Perspectives on the evolving state of the art management of gastrointestinal stromal tumours
Introduction
Gastrointestinal stromal tumours (GISTs) account to almost one fifth of all sarcomas, making them the most common mesenchymal tumour (1). GISTs can originate from any part of the gastrointestinal tract, from the oesophagus to the rectum, while extra-intestinal sites of origins are extremely rare. GISTs are thought to arise from the phenotypically similar interstitial cells of Cajal residing in the muscular layer of the digestive tract; stomach and small intestine are the most frequent locations of origin (2,3). The aggressiveness/malignant potential of GISTs vary pending on tumour size, mitotic activity, and the anatomical origin itself. Surgical resection is the only curative treatment modality for localized GIST and constitutes definitive therapy in up to two thirds of these cases (4,5). Although a significant proportion of patients will be cured with surgery alone, approximately 40% will eventually relapse, the great majority within the first 5 years (6). The Armed Forces Institute of Pathology Risk Group Classification is still one of the most widely used system for the assertion of the risk of recurrence after curative surgery (3). This classification system does not incorporate the accumulated vast knowledge in terms of mutational landscape of GISTs (7).
The discovery of the KIT tyrosine kinase receptor and subsequently that of the mutually exclusive KIT and platelet-derived growth factor receptor (PDGFRA) gain of function mutations have provided a paradigm shift in the way we classify, diagnose and treat GISTs (7,8). The different segments of the KIT transmembrane receptor (extracellular domain, transmembrane hinge, juxtamembrane domain, intracellular tyrosine kinase domain) all have a specific designated role in the process of tyrosine kinase activation. In up to 82–87% of GISTs activating mutations in either the KIT (69–83%) or the homologous PDGFRA receptor (12–14%) lead to constitutive, ligand-independent activation ultimately leading to increased cell proliferation, apoptosis inhibition. The most frequent KIT ‘hot spot’ mutations are in exon 11 (encoding the juxtamembrane domain), less frequently in exon 9 (extracellular domain) and rarely in exon 13 (ATP-binding region) and exon 17 (activation loop of the kinase) (7,9). Deletions affecting codons 557–558 of exon 11 of the c-KIT gene account to around a quarter of all GIST cases (10). KIT mutations in exon 9 (7–15% of all GIST cases) are characterized by A502-Y503 codon duplications, with up to 80% of the cases originating outside the stomach, mainly from the small intestine (11,12). The frequency of primary exon 13 and 17 mutations is around 1–2%, these tumours mostly arising from the small bowel rather than the stomach (13). The 60–65% of PDGFRA mutations are p.D842V substitutions involving the second kinase domain (corresponding to exon 17 of KIT) (14).
The 10–15% of all adult GISTs have no detectable mutations in KIT or PDGFRA receptors and historically are referred to as ‘wild-type’ (WT) GISTs. About half of all KIT/PDGFRA WT-GISTs have inactivating mutations in the genes coding one of the four (SDHA, SDHB, SDHC and SDHD) subunits of the succinate dehydrogenase (SDH) complex (15,16). The lack of KIT mutations does not affect the KIT protein expression of these tumours; they have a predilection for the stomach, display a multi-lobulated/multi-nodular growth pattern and are prone to metastasize to the lymph nodes (16). In the absence KIT/PDGFRA mutations, most infrequently further mutations in the BRAF (V600E), HRAS, NRAS or PIK3CA genes of the downstream signalling pathway have been detected (7,17). NF1-associated GISTs characterized by the somatic inactivation of the WT NF1 allele constitute a small subgroup of KIT/PDGFRA WT-GISTs. These frequently multicentric, mostly small intestinal tumours present as small, low mitotic index lesions and display a favourable long-term outcome with low recurrence and metastases rates (7). Most recently a small subgroup of “Quadruple WT-GIST” has been identified, characterized by lack of mutations in any of the known KIT, PDGFRA, BRAF, RAS or NF1 genes while retaining an intact SDH complex (SDHB IHC positive, and no mutations in SDH). The detailed characterization of this sub-group is still ongoing (18).
With its ubiquitous role in the pathogenesis of GISTs, KIT emerged as a universal therapeutic target. The pronounced clinical efficacy of imatinib (a competitive inhibitor of the ATP-binding domain) in GIST was confirmed one and a half decades ago (8). In the advanced/metastatic setting imatinib offers an overall 80% disease control rate (objective response or stable disease), a median progression-free survival (PFS) of approximately 20 months and a median overall survival (OS) of around 50 months (19).
