
Barrett’s esophagus: current standards in advanced imaging
Introduction
Esophageal adenocarcinoma (EAC) is a growing threat worldwide with a global incidence of 0.7 per 100,000 person years (1.1 in men, 0.3 in women) (1). EAC has become the predominant subtype in many Western Countries where it is now more common than esophageal squamous cell carcinoma (2). In the US, the incidence of EAC is on the rise with an estimated 18,440 new cases and 16,170 EAC deaths projected in 2020Jeny (3-5). EAC typically affects older adults with identifiable risk factors including male gender, Caucasian, gastroesophageal reflux disease, smoking, and central obesity. The prognosis for patients with EAC is strongly related to stage at diagnosis, but unfortunately the majority of patients with EAC present with late stage disease and have limited options. Despite implementation of screening programs and greater recognition of this deadly cancer, we have unfortunately still not impacted outcomes related to this cancer on a population level (4). Advancements in endoscopic and minimally invasive surgical interventions have led to improved outcomes for those patients who present with earlier stage disease; thus, prompt identification of patients with risk factors for BE/EAC is critical.
Rationale for Barrett’s esophagus (BE) screening and surveillance endoscopy
BE is the only identifiable precursor lesion of EAC and affects up to 5% of the general population (6). It results from chronic acid exposure of the stratified squamous epithelium in the distal esophagus leading to metaplasia and replacement with intestinal type columnar epithelium. BE is believed to progress from non-dysplastic (NDBE) → low grade dysplasia (LGD) → high grade dysplasia (HGD) → intramucosal carcinoma (IMC) and finally to invasive EAC in a stepwise fashion. Robust screening programs for those at high risk, coupled with guideline supported surveillance strategies for BE patients, offer an opportunity to intervene on this process and change the patient’s trajectory. At the present time, there is no level 1 evidence that screening and surveillance reduces incidence, morbidity, or mortality related to BE/EAC. However, there are important case control and cohort studies that address this question. A recent systematic review and meta-analysis demonstrated lower EAC-related mortality associated with regular BE surveillance (RR 0.60, 95% CI, 0.50–0.71) as well as with surveillance detected EAC vs. symptom detected EAC (RR 0.73, 95% CI, 0.57–0.94) (7). Barrett’s esophagus surveillance allows for detection of EAC at earlier stages, which is associated with better outcomes. Randomized trials are needed to assess the impact of surveillance and potential to decrease the burden and mortality of EAC.
A high-quality endoscopic exam, detection of neoplastic lesions and endoscopic eradication therapy (EET) or minimally invasive surgical procedures for dysplastic lesions and early cancers are critical steps for effective surveillance programs. Several advanced imaging modalities have been investigated with numerous studies on chromoendoscopy (dye-based and virtual), confocal laser endomicroscopy (CLE), volumetric laser endoscopy (VLE), and more recently artificial intelligence. Adjunct use of these technologies during endoscopy allows for real-time diagnosis and prediction of histology which can effectively guide EET (8).
The objectives of this review include to (I) review best practices in Barrett’s Esophagus surveillance; (II) examine the role of advanced imaging techniques; (III) highlight guidelines and quality indicators.
Principles for a high-quality endoscopic examination of BE
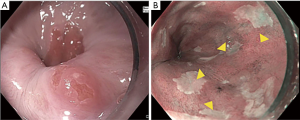
The first step to a high-quality examination requires documentation of esophageal landmarks including the presence of a hiatal hernia (9). Use of a distal attachment cap can facilitate improved visualization. The mucosa should be carefully cleaned with the water jet and gently suctioned to remove any debris or mucus without damaging the mucosa. Precise manipulation of insufflation and desufflation can adjust the luminal view to detect subtle abnormalities. The endoscopist should spend enough time (consider 1 minute per cm) carefully inspecting the Barrett’s segment (10). Findings should be reported in a systematic fashion. The Barrett’s segment should be carefully measured and reported using the Prague classification (Circumferential and Maximal extent of Barrett’s) (11). Any superficial lesions should be described using the Paris classification (12,13) (Figure 1). A ten-step approach for a high-quality examination of BE is provided (Table 1).

