Minimally invasive surgery for gastric cancer in USA: current status and future perspectives
Introduction
Worldwide there are nearly 1 million new cases of gastric cancer each year and it remains the third leading cause of cancer related deaths worldwide (1). There is wide geographical variation in the incidence of gastric cancer with the highest incidence seen in Eastern Asia, Eastern Europe, and some Latin American countries. In Western countries, like the United States, the incidence of gastric cancer is lower with approximately 21,000 new cases diagnosed each year (2). Many studies have been done in both the East and West with regards to the use of minimally invasive techniques for gastrectomy for gastric cancer. It is important to keep in mind, however, that there are differences in the presentation of disease between the East and West which may limit the generalizability of Eastern results to Western patients.
Tumors located in the proximal third of the stomach are more common in Western countries and patients typically present with more advanced stage disease often due to lack of government supported screening programs (3-8). The incidence of proximal gastric cancer is on the rise in the United States, most likely related to the obesity epidemic in the United States and prevalence of gastroesophageal reflux disease (9,10). While the incidence of non-cardia gastric cancer has been declining in almost all race and age groups, the incidence of distal gastric cancer has been going up in younger Caucasian patients in the 25 to 39 years old age group (11).
Presentation with earlier stage disease is a well-established observation in the East, likely due to higher incidence of disease and more robust screening protocols. The incidence of earlier stage presentation of gastric cancer is, however, increasing in the United States. Our high volume institution in the United States has observed nearly a doubling in the presentation of early stage disease, from 20% to 40%, since 1985 (12). This increase in early stage presentation has given way to the more widespread application and utilization of minimally invasive techniques to treat gastric cancer, as surgery remains the only curative option for early gastric cancer. There are multiple retrospective and prospective studies as well as meta-analyses from both the East and the West which have shown oncologic equivalency between minimally invasive and open gastrectomy (OG) for gastric cancer (13). Many of these studies have suggested improvements in areas such as estimated blood loss, length of hospital stay, return of bowel function, analgesic requirements, recovery time, and quality of life in patients who undergo minimally invasive gastrectomy compared to OG. Perhaps the most important observation in the United States is more rapid postoperative recovery seen after minimally invasive gastrectomy which has allowed patients to go on to receive indicated adjuvant systemic chemotherapy rather than being limited by the morbidities associated with open surgery (14). Despite a fairly robust body of data supporting minimally invasive techniques as an excellent option for appropriately selected patients, factors such as learning curves associated with advanced minimally invasive techniques and patient selection particularly at the beginning stages of surgeon experience are still extremely important considerations when selecting surgical approach. Since the disease is still rarely seen in the United States outside of high-volume centers, the widespread acceptance of minimally invasive approaches for gastric cancer has been limited by the learning curve associated with both laparoscopic and robotic gastrectomy and the technical skills required to achieve acceptable oncologic outcomes including extent of gastric resection and lymphadenectomy.
Laparoscopic gastrectomy (LAG)
Early gastric cancer
The use of laparoscopic-assisted distal gastrectomy for early gastric cancer was first described by Kitano et al. in 1994 (15). This technique became the focus for many retrospective studies out of the East in the early 2000’s which supported the oncologic adequacy and feasibility of the laparoscopic approach. These studies showed promise for laparoscopy in the management of early gastric cancer, particularly in terms of decreasing length of hospital stay, analgesic use, and estimated blood loss (16-20). Following this series of retrospective studies, a number of randomized control trials (RCTs) were performed to prospectively compare OG to LAG. In 2002, Kitano et al. published the first RCT for laparoscopic distal gastrectomy (LADG). This study included 28 patients with early gastric cancer who were randomized to either open distal gastrectomy (ODG) or LADG. This trial found that that the LADG group had less estimated blood loss, earlier return of bowel function, and improved pain scores (21). Subsequent RCTs showed similar trends to both the retrospective observations and the results from the Kitano et al. RCT with regards to blood loss, pain medication requirements, and length of hospital stay (22-24).
