E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94. [Crossref] [PubMed]
- Kumada H, Okanoue T, Onji M, et al. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatol Res 2010;40:8-13. [Crossref] [PubMed]
- Bak LK, Iversen P, Sorensen M, et al. Metabolic fate of isoleucine in a rat model of hepatic encephalopathy and in cultured neural cells exposed to ammonia. Metab Brain Dis 2009;24:135-45. [Crossref] [PubMed]
- Plauth M, Schutz T. Branched-chain amino acids in liver disease: new aspects of long known phenomena. Curr Opin Clin Nutr Metab Care 2011;14:61-6. [Crossref] [PubMed]
- Als-Nielsen B, Koretz RL, Kjaergard LL, et al. Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst Rev 2003. [PubMed]
- Gluud LL, Dam G, Les I, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev 2017;5. [PubMed]
- Kanematsu T, Koyanagi N, Matsumata T, et al. Lack of preventive effect of branched-chain amino acid solution on postoperative hepatic encephalopathy in patients with cirrhosis: a randomized, prospective trial. Surgery 1988;104:482-8. [PubMed]
- Les I, Doval E, García-Martínez R, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 2011;106:1081-8. [Crossref] [PubMed]
- Gluud LL, Dam G, Borre M, et al. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence? Metab Brain Dis 2013;28:221-5. [Crossref] [PubMed]
- Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr 2006;136:277S-80S. [Crossref] [PubMed]
- Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166-73.e1. [Crossref] [PubMed]
- Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis 2012;16:95-131. [Crossref] [PubMed]
- Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015;31:193-9. [Crossref] [PubMed]
- Welle S, Thornton C, Jozefowicz R, et al. Myofibrillar protein synthesis in young and old men. Am J Physiol 1993;264:E693-8. [PubMed]
- Hasten DL, Pak-Loduca J, Obert KA, et al. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds. Am J Physiol Endocrinol Metab 2000;278:E620-6. [Crossref] [PubMed]
- Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250-8. [Crossref] [PubMed]
- Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest 1976;57:987-99. [Crossref] [PubMed]
- Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 2000;130:2413-9. [Crossref] [PubMed]
- Anthony TG, Anthony JC, Yoshizawa F, et al. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr 2001;131:1171-6. [Crossref] [PubMed]
- Dardevet D, Sornet C, Balage M, et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr 2000;130:2630-5. [Crossref] [PubMed]
- Tsien C, Davuluri G, Singh D, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015;61:2018-29. [Crossref] [PubMed]
- Kitajima Y, Takahashi H, Akiyama T, et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J Gastroenterol 2018;53:427-37. [Crossref] [PubMed]
- Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769-75. [Crossref] [PubMed]
- Agergaard J, Bulow J, Jensen JK, et al. Effect of light-load resistance exercise on postprandial amino acid transporter expression in elderly men. Physiol Rep 2017;5. [Crossref] [PubMed]
- Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2017;29:1416-23. [Crossref] [PubMed]
- Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Ann Hepatol 2010;9 Suppl:112-8. [PubMed]
- Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem 2009;16:4843-57. [Crossref] [PubMed]
- Kawaguchi T, Izumi N, Charlton MR, et al. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011;54:1063-70. [Crossref] [PubMed]
- Tabaru A, Shirohara H, Moriyama A, et al. Effects of branched-chain-enriched amino acid solution on insulin and glucagon secretion and blood glucose level in liver cirrhosis. Scand J Gastroenterol 1998;33:853-9. [Crossref] [PubMed]
- Korenaga K, Korenaga M, Uchida K, et al. Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res 2008;38:1087-97. [Crossref] [PubMed]
- Sakaida I, Tsuchiya M, Okamoto M, et al. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol Res 2004;30S:67-72. [Crossref] [PubMed]
- Kawaguchi T, Taniguchi E, Itou M, et al. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int 2007;27:1287-92. [PubMed]
- Miyake T, Abe M, Furukawa S, et al. Long-term branched-chain amino acid supplementation improves glucose tolerance in patients with nonalcoholic steatohepatitis-related cirrhosis. Intern Med 2012;51:2151-5. [Crossref] [PubMed]
- Takeshita Y, Takamura T, Kita Y, et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism 2012;61:1388-94. [Crossref] [PubMed]
- Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017. [Epub ahead of print]. [PubMed]