Full table
Sampling BE
The Seattle protocol (four-quadrant biopsies using the “turn and suction technique” at 1–2 cm intervals along the entire length of the Barrett’s segment) remains the standard of care for tissue sampling (14). Any suspicious areas (nodularity, erythema, erosion, ulceration) should be biopsied and samples placed in separate jars as these mucosal abnormalities can be associated with dysplasia (15). All visible lesions, no matter how subtle, should be completely removed endoscopically (16).
The major limitation in current BE surveillance practice is the need for random biopsies. Despite international guideline recommendations to follow a systematic approach (the Seattle protocol), non-adherence is common, and increases by 31% for every 1cm increase in length of the Barrett’s segment (17). The rate of guideline adherence in the community setting is only about 50%, which significantly decreases dysplasia detection (OR 0.53, 95% CI, 0.35–0.82) (18). This may contribute to high miss rates for EAC. Indeed, a systematic review and meta-analysis of 820 patients (24 studies) with index endoscopy with NDBE or BE-LGD demonstrated up to 25% (95% CI, 16.4–36.8%) of EAC were missed (19). Most neoplasia in BE is nonpolypoid making it difficult to detect, and random biopsy is prone to sampling error as the distribution of neoplasia is highly variable and focal so can be easily missed (20). These limitations inherent in use of white light endoscopy (WLE) with random biopsies may explain why earlier studies underestimated the mortality benefit of endoscopic surveillance (21).
Imaging modalities in Barrett’s esophagus
Imaging modalities have the potential to overcome these challenges. They can help identify suspicious areas for additional biopsies on top of the routine, guideline supported approach. Use of additional technologies does not replace the Seattle protocol. We emphasize their value to facilitate high quality examinations with closer inspection and increased identification of suspicious areas, a strategy of “Look more, biopsy appropriately.” Furthermore, advanced imaging offers the chance to detect and diagnose dysplasia in real-time and guide EET (Figure 2).
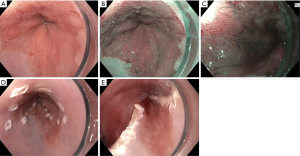
High definition white light endoscopy (HD-WLE)
At a minimum, HD-WLE should be used. Cohort studies have demonstrated clear superiority of HD-WLE compared to standard WLE for dysplasia detection (22). Although there are no head to head randomized controlled trials, HD-WLE is now considered standard of care and endorsed by societal guidelines and consensus recommendations (23-25).
Conventional and virtual chromoendoscopy
Chromoendoscopy, either dye-based or virtual, is the most extensively studied advanced imaging modality for improving neoplasia detection in BE. A comprehensive review of 14 studies (843 patients) demonstrated a 34% (95% CI, 20–56%) increase in the diagnostic yield of dysplasia or cancer using chromoendoscopy compared to WLE (26). Importantly, there was no difference between dye based or virtual chromoendoscopy (P=0.45).
Dye-based chromoendoscopy
The most commonly used dyes for BE surveillance are acetic acid, methylene blue, and indigo carmine. Application of these agents to the surface mucosa within the esophagus can enhance surface vasculature and the appearance of neoplasia. They are applied via a spray catheter through the working channel of the endoscope to lightly coat the surface mucosa in an even fashion. General limitations include cost, need for special dye spraying equipment, additional procedure time, and difficulty in adequate application. Acetic acid is a colorless chemical that effectively stains BE a whitish color. It works by disrupting cells in the surface epithelium leading to changes in the mucosal patterns. A clue to dysplasia is an area that loses the whitening effect earlier than the surrounding mucosa. In the hands of expert endoscopists, acetic acid chromoendoscopy performs well and meets the ASGE Preservation and Incorporation of Valuable Innovations (PIVI) thresholds (sensitivity 96.6%, negative predictive value 98.3%, specificity 84.6%) (27). Methylene blue is a vital dye that is quickly absorbed by intestinal and colonic epithelium with effects lasting up to 20 minutes. In BE, areas of intestinal metaplasia appear dark blue (stained) compared to unstained esophageal squamous epithelium. Areas with irregular uptake of blue stain (dark and light areas) are suspicious for neoplasia. A meta-analysis of 9 studies (450 patients) showed no increased benefit with methylene blue chromoendoscopy compared to WLE with random biopsy for BE (28). The performance characteristics also do not meet PIVI thresholds (sensitivity 64%, negative predictive value 70%, specificity 96% (27). In light of inadequate metrics and a potential risk of causing DNA damage, methylene blue chromoendoscopy is not currently recommended for use (29). Indigo carmine is a non-vital dye that collects in the pits and grooves of the surface mucosa. It has been studied mostly in conjunction with high resolution magnification endoscopy.