Other important measures highlighted by these studies were volume of lymph nodes retrieved, overall survival (OS), disease free survival, and quality of life. An RCT published by Lee et al. in 2005 showed an increased number of lymph nodes retrieved with ODG compared to LADG (38.1 vs. 31.8) but both techniques retrieved an adequate number of lymph nodes for accurate staging (22). The only RCT from the West was published by Huscher et al. in 2005 and this study showed no statistically significant difference in the 5-year OS (59% vs. 56%) or disease-free survival (57% vs. 55%) between the two groups, supporting the oncologic equivalency between the two techniques (23). In 2008 Kim et al. randomized 164 patients to LADG vs. ODG and again noted decreased analgesic requirements, estimated blood loss, and length of hospital stay in the LADG and increased number of nodes harvested in the ODG group (45.1 vs. 29). Similar to the Kim et al. study from 2005, however, the number of harvested nodes was adequate for staging with both techniques. A new finding in this trial was an improved quality of life observed in the LADG group compared to ODG group (24).
The first multicenter RCT was carried out by the Korean Laparoscopic Gastrointestinal Group (KLASS). This was a phase III, multicenter, prospective RCT to evaluate short and long term outcomes in patients with early gastric cancer who underwent either LADG or ODG. Published analysis of preliminary outcomes in 2010 showed lower estimated blood loss in LADG group. There was no statistically significant difference in the postoperative complication rate or mortality between the two groups (25). The short-term outcome analysis was later published in 2016 which revealed that LADG was associated with decreased estimated blood loss (190 vs. 156 mL, P<0.001), shorter length of hospital stay (7.1 vs. 7.9 days, P<0.001), and that ODG was associated with higher lymph node retrieval (43.7 vs. 40.5, P=0.001). More clinically significant, however, was the finding of a lower overall complication rate associated with LADG compared to ODG (13% vs. 19.9%, P=0.001). The mortality rate was similar between the two groups and was not found to be statistically significant (26).
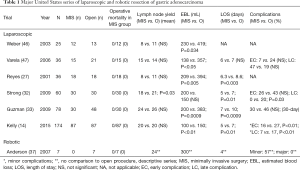
In the United States, Reyes et al. were the first group to publish their experience with LAG for gastric cancer. This was a retrospective case matched series of 36 patients, 18 of whom underwent LAG and 18 of whom underwent OG. Cases were matched for age and surgical indication. This study showed that LAG was associated with lower estimated blood loss, two days shorter length of hospital stay, earlier return of bowel function, and decreased incidence of postoperative ileus. There were no differences in the extent of lymphadenectomy between the two groups or in the incidence of intraoperative complications. The study did find a significantly longer operative time associated with the laparoscopic group compared to the open group (4.2 vs. 3.0 hours), likely due to the known learning curve associated with minimally invasive techniques (27).
Advanced gastric cancer
The first laparoscopic total gastrectomy for cancer was reported in 1999 by Azagra et al. (28). The use of LAG for advanced gastric cancer has since been evaluated by multiple large retrospective studies, largely from Eastern countries. Initial concerns regarding laparoscopic total gastrectomy revolved around status of the proximal resection margin, technical limitations in restoring gastrointestinal continuity with the esophagojejunal anastomosis, and ability to achieve adequate lymphadenectomy using laparoscopic techniques for advanced gastric cancer. Shinohara et al. reported on 336 patients who underwent OG vs. LAG for clinical stage T2–T4 gastric cancer. The LAG group had less estimated blood loss and shorter hospital stay, findings similar to previous studies comparing LADG and ODG. There was no statistically significant difference observed in mortality, complication rate, or 5-year disease free survival between the two groups (29). Park et al. showed similar outcomes associated with LADG for advanced gastric cancer (30). A prospective randomized trial done by Cai et al. in 2011 compared OG and LAG for patients with advanced gastric cancer. The morbidity rate in the LAG group was 12.2% compared to 19.1% in the OG group but this was not statistically significant (P=0.357). There was also no difference in OS between the two groups (67.1% in LAG group vs. 53.8% in OG group, P=0.911) (31).