Neoadjuvant treatment
While assessing the upfront resectability of GISTs is strictly speaking a surgical decision, multidisciplinary teams specialized in the management of GISTs need to establish the potential beneficial effects of down-staging prior to curative surgery. There are several clinical scenarios where neoadjuvant treatment can be indicated, including prospect of multi-visceral resection, risk of a mutilative surgery and low chances of a complete R0 resection. Current guidelines recommend neoadjuvant treatment for unresectable GISTs or those resectable tumors where there is a risk of significant morbidity (5). Up so far the role of neoadjuvant imatinib treatment has not been assessed within the frame of randomized phase III trials, nevertheless retrospective series and some prospective phase II trials have demonstrated the efficacy and safety of neoadjuvant imatinib in locally advanced GISTs (20-22).
We consider mutational status analysis essential in tailoring a neoadjuvant treatment plan. The standard treatment dose is 400 mg of imatinib once a day (5). A higher dosage of imatinib 800 mg/day) for KIT exon 9-mutated GISTs is suggested by international guidelines as a significant PFS advantage has been demonstrated in the metastatic setting (5,23). There is no randomized clinical trial evidence in regard of the optimal length of neoadjuvant imatinib therapy; nevertheless, a 6–9 months’ treatment interval can be recommended to minimize the risk of tumour progression on treatment (5,21,22).
The EORTC sarcoma centers study analyzed disease-free survival (DFS) and disease-specific survival (DSS) in 161 patients with locally advanced, non-metastatic GISTs who received neoadjuvant imatinib. The tumor resection after preoperative imatinib, with a median time on therapy of 40 weeks was R0 in 83% of the patients. Only two patients out of the 161 have demonstrated disease progression during neoadjuvant therapy. Five-year DSS/DFS rates were 95/65%, respectively with a median OS of 104 months. The most common mutations affected exon 11 KIT (65%) (23). In terms of postoperative treatment, locally advanced GISTs are all to be considered high risk for relapse, regardless the postoperative pathology readings. In the EORTC sarcoma centers study poorer DFS was related not only to primary tumor location in small bowel but also to lack of postoperative treatment (23). In another more recently published, smaller series of 76 patients whom received neoadjuvant imatinib, lack of adjuvant imatinib was the only factor related to inferior PFS and OS (24). The authors of this review would suggest strongly considering adjuvant imatinib therapy for all patients whom received neoadjuvant treatment prior to primary tumour resection.
Within the frame of a desperate need of pronounced response for tumour down-staging, one needs to maximize the chances of efficacious treatment. A Cmin threshold of 760 ng/mL was associated with prolonged PFS in the treatment of advanced GIST patients. There is an established relationship between imatinib trough concentration and outcomes in the treatment of advanced GISTs (25). Therapeutic dose monitoring shall be considered for all patients undergoing neoadjuvant treatment, wherever such a service is at hand. It must be noted that there is a low, around 3% risk of intratumoural bleeding with upfront neoadjuvant imatinib treatment, thus patients need close clinical monitoring in the first few weeks of therapy (26).
The neoadjuvant treatment for GISTs with genotypes refractory/resistant to licensed tyrosine kinase inhibitors (TKIs) is very difficult. More recently, BLU-285, a mutation-specific inhibitor of kinases with mutations in KIT D816V and PDGFRA D842V showed promising clinical activity in the advanced setting. The results of the dose escalation part of a phase I study confirmed strong clinical activity of BLU-285 in PDGFRA D842-mutant advanced GISTs with an overall response rate (ORR) of 60%(27).
Another oral small-molecule inhibitor crenolanib also demonstrated some, as compared to BLU-285 less impressive, clinical activity in metastatic PDGFRA-mutant (including D842V-mutated kinase) with a single objective response out of seven patients treated (28).
Adjuvant treatment
Microscopic complete resection with histologically negative margins (R0) without rupturing the tumor is the optimal standard treatment for localized GISTs. Although a significant proportion of patients will be cured with surgery alone, approximately 40% will eventually relapse, the great majority within the first 5 years after the operation (5,6). With the proven impressive efficacy of imatinib in controlling advanced disease, its’ use was swiftly extended into to the adjuvant treatment of GIST (29).
The Z9001 trial data suggests that high-risk patients (tumor size >10 cm and high mitotic rate) derive a greater efficacy from adjuvant therapy (30). The use of adjuvant imatinib is not recommended for low and very low risk GISTs, however there is no consensus for those with intermediate risk tumours (5). Based on the results of the three pivotal Phase III adjuvant trials regardless of the classification scheme used, patients identified as intermediate risk had a clinical course similar to that of the low-risk group (5). For patients with intermediate risk tumours the risks and benefits of treatment and the lack of clinical data unequivocally supporting a survival benefit in this subgroup should be thoroughly explained and debated. The currently recruiting French GIST randomized phase II trial comparing imatinib over 3 years versus surveillance in intermediate-risk patients with a high-risk genomic grade index might help us defining a sub-group meriting adjuvant treatment (31).