Virtual chromoendoscopy
Virtual chromoendoscopy uses technology built into the endoscope to achieve a similar result as dye application with the ease of only pressing a button. The most widely used system is narrow band imaging (NBI, Olympus) which applies a red-green-blue light filter to the target mucosa. NBI is based on the optical phenomenon that the depth of light penetration into tissue depends on the wavelength; the shorter the wavelength, the more superficial the penetration. Whereas standard WLE uses light at wavelengths of 400–700 nm, NBI applies shorter wavelengths (400–540 nm) to maximally highlight the surface mucosa and vascular pattern (30). Additionally, this narrower spectrum is matched to the maximum absorption of hemoglobin, so that structures with high hemoglobin content will appear dark (surface capillaries appear brown, submucosal vessels appear cyan) compared to the brighter surrounding mucosa. Other platforms for virtual chromoendoscopy are The Fujinon Intelligent Color Enhancement (FICE, Fujinon Inc., Japan) and iScan (Pentax, Japan) systems which capture the full spectrum of white light then apply digital processing programs to enhance the surface mucosa and vascular patterns (31). The newer iScan Optical Enhancement system (OE) uses specific wavelengths of light matched to hemoglobin absorption to highlight the microvasculature in combination with magnification endoscopy for closer inspection, and offers potential to improve dysplasia detection in BE (32). Another more recent system is ELUXEO 7000 (Fujifulm, Japan) which uses 4 LED multi light technology that facilitates visualization using blue light imaging (BLI) and can aid in visualization of BE neoplasia (33).
Virtual chromoendoscopy adds no cost (already built into the endoscope), negligible time (pressing a button), and no additional risk to the patient. Results from an international crossover randomized controlled trial showed higher detection of dysplasia (30% vs. 21%, P=0.01) and fewer biopsies required per patient (3.6 vs. 7.6, P<0.0001) with NBI compared to HD-WLE (34). The ASGE recently updated previous meta-analyses and provided strong data supporting the use of chromoendoscopy in BE. They noted a 9% absolute increase in dysplasia detection (95% CI, 4.1–14%) and 30.3% relative increase (95% CI, 16.2–44.3%), with no difference between virtual or dye based chromoendoscopy (35).
Additional advanced imaging modalities
Several other imaging modalities have been investigated for use in BE but most are not ready for clinical application at this time (14).
CLE
CLE is a technology based on principles of light microscopy that process reflected light into a high-resolution gray scale image for mucosal visualization at the cellular level (36). Endoscopy based (eCLE) is no longer commercially available but probe based (pCLE) can be used. It has the potential for in vivo histology, which could facilitate same-session endoscopic therapy and resection. There is also potential for machine learning in endomicroscopy (37). However, it is time consuming, costly, requires IV contrast agents, and evaluation is limited to the mucosa. A recent systematic review and meta-analysis by the ASGE noted a marginal increase in diagnostic yield (absolute increase in dysplasia detection 10.2%, 95% CI, 1.4–19.1%) and a non-significant relative increase in dysplasia detection (36%, 95% CI, –5.4–77.5%), informing their recommendation against routine use of CLE (conditional recommendation, low quality of evidence (35).
VLE
Optical coherence tomography (OCT) is a probe-based platform that investigates areas of interest with infrared light and creates high resolution cross-sectional images. VLE employs a technology similar to OCT where the probe is passed through a balloon designed for use in the esophagus and images the esophageal wall layers. VLE scans 6 cm of the esophagus over 90 seconds and provides a resolution of 10 mm and an imaging depth of 3 mm (35). Continuous adaptations to the platform make it difficult to study outcomes, but certain new features such as a laser to tag areas of suspected dysplasia and target them for biopsies, offer great potential to strengthen this technology for BE (38). VLE is time consuming and learning how to interpret images is complex, but computer-aided detection (CAD) may enhance application of this technology. European studies demonstrated strong ability to detect BE neoplasia using a computer-assisted algorithm for VLE (Area under the receiver operating characteristic curve AUC 0.95), and greater AUC with multi-frame analysis (39,40). As the technology continues to evolve, additional studies on diagnostic accuracy and cost effectiveness will be important before it can be recommended (41).