- Ravaioli F, Conti F, Brillanti S, et al. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig Liver Dis 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kawanaka M, Nishino K, Nakamura J, et al. Quantitative Levels of Hepatitis B Virus DNA and Surface Antigen and the Risk of Hepatocellular Carcinoma in Patients with Hepatitis B Receiving Long-Term Nucleos(t)ide Analogue Therapy. Liver Cancer 2014;3:41-52. [Crossref] [PubMed]
- Saito Y, Saito H, Nakamura M, et al. Effect of the molar ratio of branched-chain to aromatic amino acids on growth and albumin mRNA expression of human liver cancer cell lines in a serum-free medium. Nutr Cancer 2001;39:126-31. [Crossref] [PubMed]
- Wubetu GY, Utsunomiya T, Ishikawa D, et al. Branched chain amino acid suppressed insulin-initiated proliferation of human cancer cells through induction of autophagy. Anticancer Res 2014;34:4789-96. [PubMed]
- Miuma S, Ichikawa T, Arima K, et al. Branched-chain amino acid deficiency stabilizes insulin-induced vascular endothelial growth factor mRNA in hepatocellular carcinoma cells. J Cell Biochem 2012;113:3113-21. [Crossref] [PubMed]
- Nishitani S, Horie M, Ishizaki S, et al. Branched chain amino acid suppresses hepatocellular cancer stem cells through the activation of mammalian target of rapamycin. PLoS One 2013;8. [Crossref] [PubMed]
- Shimizu M, Shirakami Y, Hanai T, et al. Pharmaceutical and nutraceutical approaches for preventing liver carcinogenesis: chemoprevention of hepatocellular carcinoma using acyclic retinoid and branched-chain amino acids. Mol Nutr Food Res 2014;58:124-35. [Crossref] [PubMed]
- Imanaka K, Ohkawa K, Tatsumi T, et al. Impact of branched-chain amino acid supplementation on survival in patients with advanced hepatocellular carcinoma treated with sorafenib: A multicenter retrospective cohort study. Hepatol Res 2016;46:1002-10. [Crossref] [PubMed]
- Yoshiji H, Noguchi R, Kitade M, et al. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J Gastroenterol 2009;44:483-91. [Crossref] [PubMed]
- Iwasa J, Shimizu M, Shiraki M, et al. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci 2010;101:460-7. [Crossref] [PubMed]
- Kikuchi Y, Hiroshima Y, Matsuo K, et al. A Randomized Clinical Trial of Preoperative Administration of Branched-Chain Amino Acids to Prevent Postoperative Ascites in Patients with Liver Resection for Hepatocellular Carcinoma. Ann Surg Oncol 2016;23:3727-35. [Crossref] [PubMed]
- Hayaishi S, Chung H, Kudo M, et al. Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis. Dig Dis 2011;29:326-32. [Crossref] [PubMed]
- Meng WC, Leung KL, Ho RL, et al. Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg 1999;69:811-5. [Crossref] [PubMed]
- Togo S, Tanaka K, Morioka D, et al. Usefulness of granular BCAA after hepatectomy for liver cancer complicated with liver cirrhosis. Nutrition 2005;21:480-6. [Crossref] [PubMed]
- Ichikawa K, Okabayashi T, Maeda H, et al. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today 2013;43:720-6. [Crossref] [PubMed]
- Meng J, Zhong J, Zhang H, et al. Pre-, peri-, and postoperative oral administration of branched-chain amino acids for primary liver cancer patients for hepatic resection: a systematic review. Nutr Cancer 2014;66:517-22. [Crossref] [PubMed]
- Kuroda H, Ushio A, Miyamoto Y, et al. Effects of branched-chain amino acid-enriched nutrient for patients with hepatocellular carcinoma following radiofrequency ablation: a one-year prospective trial. J Gastroenterol Hepatol 2010;25:1550-5. [Crossref] [PubMed]
- Ishikawa T, Michitaka I, Kamimura H, et al. Oral branched-chain amino acids administration improves impaired liver dysfunction after radiofrequency ablation therapy for hepatocellular carcinoma. Hepatogastroenterology 2009;56:1491-5. [PubMed]
- Nishikawa H, Osaki Y, Iguchi E, et al. The effect of long-term supplementation with branched-chain amino acid granules in patients with hepatitis C virus-related hepatocellular carcinoma after radiofrequency thermal ablation. J Clin Gastroenterol 2013;47:359-66. [Crossref] [PubMed]
- Nishikawa H, Osaki Y, Inuzuka T, et al. Branched-chain amino acid treatment before transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2012;18:1379-84. [Crossref] [PubMed]
- Lee IJ, Seong J, Bae JI, et al. Effect of Oral Supplementation with Branched-chain Amino Acid (BCAA) during Radiotherapy in Patients with Hepatocellular Carcinoma: A Double-Blind Randomized Study. Cancer Res Treat 2011;43:24-31. [Crossref] [PubMed]
Cite this article as: Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. Transl Gastroenterol Hepatol 2018;3:47.