Molecular profiling as prognostic and predictive tool in adjuvant decision making
There are several risk-stratification schemes in use for operable GISTs which help determine the need of adjuvant treatment for high-risk tumours. The most widely used are the National Institutes of Health consensus classification and its modified version, alongside the Armed Forces Institute of Pathology (AFIP) criteria. The AFIP classification is currently still the most frequently used one in the everyday practice in Europe. Large tumour size, high mitosis count, non-gastric location, presence of rupture, and male sex have all been proven independent adverse prognostic factors (6).
Over the last decade a vast amount of knowledge has built up in terms of understanding the prognostic and predictive relevance of different genetic subtypes of GIST (7). Deletions affecting codons 557-558 of exon 11 of the c-KIT gene have been associated with an aggressive, metastasizing phenotype and indicate an overall poor prognosis (10). In the Polish registry study 80% of 557/558 codon deleted GISTs stratified as high-risk tumours, with a lower 5-year relapse free survival (RFS) rate as compared with any other KIT exon 11 mutations but also with other exon 11 deletions that have not involved codons 557/558 (12). In the Multinational European Contica GIST Database analysis the poor prognostic impact of KITdel-inc557/558 on patients’ survival was only significant in GIST localized to the stomach. Twice as many patients with gastric GIST harbouring KITdel-inc557/558 relapsed 5 years after surgery than with other KIT exon 11 mutations (61% vs. 29%). It is important to remember that in stomach GISTs classified as AFIP non–high risk the presence of KIT del-inc557/558 remained an important prognosticator for poor outcome in comparison with other KIT exon 11 mutations, KIT exon 9 and PDGFRA exon 18 mutations (14). In the ACOSOG Z 9001 trial the 1 year of imatinib arm was only superior to placebo in terms of RFS in the KIT exon 11 deleted subgroup; none of the GISTs with KIT exon 11 point mutations or insertions, exon 9 mutations, PDGFRA-mutant tumors or WT-GISTs had a statistically significant delay in recurrence as compared to placebo (32). The underrepresentation of KIT exon 11 substituted GISTs in advanced/metastatic trials (1.6–1.86%) as compared to population-based studies (15.5–28.6%) are most probably in keeping with a more indolent clinical behaviour of this genotype. These tumours are characterised by low mitotic activity and small size at presentation with 5-year RFS rate of 50.7% as opposed to KIT deleted (28.1%) or duplicated GISTs (40.0%) (7,12,14). Duplications in KIT exon 11 have been associated with gastric tumour location and a more favourable clinical course (12,33).
In contrast to some historical observations (34) KIT exon 9 mutations per se do not have a relative (as compared with KIT exon 11 mutations of non-gastric origin) negative prognostic relevance. The worse prognosis of KIT exon 9 mutants is related to the non-gastric tumour location itself rather than to an intrinsic aggressive biologic nature of this mutation (11,14). In the meta GIST pooled analysis of two pivotal Phase III trials comparing 400 vs. 800 mg daily imatinib dose in the advanced setting, the sole predictive factor of response was the presence of KIT exon 9 mutation. The estimated risk of progression or death was reduced by 42% in the high-dose arm (compared with the standard-dose arm) in the KIT exon 9 mutated subgroup, without an OS advantage (19). Given the data supporting the use of a higher dose of imatinib in the case of an exon 9 KIT mutation in advanced GIST, many clinicians prefer to use the 800 mg/day imatinib dose even in the adjuvant treatment of high-risk patients. Beyond the obvious regulatory/licensing restrictions limiting this practice, it must be emphasized that no controlled trial supports higher dosing in the adjuvant setting.
Gastric KIT exon 13 mutant GISTs are slightly larger and of a higher risk group than gastric GISTs on average, while the clinical behaviour of small intestinal GISTs with KIT exon 13 or KIT exon 17 mutations are similar to other small intestinal GISTs (7).
The markedly lower representation of PDGFRA-mutated GISTs in advanced clinical trials (around 2% vs. 12–14% in population studies) is in keeping with a comparatively benign clinical behaviour of these tumours. Moreover, PDGFRA mutant GISTs are almost exclusively of gastric origin (90–93%), thus belonging to a better prognostic group (7,12). PDGFRA exon 18 mutation status correlates with excellent 5-year DFS of 75%, in contrast to KIT exon 9 and KITdel-inc557/558 mutated tumours. Interestingly, no significant difference in DFS figures was observed between PDGFRA p.D842V versus other PDGFRA exon 18 mutations (12). There is consensus that PDGFRA D842V mutated GIST should not be treated with any adjuvant therapy, given the lack of sensitivity of this genotype both in vitro and in vivo (7). The excellent survival of patients with tumours harbouring PDGFRA mutations in the placebo group of the ACOSOG Z 9001 trial provide a good additional argument that these patients may not require adjuvant treatment (32).