Other imaging modalities
Autofluorescence imaging (AFI) is a technology that depends on endogenous fluorophores within the GI mucosa that absorb light of varying wavelengths depending on their metabolic activity, blood flow, and biochemical characteristics. This provides an opportunity to distinguish between normal tissue and tissue which is inflamed or neoplastic. AFI is limited by a high false positive rate and minimal incremental diagnostic yield over Seattle protocol (42). Molecular endoscopy uses targeted probes directed to specific molecules in the GI tract and has the potential for highly specific in vivo diagnosis (43). It remains limited by cost, time, and special equipment that is not available for routine clinical use.
Artificial intelligence
CAD systems have arrived and offer promising technology that could transform our ability to endoscopically detect BE related neoplasia (44,45). The ARGOS project was developed in collaboration by expert endoscopists at BE referral centers in the Netherlands, an imaging analysis group, and commercial enterprises with the goal to create the first CAD for BE neoplasia detection. Their initial work focused on training their algorithm using endoscopic still images in WLE and results showed high accuracy (92%), sensitivity (95%), and specificity (85%) for detecting and localizing dysplasia in BE (46). They used over 494,364 still images to train their system, further trained and refined their system on datasets of retrospectively and then prospectively collected images of BE neoplasia (with internal validation), and then a fourth dataset was used for external validation (47). Their CAD system currently shows high accuracy for detecting BE neoplasia and next steps include deeper learning with video recordings to refine an algorithm for real-time analysis during endoscopy. A US group has also developed an artificial intelligence algorithm using convolutional neural networks that detected BE neoplasia with accuracy 93.7%, sensitivity 95.6%, specificity 91.8% for an AUC 0.94 (48). Artificial intelligence is also being developed for VLE and BE (49).
Incorporating imaging modalities into practice: guidelines & quality indicators
Gastroenterology societies worldwide provide guidelines on use of imaging modalities (Table 2) as well as quality indicators in BE (Table 3).
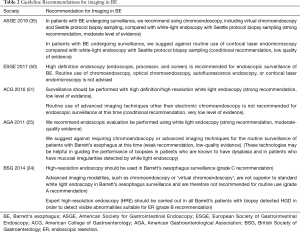
Full table

Full table
The ASGE set minimum PIVI thresholds for when advanced imaging modalities should be recommended in clinical practice. To eliminate random biopsies in BE surveillance, an imaging technology with targeted biopsies should meet the following performance threshold: (I) per-patient sensitivity of 90% or greater and a negative predictive value (NPV) of 98% or greater for detecting HGD/early EAC, compared with the current standard protocol (WLE with targeted and random 4-quadrant biopsies every 2 cm); (II) specificity should be sufficiently high (80%) to allow a reduction in the number of biopsies (compared with random biopsies) (53). Currently, only acetic acid chromoendoscopy, virtual chromoendoscopy with NBI, and endoscope-based confocal laser endomicroscopy meet these thresholds, but only when performed by expert endoscopists (27). Additionally, an international survey of BE experts sought to determine the minimum incremental diagnostic yield for detection of dysplasia or cancer of an advanced imaging modality compared to the standard Seattle Protocol. Results showed the incremental diagnostic yield for virtual chromoendoscopy to be fairly low (15%, IQR 10–29%) whereas for VLE it was much higher (30%, IQR 20–50%). These results provide benchmarks that need to be met for guidelines to recommend routine use of these technologies (54).
The need for standardized grading systems has also limited uptake of advanced imaging modalities. Several classification systems have been proposed using NBI, but the criteria were complex and validation studies were poor. To address this gap, The Barrett’s International NBI Group (BING) brought together experts from the US, Europe, and Japan to develop a simple, validated system for identification of dysplasia and EAC in patients with BE (55). The BING criteria distinguishes regular mucosal patterns (circular, ridged/villous, or tubular patterns) from irregular mucosal patterns (absent or irregular surface patterns), and regular vascular patterns (regularly positioned blood vessels along or between mucosal ridges and/or characterized by normal, long branching patterns) from irregular vascular patterns (focally or diffusely distributed vessels in discordance with the normal architecture). The BING criteria demonstrated 85% overall accuracy for identification of dysplasia (sensitivity 80%, specificity 88%, 81% positive predictive value, 88% negative predictive value) and high interobserver agreement (k=0.681) (55).