Two of the largest early studies from the West were published by Strong et al. and Guzman et al. in 2009, both from high volume cancer centers in the United States. The first study reported on the initial laparoscopic experience of Memorial Sloan Kettering Cancer Center. The study matched 30 patients who underwent LADG with 30 patients who underwent ODG. The two groups did not differ in regards to demographics or stage. Approximately half of the patients in this study were either stage Ia or Ib (55%). LADG was associated with a decreased length of stay and decreased complications, both early (26% vs. 43%, P=0.07) and late (0% vs. 20%, P=0.03). Oncologic outcomes were comparable between the two approaches in terms of margin status and lymph node retrieval. Median operative time for the laparoscopic approach was 270 minutes compared to 126 minutes in the open group (P<0.01) (32). The second series reported on the experience of City of Hope. In this study 78 consecutive patients were evaluated. There were 48 patients in the OG group and 30 patients in the minimally invasive group. LAG was associated with decreased estimated blood loss and decreased length of hospital stay, consistent with findings of previous studies. This study also showed increased operative time associated with the minimally invasive approach compared to open (399 vs. 298 minutes, P<0.0001). Important to this series was the observation that all laparoscopic resections had negative resection margins and adequate number of lymph nodes retrieved for accurate staging. There was no statistically significant difference in the number of lymph nodes retrieved between the two approaches, likely owning to surgeon experience at this high volume center (33).
A meta-analysis by Viñuela et al. looked at RCTs and high quality non-randomized trials evaluating LADG vs. ODG published between 2000 and 2009. Included in this study were 6 RCTs and 32 high quality non-RCTs, which amounted to the evaluation of 3,055 patients in total. Of the 3,055 patients included, 1,658 underwent LADG and 1,397 underwent ODG. LADG was associated with lower overall complications (OR 0.59; P<0.001), medical complications (OR 0.49; P=0.002), and decreased length of hospital stay. There was no significant difference in the postoperative mortality or major surgical complications (13). A more recent study by Kelly et al. in 2015 reported on 87 patients undergoing LAG and 87 patients undergoing OG. Almost 40% of the patients in this study had locally advanced disease and approximately 20% of patients had proximally located tumors. This study revealed that LAG was associated with lower estimated blood loss, decreased duration of narcotic and epidural use, and decreased incidence of minor complications in the early postoperative period (27% vs. 16%; P<0.01) and late postoperative period (17% vs. 7%; P<0.01). Extremely relevant and important to this finding of decreased morbidity was the observation that patients who underwent LAG had a higher likelihood of going on to receive adjuvant systemic chemotherapy when indicated (14). Receipt of adjuvant chemotherapy is extremely important in patients with advanced gastric cancer in the West, as patients typically present with more advanced stage disease. The multidisciplinary management of locally advanced gastric cancer in the United States has largely been governed by the findings of both the MacDonald trial in 2001 (34) and MAGIC trial in 2006 (35), with these patients receiving either neoadjuvant or adjuvant chemotherapy. The importance of the finding of increased receipt of indicated adjuvant chemotherapy following LAG in the study by Kelly et al. is that previous studies had shown that not all patients eligible for adjuvant therapy actually go on to receive this therapy due to prolonged recovery from their operation.
Robotic surgery for gastric cancer
The first published use of robotic gastrectomy for cancer was by Hashizume et al. in 2003 (36). Anderson et al. published the first series of robotic assisted laparoscopic subtotal gastrectomies with extended lymphadenectomy from the United States in 2007. While this series was small (7 patients), the study demonstrated acceptable lymph node retrieval (24), 0% 30-day mortality, and no evidence of disease recurrence at the time of publication. Median length of stay (4 days) was consistent with other published case control studies from the United States evaluating laparoscopic versus OG (37). In 2009, Song et al published their experience with their first 100 consecutive robotic gastrectomies with lymphadenectomy at a center in Korea. The goal of the study was to evaluate effectiveness, safety, and technical feasibility of the robotic approach. The series included 33 total gastrectomies and 67 subtotal gastrectomies. The study showed a postoperative morbidity rate of 13%, postoperative mortality rate of 1%, average length of hospital stay of 7.8 days, and mean number of lymph nodes retrieved of 36.7. All surgical margins were microscopically negative. Overall, this study demonstrated the feasibility and safety of robotic gastrectomy in the management of gastric cancer (38).