It must be emphasized that the prognosis of SDH deficient GISTs cannot be predicted by size and mitotic rate as even small, mitotically inactive SDH deficient GISTs may metastasize. Interestingly when metastases do occur they may be strikingly indolent, sometimes remaining stable for years or decades (15). In a recently published study SDHA mutations were associated with statistically significant better clinical outcome as compared with KIT/PDGFRA mutations and KIT/PDGFRA WT without SDH deficiency. All survival analyses (from diagnosis of primary tumours and from diagnosis of metastatic disease) confirmed a far more indolent course of disease for patients with SDHA mutated WT-GISTs (35).
The majority of NF1-associated GISTs present as small, low mitotic index lesions and they are associated with quite favourable long-term clinical outcomes reflected in low recurrence and metastases rates (36). Interestingly NF-1 associated GISTs arising from the duodenum display an aggressive behaviour, being large mitotically active tumours with pronounced metastatic potential (37).
More recently, the combined prognostic value of the most relevant genetic GIST subtypes has been analyzed in a series of 451 untreated primary localized GISTs for KIT, PDGFRA and BRAF mutations and offered further proof that mutational status is a significant prognostic indicator of OS in treatment naive, localized GISTs. Based on multivariable Cox regression models the authors identified three distinct molecular risk groups. Group I, consisting of PDGFRA exon 12, BRAF and KIT exon 13-mutated cases, exhibited the best clinical outcome. The intermediate risk (HR =3.06) Group II, included ‘Triple-Negative’, KIT exon 17, PDGFRA exon 18 D842V, and PDGFRA exon 14-mutated GISTs. Group III, comprised of KIT exon 9 and exon 11 and PDGFRA non-D842V exon 18 mutant GISTs, displayed the worst clinical outcome (HR =4.52) (38). This study clearly highlighted the combined prognostic impact of mutational status on the natural history of GIST.
Inclusion of molecular prognostic grouping into currently used clinico-pathologic risk stratification criteria could clearly fine tune the decision-making process for adjuvant therapy. The authors of this review strongly believe that these important findings will have to be swiftly incorporated into international guidelines directing treatment (Table 1).
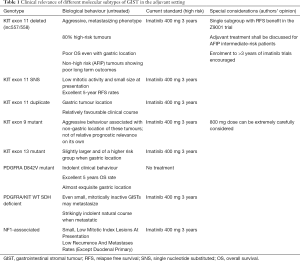
Full table
Optimal length of adjuvant treatment
According to the OS findings in the SSGXVIII/AIO trial, 3 years of adjuvant imatinib therapy are the recommended standard of care for patients with GIST with high-risk features. At a median follow-up of 90 months, patients randomized to 3-year imatinib dosing had a significantly improved OS (92% vs. 85% for the 1-year arm) (39). The results of 2 years vs. surveillance EORTC phase III trial examined the so called imatinib failure-free survival (IFFS) the time to initiation of a different TKI following recurrence was no different in between treatment and control arms (87% vs. 84% respectively). There was a slight trend toward IFFS benefit in the high-risk subgroup (40).
With the never-ending debates around the ability of imatinib to actually eradicate disease altogether (as opposed to a ‘cytostatic effect’ of the drug), there is a drive to extend the length of treatment well beyond 3 years. In a phase II trial of 91 high-risk GIST patients with sensitive mutations 5 years of adjuvant imatinib treatment led to 5-year 90% RFS and 95% OS rates. 7 “true” recurrences were recorded, with 6 occurring 7.4 to 23.1 months after imatinib discontinuation. Only one patient with a high-risk PDGFRA D842V mutant gastric GIST recurred while on treatment (41). It is worth noting that half of the patients discontinued treatment early, though only a minority of all patients (16%) discontinued due to adverse events. Two currently recruiting randomized trials for high-risk GIST patients compare the current 3-year long standard of care with 5 and 6 years of treatment respectively (42,43).
The SSGXVIII/AIO study proved that tumor rupture is a highly unfavourable prognostic factor, with the overwhelming majority of these patients relapsing at 5 years after surgery (39). Independently from conventional risk-stratification schemes, these patients all should be offered adjuvant treatment. The optimal length of “adjuvant” treatment in these virtually metastatic patients, is as uncertain as for other high-risk groups; nevertheless, one would offer indefinite imatinib treatment pending on other high risk features of the tumours.
Management of advanced GIST
Almost half of advanced GIST patients treated with imatinib have a survival longer than 5 years, with approximately one fifth of patients showing a long-lasting disease control for more than 10 years. It must be noted that patients with advanced GISTs who have a KIT exon 11 mutation have a superior prognosis as compared with all other mutation subtypes (44,45). The SWOG Intergroup Trial S0033 univariate analysis of OS by KIT exon 11mutant, KIT exon 9 mutant, and KIT/PDGFRA WT genotypes revealed median survival times of 66, 38, and 40 months, respectively. In long-term survivors more responses [complete response (CR) + partial response (PR)], were seen in the KIT exon 11 mutant genotype group than in the KIT/PDGFRA WT or KIT exon 9 groups: 70%, 48% and 50% respectively. The median PFS for patients with KIT exon 11 mutations was 25 months compared to 17 months for patients with KIT exon 9 mutations and 13 months for those with WT genotype. Interestingly no difference in PFS/OS were found amongst patients whose GIST had different classes of KIT exon 11 mutations (45).