Training in advanced imaging modalities
Significant advances in science and technology have tremendously improved our ability to recognize early neoplasia in the gastrointestinal tract. Image enhanced endoscopy is clearly a valuable and efficacious tool to increase detection of dysplasia in BE as demonstrated by strong clinical trial data. So why is it that uptake remains limited and competency so difficult to achieve? Certainly, resources may be limited in particular practice settings. Time and cost may be a factor. The major driver is likely the lack of training available in these advanced imaging modalities. Technology has evolved quicker than our ability as a society and community to create robust, high-quality educational programs for endoscopists of all levels. To overcome challenges with advanced imaging in BE we propose two major goals: (I) to train non-expert endoscopists to achieve PIVI thresholds; (II) to refine our techniques to exceed the PIVI thresholds at the expert level. The second goal is somewhat longer term as it may rely on technological developments and clinical trial data.
The first task can be addressed in the short term and requires training the larger community of gastroenterologists around the world. For these technologies to be incorporated into routine clinical practice, providers need to feel comfortable and competent in recognizing BE related neoplasia (54). This may require leveraging societal efforts and linking performance to quality metrics. The need for validated educational programs is now being recognized. A group in the UK developed and validated a training module that was effective at improving endoscopist performance in use of acetic acid chromoendoscopy for Barrett’s detection (56). More recently, an international working group developed an interactive, web based tool for detection and delineation of Barrett’s esophagus-related neoplasia (BORN) (57). The BORN training module is freely available on the internet to endoscopists of all levels and is Continuing Medical Education-accredited. The program delivers high quality endoscopy videos through an interactive platform that provides scoring and feedback to participants.
Finally, the future of endoscopy lies in our current trainees. The traditional structure of gastroenterology (GI) fellowship has been an apprenticeship-based model much like the rest of medicine, where trainees learn clinical medicine and endoscopy on the job. With implementation of the Next Accreditation System through the ACGME, there has been a shift towards Competency Based Medical Education (CBME), an approach that recognizes trainee progression through a series of clinical milestones. This system relies on defined quality and competency metrics in the cognitive and technical aspects of endoscopy, many of which have been established in the GI core curriculum. As technology evolves and the endoscopic armamentarium grows, it will be critical to incorporate training in advanced imaging modalities into fellowship programs (58). At the present time, there is no data on trainee learning curves or the impact of training on achieving competency in advanced imaging modalities. Training the next generation can be achieved through use of online modules or cloud-based curriculum, flipped classroom learning, and a case-based approach (59).
Conclusions
Improving our ability to detect and diagnose early BE related neoplasia can guide EET and may help decrease the burden of EAC. This starts with a high-quality endoscopic examination and careful inspection of the Barrett’s segment. HD-WLE is considered minimum standard of care. Additionally, we recommend use of chromoendoscopy (including virtual chromoendoscopy) and the Seattle protocol for biopsy sampling. Practicing endoscopists and trainees should follow guideline recommendations and understand key quality indicators for the management of BE related neoplasia. With evolving technology and integration of artificial intelligence into endoscopy, it will be critical to design educational programs to graduate competent fellows and train practicing endoscopists.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Krish Ragunath, Philip WY Chiu) for the series “Advanced Endoscopic Imaging of the GI Tract” published in Translational Gastroenterology and Hepatology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh.2020.02.10). The series “Advanced Endoscopic Imaging of the GI Tract” was commissioned by the editorial office without any funding or sponsorship. JM Kolb received funding from the National Institutes of Health (NIH) T32-DK007038. S Wani is supported in part by the Department of Medicine Outstanding Early Scholars Program, and is a consultant for Medtronic, Boston Scientific, and Interspace.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390-405. [Crossref] [PubMed]