In 2014, a meta-analysis was published comparing robotic gastrectomy (RAG) vs. LAG for gastric cancer. This study evaluated 1,875 patients who underwent either robotic gastrectomy or LAG. This analysis showed that RAG was associated with lower estimated blood loss, longer distal margin, and similar volume of harvested lymph nodes (39). In 2015, Coratti et al. published a series of 98 consecutive robotic gastrectomies with D2 lymphadenectomy performed exclusively by two surgeons at a center in Italy. This study looked at perioperative, postoperative, and long-term outcomes. The series included 38 total gastrectomies, 59 distal gastrectomies, and one proximal gastrectomy. Pathologic staging revealed early disease in 46.9% of the patients. Average follow-up time for all patients was 46.9 months. Perioperative measures of importance revealed an average estimated blood loss of 105.4 mL and an intraoperative complication rate of 2.04%. In terms of postoperative outcomes, the average length of hospital stay was 7 days, postoperatively morbidity was 12.2%, and postoperative mortality was 4.1%. Analysis of long-term follow-up data revealed a 5-year OS rate of 73.3%. Five-year DSS was subdivided by stage with a 100% 5-year DSS for stage IA patients, 84.6% for stage IB patients, 76.9% for stage II patients, 21.5% for stage III patients, and 0% for stage IV patients (of which there was only a single patient). Overall this study showed promising long-term outcomes after robotic gastrectomy compared to historic evidence from both open and laparoscopic series (40).
Another presumed advantage of robotic gastrectomy is that the learning curve for robotic gastric surgery may be easier to learn compared to laparoscopic gastric surgery (41). The published number of cases representing the learning curve for laparoscopic gastric surgery is anywhere from 40 to 100 cases (42-45). It is thought that robotic gastric surgery may have an easier learning curve due to the benefits of three-dimensional imaging, articulating instruments for more precise dissection, and functional imaging. Since gastric cancer is relatively rare in Western countries, the widespread use of robotic platforms for gastric cancer is likely limited by the relatively lower number of gastric cancer cases in most institutions. The hope is that the experience with the robotic platform at high volume centers in the United States will facilitate better understanding of the necessary learning curve for proficiency with robotic gastric surgery and will allow for adequately powered retrospective and prospective studies to better evaluate the oncologic adequacy, perioperative benefits, and postoperative outcomes.
Conclusions
Minimally invasive gastrectomy has emerged as a safe, feasible, and oncologically acceptable approach for appropriately selected patients with gastric cancer. Benefits associated with minimally invasive techniques for gastrectomy include lower estimated blood loss, shorter length of hospital stay, comparable or fewer perioperative complications, and quicker postoperative recovery for patients. Many of the supporting studies for minimally invasive gastrectomy have come out of the East and the applicability of these findings to patients in the West has been questioned due to the increased incidence of gastric cancer, earlier stage of presentation, and higher case volumes in the East as well as the known heterogeneity of disease between the two regions. Despite these differences, published studies from the United States (summarized in Table 1) have shown similar outcomes to Eastern studies and results have been very promising thus far. Perhaps the most significant finding from recent Western studies is the more rapid postoperative recovery following LAG. Quicker recovery after surgery was noted to be associated with an improvement in the receipt of indicated adjuvant systemic chemotherapy in patients with advanced gastric cancer. The widespread acceptance and utilization of minimally invasive techniques in the West will likely continue to be limited by the decreased incidence of gastric cancer and, outside of a few high volume centers, low volume of gastrectomy cases for surgeons in the West. The hope, however, is that the indications for minimally invasive gastrectomy will continue to expand with an enlarging body of data from future studies. With the emergence of robotic assisted procedures as another minimally invasive technique suitable for the management of gastric cancer, the hope is that future studies and the experiences of high volume centers will provide more information regarding the learning curve of robotic gastrectomy as well as the oncologic equivalency, safety, and perioperative and postoperative outcomes following this technique.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology 1993;104:510-3. [Crossref] [PubMed]
- Theuer CP. Asian gastric cancer patients at a southern California comprehensive cancer center are diagnosed with less advanced disease and have superior stage-stratified survival. Am Surg 2000;66:821-6. [PubMed]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. [Crossref] [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [Crossref] [PubMed]