In the pooled analysis of the two pivotal Phase III trials comparing 400 vs. 800 mg daily imatinib dose the sole predictive factor of response was the presence of KIT exon 9 mutation. The estimated risk of progression or death was reduced by 42% in the high-dose arm (compared with the standard-dose arm) in patients with KIT exon 9 mutated tumours. However, no significant difference in OS was seen between patients treated with 400 and 800 mg imatinib, irrespective of mutational status. The study concluded that for most patients, the recommended daily dose is 400 mg daily, with the exception of KIT exon 9 mutated tumours where the 800 mg dose can be considered (19).
PDGFRA exon 18 D842 V mutant GISTs have been long considered as insensitive to imatinib treatment (46). In contrast to the common perception, in a recent large multicenter observational study reported objective response to imatinib in a small proportion of patients with PDGFRA D842V-mutated GISTs. The 12.5% of 16 patients with the mutation had partial response, 18.8% had stable disease and 56.3% had progressive disease (PD) as best response according to Choi criteria, with a median TTP of 8.0 months (47). We can conclude that as patients with PDGFRA D842V-mutated GISTs should not be universally denied imatinib, especially in clinical scenarios where no other TKI is available.
In a small series of seven metastatic NF1-associated GIST patients, three out of the four imatinib treated patients showed primary resistance to the treatment (all 3 tumours were KIT/PDGFRA WT-GISTs). The fourth metastatic patient with an exon 18 mutated tumour had temporary stable disease (SD). Median OS for this 4-patient cohort was 21 months (48).
The pivotal BFR14 trial addressed the very important clinical question whether imatinib dosing interruption in the metastatic setting would have a detrimental effect on outcomes, or not. According to their results the interruption of imatinib leads to a very rapid progression in most patients, thus such an approach is inappropriate, unless excessive toxicity occurs (49). There is some clinical benefit of increasing the dose of imatinib to 800 mg/day upon PD as some further disease control can be achieved in a third of the patients (50,51).
It must be noted that disease progression in the real-life setting might be due to the suboptimal pharmacological serum concentration of imatinib. A Cmin imatinib concentration of above 760 ng/mL was associated with a prolonged PFS (24).
It is highly debatable whether replacing imatinib (which has got an incredibly mild side effect profile) in the first line setting with a more toxic combination of several agents will ever be of clinical relevance. Nevertheless, there are attempts to explore whether alternating different, potent TKIs in the first line setting would be of any effect on delaying emergence of secondary resistance. The ongoing phase II, randomised, open-label ALT-GIST study evaluates the use of an alternating regimen of imatinib and regorafenib for the first-line treatment of advanced GIST (52).
Secondary imatinib resistance
Acquisition of KIT or PDGFRA secondary mutations represent the most frequent mechanism of imatinib resistance in GIST. Radiological evidence of clonal resistance can be detected as the appearance of one or more areas of increased vascularity within a previously responding or stable lesion. These lesions can precede by several months’ PD according to RECIST (53). Secondary mutations are more likely to be found in patients who initially harboured KIT exon 11 mutations (73%) as compared to KIT exon 9 mutations (19%) (54).
Sunitinib and regorafenib have been approved for imatinib resistant GIST based on placebo controlled randomized Phase III trials (55,56).
Sunitinib is a potent multi-target (including KIT and PDGFRA) TKI licensed for second-line therapy after imatinib failure, given at 50 mg/day dose in 4 weeks on/2 weeks off treatment schedule (55). Unfortunately, the side effect profile of sunitinib is far less advantageous as compared to imatinib, leading to frequent dose reductions and treatment discontinuations. A continuously administered dosed daily oral regimen of 37.5 mg lower daily dose has proved itself as an effective and well tolerated alternative of administration, and can be supported as an on an individualized basis (57).
Primary mutational status has proved to be of a predictive value in the second line setting as well. Sunitinib treatment of KIT exon 9 mutants and KIT/PDGFRA WT-GISTs led to extended PFS and median OS as compared to other genotypes, including KIT exon 11 mutants. Secondary mutations had also a predictive value, whereas gatekeeper mutations in the KIT ATP-binding pocket were associated with increased sunitinib sensitivity. Activation loop mutations involving exon 17 KIT are mostly insensitive to sunitinib (54).