- National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2015. Available online: https://seer.cancer.gov/csr/1975_2015/
- Thrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci 2018;63:1988-96. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett's esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus 2010;23:451-7. [Crossref] [PubMed]
- Codipilly DC, Chandar AK, Singh S, et al. The Effect of Endoscopic Surveillance in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Gastroenterology 2018;154:2068-86.e5. [Crossref] [PubMed]
- Boerwinkel DF, Swager A, Curvers WL, et al. The clinical consequences of advanced imaging techniques in Barrett's esophagus. Gastroenterology 2014;146:622-9.e4. [Crossref] [PubMed]
- Gorrepati VS, Sharma P. How Should We Report Endoscopic Results in Patient's with Barrett's Esophagus? Dig Dis Sci 2018;63:2115-21. [Crossref] [PubMed]
- Gupta N, Gaddam S, Wani SB, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus. Gastrointest Endosc 2012;76:531-8. [Crossref] [PubMed]
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Paris Workshop on Columnar Metaplasia in the Esophagus and the Esophagogastric Junction, Paris, France, December 11-12 2004. Endoscopy 2005;37:879-920. [Crossref] [PubMed]
- Wani S, Gaddam S.. Editorial: Best Practices in Surveillance of Barrett's Esophagus. Am J Gastroenterol 2017;112:1056-60. [Crossref] [PubMed]
- Reid BJ, Blount PL, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett's high-grade dysplasia. Am J Gastroenterol 2000;95:3089-96. [Crossref] [PubMed]
- Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett's esophagus: the TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett's Esophagus) Consortium. Gastrointest Endosc 2017;86:1-17.e3. [Crossref] [PubMed]
- Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett's esophagus: an analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90:732-41.e3. [Crossref] [PubMed]
- Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736-42. [Crossref] [PubMed]
- Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of Missed Esophageal Adenocarcinoma After Barrett's Esophagus Diagnosis: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:599-607.e7. [Crossref] [PubMed]
- Pech O, Gossner L, Manner H, et al. Prospective evaluation of the macroscopic types and location of early Barrett's neoplasia in 380 lesions. Endoscopy 2007;39:588-93. [Crossref] [PubMed]
- Kolb JM, Kaltenbach T, Soetikno R. Implementation of new knowledge, technique, and technology to survey Barrett's is urgently needed. Gastroenterology 2014;146:587. [Crossref] [PubMed]
- Sami SS, Subramanian V, Butt WM, et al. High definition versus standard definition white light endoscopy for detecting dysplasia in patients with Barrett's esophagus. Dis Esophagus 2015;28:742-9. [Crossref] [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- American Gastroenterological Association, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562-70.e1-2.
- Committee AT, Thosani N, Abu Dayyeh BK, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett's esophagus. Gastrointest Endosc 2016;83:684-98.e7. [Crossref] [PubMed]
- Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc 2009;69:1021-8. [Crossref] [PubMed]
- Olliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet 2003;362:373-4. [Crossref] [PubMed]
- Mizuno H, Gono K, Takehana S, et al. Narrow band imaging technique. Tech Gastrointest Endosc 2003;5:78-81. [Crossref]
- Kodashima S.. Novel image-enhanced endoscopy with i-scan technology. World Journal of Gastroenterology 2010;16:1043. [Crossref] [PubMed]
- Everson MA, Lovat LB, Graham DG, et al. Virtual chromoendoscopy by using optical enhancement improves the detection of Barrett's esophagus-associated neoplasia. Gastrointest Endosc 2019;89:247-56.e4. [Crossref] [PubMed]
- de Groof AJ, Swager AF, Pouw RE, et al. Blue-light imaging has an additional value to white-light endoscopy in visualization of early Barrett's neoplasia: an international multicenter cohort study. Gastrointest Endosc 2019;89:749-58. [Crossref] [PubMed]
- Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut 2013;62:15-21. [Crossref] [PubMed]
- Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointestinal Endoscopy 2019;90:335-59.e2. [Crossref] [PubMed]
- ASGE Technology Committee. Confocal laser endomicroscopy. Gastrointest Endosc 2014;80:928-38. [Crossref] [PubMed]
- Hong J, Park BY, Park H. Convolutional neural network classifier for distinguishing Barrett's esophagus and neoplasia endomicroscopy images. Conf Proc IEEE Eng Med Biol Soc 2017;2017:2892-5. [Crossref] [PubMed]
- Alshelleh M, Inamdar S, McKinley M, et al. Incremental yield of dysplasia detection in Barrett's esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsy protocol. Gastrointest Endosc 2018;88:35-42. [Crossref] [PubMed]
- Swager AF, van der Sommen F, Klomp SR, et al. Computer-aided detection of early Barrett's neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc 2017;86:839-46. [Crossref] [PubMed]
- Struyvenberg MR, van der Sommen F, Swager AF, et al. Improved Barrett's neoplasia detection using computer-assisted multiframe analysis of volumetric laser endomicroscopy. Dis Esophagus 2020;33:doz065. [PubMed]
- Trindade AJ, Leggett CL, Chang KJ. Volumetric laser endomicroscopy in the management of Barrett's esophagus. Curr Opin Gastroenterol 2017;33:254-60. [Crossref] [PubMed]
- Muthusamy VR, Kim S, Wallace MB. Advanced Imaging in Barrett's Esophagus. Gastroenterol Clin North Am 2015;44:439-58. [Crossref] [PubMed]
- Nagengast WB, Hartmans E, Garcia-Allende PB, et al. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut 2019;68:7-10. [Crossref] [PubMed]
- van der Sommen F, Zinger S, Curvers WL, et al. Computer-aided detection of early neoplastic lesions in Barrett's esophagus. Endoscopy 2016;48:617-24. [Crossref] [PubMed]
- de Souza LA Jr, Palm C, Mendel R, et al. A survey on Barrett's esophagus analysis using machine learning. Comput Biol Med 2018;96:203-13. [Crossref] [PubMed]
- de Groof J, van der Sommen F, van der Putten J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett's neoplasia on white light endoscopy. United European Gastroenterol J 2019;7:538-47. [Crossref] [PubMed]
- de Groof J, Struyvenberg MR, van der Putten J, et al. 640 The ARGOS Project: First Deep Learning Algorithm for Detection of Barrett’s Neoplasia Outperforms Conventional Computer Aided Detection Systems in a Multi-Step Training and Validation Study. Gastrointest Endosc 2019;89:AB99. [Crossref]
- Hashimoto R, Lugo M, Mai D, et al. 641 Artifical Intelligence Dysplasia Detection (AIDD) Algorithm for Barrett’s Esophagus. Gastrointest Endosc 2019;89:AB99-100. [Crossref]
- Trindade AJ, McKinley MJ, Fan C, et al. Endoscopic Surveillance of Barrett's Esophagus Using Volumetric Laser Endomicroscopy With Artificial Intelligence Image Enhancement. Gastroenterology 2019;157:303-5. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Sharma P, Katzka DA, Gupta N, et al. Quality indicators for the management of Barrett's esophagus, dysplasia, and esophageal adenocarcinoma: international consensus recommendations from the American Gastroenterological Association Symposium. Gastroenterology 2015;149:1599-606. [Crossref] [PubMed]
- Sharma P, Savides TJ, Canto MI, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. Gastrointest Endosc 2012;76:252-4. [Crossref] [PubMed]
- Machicado JD, Han S, Yadlapati RH, et al. A Survey of Expert Practice and Attitudes Regarding Advanced Imaging Modalities in Surveillance of Barrett's Esophagus. Dig Dis Sci 2018;63:3262-71. [Crossref] [PubMed]
- Sharma P, Bergman JJ, Goda K, et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology 2016;150:591-8. [Crossref] [PubMed]
- Chedgy FJQ, Kandiah K, Barr H, et al. Development and validation of a training module on the use of acetic acid for the detection of Barrett's neoplasia. Endoscopy 2017;49:121-9. [Crossref] [PubMed]
- Bergman J, de Groof AJ, Pech O, et al. An Interactive Web-Based Educational Tool Improves Detection and Delineation of Barrett's Esophagus-Related Neoplasia. Gastroenterology 2019;156:1299-308.e3. [Crossref] [PubMed]
- Kolb JM, Wagh MS. How to ACE your endoscopy training: let competency speak volumes. Gastrointestinal Endoscopy 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Soetikno R, Kolb JM, Nguyen-Vu T, et al. Evolving endoscopy teaching in the era of the millennial trainee. Gastrointest Endosc 2019;89:1056-62. [Crossref] [PubMed]
Cite this article as: Kolb JM, Wani S. Barrett’s esophagus: current standards in advanced imaging. Transl Gastroenterol Hepatol 2021;6:14.