- Schwarz RE, Zagala-Nevarez K. Ethnic survival differences after gastrectomy for gastric cancer are better explained by factors specific for disease location and individual patient comorbidity. Eur J Surg Oncol 2002;28:214-9. [Crossref] [PubMed]
- Yao JC, Schnirer II, Reddy S, et al. Effects of sex and racial/ethnic group on the pattern of gastric cancer localization. Gastric Cancer 2002;5:208-12. [Crossref] [PubMed]
- Verdecchia A, Mariotto A, Gatta G, et al. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer 2003;39:1603-9. [Crossref] [PubMed]
- Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287-9. [Crossref] [PubMed]
- Hansson LE, Sparén P, Nyrén O. Increasing incidence of carcinoma of the gastric cardia in Sweden from 1970 to 1985. Br J Surg 1993;80:374-7. [Crossref] [PubMed]
- Anderson WF, Camargo MC, Fraumeni JF Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723-8. [Crossref] [PubMed]
- Herrera-Almario G, Strong VE. Minimally Invasive Gastric Surgery. Ann Surg Oncol 2016;23:3792-7. [Crossref] [PubMed]
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446-56. [Crossref] [PubMed]
- Kelly KJ, Selby L, Chou JF, et al. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann Surg Oncol 2015;22:3590-6. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Adachi Y, Shiraishi N, Shiromizu A, et al. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 2000;135:806-10. [Crossref] [PubMed]
- Shimizu S, Uchiyama A, Mizumoto K, et al. Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery? Surg Endosc 2000;14:27-31. [Crossref] [PubMed]
- Tanimura S, Higashino M, Fukunaga Y, et al. Hand-assisted laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Laparosc Endosc Percutan Tech 2001;11:155-60. [Crossref] [PubMed]
- Yano H, Monden T, Kinuta M, et al. The usefulness of laparoscopy-assisted distal gastrectomy in comparison with that of open distal gastrectomy for early gastric cancer. Gastric Cancer 2001;4:93-7. [Crossref] [PubMed]
- Choi SH, Yoon DS, Chi HS, et al. Laparoscopy-assisted radical subtotal gastrectomy for early gastric carcinoma. Yonsei Med J 1996;37:174-80. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [Crossref] [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Reyes CD, Weber KJ, Gagner M, et al. Laparoscopic vs open gastrectomy. A retrospective review. Surg Endosc 2001;15:928-31. [Crossref] [PubMed]
- Azagra JS, Goergen M, De Simone P, et al. Minimally invasive surgery for gastric cancer. Surg Endosc 1999;13:351-7. [Crossref] [PubMed]
- Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 2013;27:286-94. [Crossref] [PubMed]
- Park DJ, Han SU, Hyung WJ, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc 2012;26:1548-53. [Crossref] [PubMed]
- Cai J, Wei D, Gao CF, et al. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 2011;28:331-7. [Crossref] [PubMed]
- Strong VE, Devaud N, Allen PJ, et al. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol 2009;16:1507-13. [Crossref] [PubMed]
- Guzman EA, Pigazzi A, Lee B, et al. Totally laparoscopic gastric resection with extended lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol 2009;16:2218-23. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am 2003;83:1429-44. [Crossref] [PubMed]
- Anderson C, Ellenhorn J, Hellan M, et al. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 2007;21:1662-6. [Crossref] [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [Crossref] [PubMed]
- Shen WS, Xi HQ, Chen L, et al. A meta-analysis of robotic versus laparoscopic gastrectomy for gastric cancer. Surg Endosc 2014;28:2795-802. [Crossref] [PubMed]
- Coratti A, Fernandes E, Lombardi A, et al. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol 2015;41:1106-13. [Crossref] [PubMed]
- Son SY, Lee CM, Jung DH, et al. Laparoscopic completion total gastrectomy for remnant gastric cancer: a single-institution experience. Gastric Cancer 2015;18:177-82. [Crossref] [PubMed]
- Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc 2009;23:1259-64. [Crossref] [PubMed]
- Hu WG, Ma JJ, Zang L, et al. Learning curve and long-term outcomes of laparoscopy-assisted distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A 2014;24:487-92. [Crossref] [PubMed]
- Jung do H. The learning curve associated with laparoscopic total gastrectomy. Gastric Cancer 2016;19:264-72. [Crossref] [PubMed]
- Kunisaki C, Makino H, Yamamoto N, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech 2008;18:236-41. [Crossref] [PubMed]
- Weber KJ, Reyes CD, Gagner M, et al. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc 2003;17:968-71. [Crossref] [PubMed]
- Varela JE, Hiyashi M, Nguyen T, et al. Comparison of laparoscopic and open gastrectomy for gastric cancer. Am J Surg 2006;192:837-42. [Crossref] [PubMed]
Cite this article as: Russo A, Strong VE. Minimally invasive surgery for gastric cancer in USA: current status and future perspectives. Transl Gastroenterol Hepatol 2017;2:38.