Regorafenib is another oral broad spectrum multi-target TKI that inhibits KIT and PDGFRA as well. In the licensing phase III trial a median PFS of 4.8 months was achieved for patients initially randomized to regorafenib compared with the placebo arm (HR 0.27, 95% CI: 0.19–0.39, P<0.0001). (56). Regorafenib proved to be efficacious in all genetic subtypes of GISTs including exon 9 and PDGFRA D842V mutants (58). Nevertheless, according to long-term follow-up results of a phase II trial KIT exon 11 mutant GISTs had an impressive median PFS of 13.4 months, while KIT/PDGFRA WT and SDH complex proficient tumors had a mere median PFS of 1.6 months (59).
In patients where the bulk of their metastatic disease is well controlled with a specific TKI and where there is solely unifocal PD detected, cytoreductive surgery can be considered as an alternative to changing course of systemic treatment. In a large two-institutional series of 400 operations the outcomes of cytoreductive surgery in unifocal progressors was similar to the expected efficacy of an eventual sunitinib switch (60).
BLU-285 is a mutation-specific inhibitor of kinases with mutations in KIT D816V and PDGFRA D842V, in which most TKIs are ineffective. The results of the dose escalation part of a phase I study were recently presented at the 2017 ASCO meeting and showed that BLU-285 is well tolerated on a QD schedule at doses up to the MTD of 400 mg and that its exposure at 300–400 mg QD provides broad coverage of primary and secondary KIT/PDGFRA mutants. BLU-285 has strong clinical activity in PDGFRA D842-mutant GISTs with an ORR of 60% per central review, whereas median PFS was not reached at the time of the report. It also demonstrates important anti-tumor activity including radiographic response and prolonged PFS in heavily pre-treated, KIT-mutant GISTs at doses of 300–400 mg QD (27). Based on these encouraging data, planning is underway for a phase III randomized study of BLU-285 in third-line metastatic GISTs.
The oral small-molecule inhibitor crenolanib exhibits activity against FLT3 and the PDGFRs (including D842V-mutated kinase). In a phase II trial of metastatic PDGFRA-mutant GIST seven patients demonstrated objective response in one-and SD in three (28). A randomized, double-blind, placebo-controlled, multicenter, phase III trial of crenolanib in subjects with advanced or metastatic GIST with D842V mutation in the PDGFRA gene is ongoing (61).
DCC-2618, a potent pan-KIT and PDGFRα kinase switch control inhibitor has shown activity across a broad range of TKI treatment-emergent mutations. In a Phase I dose-escalation study of oral 150 mg daily DCC-2618-FDG-PET scans were performed at baseline and after 3 weeks of treatment during the escalation phase and computed tomography (CT) scans were done every 2 treatment cycles. DCC-2618 was well tolerated by patients. FDG PET scans showed that 22 of the 32 (69%) patients with KIT or PDGFR -mutant GIST had a partial metabolic response according to EORTC criteria. Of the 37 evaluable patients, 5 patients achieved partial response per RECIST. Fourteen of the 24 evaluable patients receiving DCC-2618 at doses of 100 mg/day demonstrated PFS lasting more than 6 months, including 9 patients on DCC-2618 at >cycle 10 (62). As DCC-2618 showed encouraging disease control in heavily pre-treated GIST patients demonstrating objective responses and achieving prolonged stable disease, a Phase III clinical study was opened to evaluate the safety and efficacy of DCC-2618 for treating patients with advanced GISTs. The INVICTUS randomised, double-blind, placebo-controlled, international, multicentre trial seeks to assess the tolerability of DCC-2618 compared to placebo in patients with advanced GIST whose previous therapies have included imatinib, sunitinib, and regorafenib (63). A second Phase III study is planned later this year evaluating DCC-2618 in second-line GIST patients who have progressed or are intolerant to front-line therapy with imatinib.
Conclusions
GISTs are the most common type of mesenchymal tumours of the digestive tract, mainly defined by the presence or lack of mutually exclusive gain-of-function mutations in the KIT and PDGFRA receptors. WT-GISTs are a rather heterogeneous group with no detectable mutation in either the KIT or the PDGFRA receptor genes. WT-GISTs can classified into two large groups, based on SDH complex proficiency. Detailed mutational analysis in alternative pathways helps further sub-classify WT-GISTs.
KIT exon 11 mutant patients are twice as likely to respond to imatinib than those with exon 9 mutant or WT-GISTs with a progression free and OS advantage as compared to all non-KIT exon 11 mutant GISTs. KIT exon 9 mutant GISTs are more likely to respond to the higher 800 mg dose of imatinib, and in the advanced setting patients can be started ab ovo on the higher dose. The PDGFRA D842V isoform with a substitution involving codon D842 in exon 18 is generally believed to lead to primary imatinib resistance. Caution should be exercised when it comes to therapeutic decisions as recent data suggests some response to imatinib in this subset of tumours.
While SDH-deficient ‘pediatric-type’ GISTs have been previously attributed absolute primary imatinib-resistance, most recent reports suggest imatinib responsiveness in SDHA-mutated tumours. Acquisition of secondary mutations in either KIT or PDGFRA represents the most frequent mechanism of imatinib resistance in GIST. Adjuvant therapy in “high risk” WT-GIST and the optimal systemic treatment for metastatic WT-GIST remain debatable ‘hot topic’ questions, with no clear-cut clinical guidelines.
Mutational analysis of GISTs holds an equally important predictive and prognostic value in the neoadjuvant, adjuvant and palliative treatment of the disease. Genetic profiling helps tailoring the best treatment and its’ sequence, while also setting expectations of treatment response. Early enrolment into clinical trials needs to take priority in situations where efficacy of available treatment is highly questionable, saving the patient from unnecessary toxicity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ducimetière F, Lurkin A, Ranchère-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294. [Crossref] [PubMed]
- Huizinga JD, Thuneberg L, Klüppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995;373:347-49. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumours: Pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib-naive GISTs: A retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol 2011;35:1646-56. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93-107. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Sciot R, Debiec-Rychter M, Daugaard S, et al. EORTC Soft Tissue and Bone Sarcoma Group; Italian Sarcoma Group; Australasian Trials Group. Distribution and prognostic value of histopathologic data and immunohistochemical markers in gastrointestinal stromal tumours (GISTs): An analysis of the EORTC phase III trial of treatment of metastatic GISTs with imatinib mesylate. Eur J Cancer 2008;44:1855-60. [Crossref] [PubMed]
- Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumours: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23:6190-8. [Crossref] [PubMed]
- Künstlinger H, Huss S, Merkelbach-Bruse S, et al. Gastrointestinal stromal tumours with KIT exon 9 mutations: Update on genotype-phenotype correlation and validation of a high-resolution meltingassay for mutational testing. Am J Surg Pathol 2013;37:1648-59. [Crossref] [PubMed]
- Wozniak A, Rutkowski P, Piskorz A, et al. Polish Clinical GIST Registry. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann Oncol 2012;23:353-60. [Crossref] [PubMed]
- Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behaviour: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumours. Clin Cancer Res 2003;9:3329-37. [PubMed]
- Wozniak A, Rutkowski P, Schöffski P, et al. Tumour genotype is an independent prognostic factor in primary gastrointestinal stromal tumours of gastric origin: a European multicenter analysis based on ConticaGIST. Clin Cancer Res 2014;20:6105-16. [Crossref] [PubMed]
- Miettinen M, Killian JK, Wang ZF, et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumours (GISTs) signals SDHA germline mutation. Am J Surg Pathol 2013;37:234-40. [Crossref] [PubMed]
- Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: A clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol 2011;35:1712-21. [Crossref] [PubMed]
- Yamamoto H, Oda Y. Gastrointestinal stromal tumour: recent advances in pathology and genetics. Pathol Int 2015;65:9-18. [Crossref] [PubMed]
- Pantaleo MA, Nannini M, Corless CL, et al. Quadruple wild-type (WT) GIST: Defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med 2015;4:101-3. [Crossref] [PubMed]
- Gastrointestinal Stromal Tumour Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumours: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- Kurokawa Y, Yang HK, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117:25-32. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Ramaswamy A, Jain D, Sahu A, et al. Neoadjuvant imatinib: longer the better, need to modify risk stratification for adjuvant imatinib. J Gastrointest Oncol 2016;7:624-31. [Crossref] [PubMed]
- Bouchet S, Poulette S, Titier K, et al. Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumours in a real-life setting. Eur J Cancer 2016;57:31-8. [Crossref] [PubMed]
- Bechtold RE, Chen MY, Stanton CA, et al. Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with Gleevec. Abdom Imaging 2003;28:808-14. [Crossref] [PubMed]
- Heinrich M, Jones R, von Mehren M, et al. Clinical activity of BLU-285 in advanced gastrointestinal stromal tumor (GIST). J Clin Oncol 2017;35:abstr 11011.
- Matro JM, Yu JQ, Heinrich MC, et al. Correlation of PET/CT and CT RECIST response in GIST patient with PDGFRA D842V gene mutations treated with crenolanib. J Clin Oncol 2014;32:abstr 10546.
- Cohen MH, Cortazar P, Justice R, et al. Approval summary: imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. Oncologist 2010;15:300-7. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Efficacy of Imatinib in Patients With Intermediate-risk Gastrointestinal Stromal Tumor With a High-risk Genomic Grade Index (GIGIST). Available online: https://clinicaltrials.gov/ct2/show/NCT02576080
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Lasota J, Dansonka-Mieszkowska A, Stachura T, et al. Gastrointestinal stromal tumours with internal tandem duplications in 3' end of KIT juxtamembrane domain occur predominantly in stomach and generally seem to have a favorable course. Mod Pathol 2003;16:1257-64. [Crossref] [PubMed]
- Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behaviour: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumours. Clin Cancer Res 2003;9:3329-37. [PubMed]
- Pantaleo MA, Lolli C, Nannini M, et al. Good survival outcome of metastatic SDH-deficient gastrointestinal stromal tumours harbouring SDHA mutations. Genet Med 2015;17:391-5. [Crossref] [PubMed]
- Hostein I, Faur N, Primois C, et al. BRAF mutation status in gastrointestinal stromal tumours. Am J Clin Pathol 2010;133:141-8. [Crossref] [PubMed]
- Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumours in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am. J. Surg. Pathol 2006;30:90-6. [Crossref] [PubMed]
- Rossi S, Gasparotto D, Miceli R, et al. KIT, PDGFRA, and BRAF mutational spectrum impacts on the natural history of imatinib-naive localized GIST: a population-based study. Am J Surg Pathol 2015;39:922-30. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276-83. [Crossref] [PubMed]
- Raut CP, Espat NJ, Maki RG, et al. PERSIST-5: Five-year extended treatment with adjuvant imatinib for patients with intermediate/high risk primary gastrointestinal stromal tumor (GIST). J Clin Oncol 2017.
- Three Versus Five Years of Adjuvant Imatinib as Treatment of Patients With Operable GIST. Available online: https://clinicaltrials.gov/ct2/show/NCT02413736
- Efficiency of Imatinib Treatment Maintenance or Interruption After 3 Years of Adjuvant Treatment in Patients With Gastrointestinal Stromal Tumours (GIST) (ImadGist). Available online: https://clinicaltrials.gov/ct2/show/NCT02260505
- Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR 14 trial of the French Sarcoma Group. Eur J Cancer 2016;52:173-80. [Crossref] [PubMed]
- Heinrich MC, Rankin C, Blanke CD, et al. Correlation of Long-term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol 2017;3:944-52. [Crossref] [PubMed]
- Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumour: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26:5360-7. [Crossref] [PubMed]
- Farag S, Somaiah N, Choi H, et al. Clinical characteristics and treatment outcome in a large multicenter observational cohort of PDGFRA exon 18 mutated gastrointestinal stromal tumour (GIST) patients. Eur J Cancer 2017;76:76-83. [Crossref] [PubMed]
- Mussi C, Schildhaus HU, Gronchi A, et al. Therapeutic consequences from molecular biology for gastrointestinal stromal tumour patients affected by neurofibromatosis type 1. Clin. Cancer Res 2008;14:4550-5. [Crossref] [PubMed]
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25:1107-13. [Crossref] [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [Crossref] [PubMed]
- Zalcberg JR, Verweij J, Casali PG, et al. EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group; Australasian Gastrointestinal Trials Group. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 2005;41:1751-7. [Crossref] [PubMed]
- A Randomised Trial of Imatinib Alternating With Regorafenib Compared to Imatinib Alone for the First Line Treatment of Advanced Gastrointestinal Stromal Tumour (GIST) (ALT GIST). Available online: https://clinicaltrials.gov/ct2/show/NCT02365441
- Liegl B, Kepten I, Le C, Zhu M, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumour. J Clin Oncol 2008;26:5352-9. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang Y, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- George S, Blay JY, Casali PG, Le Cesne A, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959-68. [Crossref] [PubMed]
- Kollàr A, Maruzzo M, Messiou C, et al. Regorafenib treatment for advanced, refractory gastrointestinal stromal tumour: a report of the UK managed access program. Clin Sarcoma Res 2014;4:17. [Crossref] [PubMed]
- Ben-Ami E, Barysauskas CM, von Mehren M, et al. Long-term follow-up results of the multicentre phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol 2016;27:1794-9. [Crossref] [PubMed]
- Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Randomized Trial of Crenolanib in Subjects With D842V Mutated GIST; Available online: https://clinicaltrials.gov/ct2/show/NCT02847429
- Janku F, Razak ARA, Gordon MS, et al. Encouraging activity of novel pan-KIT and PDGFRα inhibitor DCC-2618 in patients (pts) with Gastrointestinal Stromal Tumor (GIST). Ann Oncol 2017;28:521-38. [Crossref]
- Phase 3 Study of DCC-2618 vs Placebo in Advanced GIST Patients Who Have Been Treated With Prior Anticancer Therapies (INVICTUS); Available online: https://clinicaltrials.gov/ct2/show/NCT03353753?term=invictus&rank=5
Cite this article as: Szucs Z, Jones RL. Perspectives on the evolving state of the art management of gastrointestinal stromal tumours. Transl Gastroenterol Hepatol 2018;3:21.


