
Diagnosis of early gastric cancer using image enhanced endoscopy: a systematic approach
Introduction
Endoscopic diagnosis of early gastric cancer mainly comprises two steps: (I) detection of cancer and (II) differentiation between cancerous and non-cancerous lesions. The usefulness of image-enhanced endoscopic observation in screening endoscopy to detect early gastric cancer remains unclear, but a large-scale clinical study on this issue is currently underway. On the other hand, the usefulness of image-enhanced magnifying endoscopy in differential diagnosis of gastric cancer has already been established (1-13). Therefore, this paper provides an overview of the diagnostic system for early gastric cancer and presents the characteristic findings of gastric mucosal lesions visualized by image-enhanced magnifying endoscopy, with a focus on differentiation between cancerous and non-cancerous lesions using image-enhanced magnifying endoscopy.
Diagnostic system
VS classification system
The vessel plus surface (VS) classification system is an established diagnostic system for differentiation between cancerous and non-cancerous lesions using magnifying endoscopy; this system was proposed by Yao et al. in 2009 (1). The usefulness of this system for diagnosing early gastric cancer has been verified with high-level evidence (4,5). The most useful features of this diagnostic system are as follows: (I) it enables the diagnosis of minute cancers ≤5 mm in size and of early gastric cancers of the superficial flat (0–IIb) type among gastritis-like cancers (superficial type of early gastric cancer, 0–II types), which cannot be diagnosed using conventional endoscopy; (II) it enables preoperatively necessary determination of the borders of early gastric cancer.
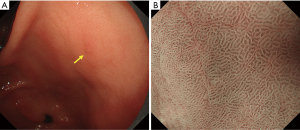
The fundamental principle of the VS classification system is to evaluate the microvascular (MV) and microsurface (MS) patterns, using anatomical terms for the analysis of findings on magnifying endoscopy of the stomach (Figure 1). The terms used for the analysis of the MV pattern include the following: (I) subepithelial capillary network (SEC), (II) collecting venule (CV), and (III) microvessels (MVs). The terms used for the analysis of the MS pattern include the following: (I) marginal crypt epithelium (MCE), (II) crypt opening (CO), (III) intervening part between crypts (IP), (IV) light blue crest (LBC), and (V) white opaque substance (WOS).
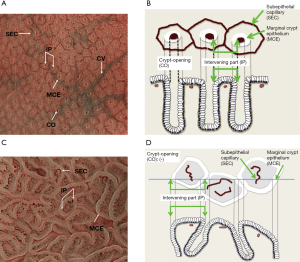
According to the VS classification system, the characteristic endoscopic findings of early gastric cancer include the presence of a clear demarcation line between cancerous and non-cancerous mucosae and the presence of an irregular MV pattern and/or irregular MS pattern inside the demarcation line. The demarcation line is defined as a borderline recognizable by the abrupt change in the MV or MS pattern between the lesion and non-lesion areas. The MV and MS patterns are evaluated separately regarding whether they are regular, irregular, or absent, and lesions that meet the following diagnostic criteria are diagnosed as cancerous, whereas those that do not are diagnosed as non-cancerous.
Diagnostic criteria
- Presence of an irregular MV pattern with a demarcation line;
- Presence of an irregular MS pattern with a demarcation line.
Lesions that meet criterion (I) or (II) (or both) are diagnosed as cancerous lesions, whereas others are diagnosed as non-cancerous lesions.
It has been reported that 97% of early gastric cancers theoretically meet these diagnostic criteria (1).
As shown previously, the MV and MS patterns can be classified into three categories: regular/irregular/absent (Figure 2). In the regular MV pattern, the capillaries under each epithelium have a closed loop (polygonal) or open loop morphology, showing a regular arrangement of symmetrically distributed homogeneous shapes. Meanwhile, the irregular MV pattern is characterized by a closed loop (polygonal), open loop, tortuous, branching, or irregular morphology. MVs have heterogeneous shapes, and they are distributed asymmetrically, showing an irregular arrangement. Cases in which there are no observable subepithelial MVs are judged as absent MV pattern. In such cases, the MS pattern (showing features such as MCE, WOS, and LBC), instead of the MV pattern, is used as the indicator for magnifying endoscopic diagnosis (2).
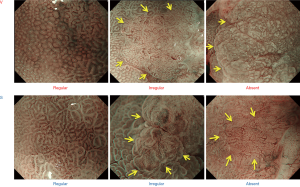
Although MCE is often used for the judgment of the MS pattern, other findings such as CO, IP, LBC, and WOS can also be used if they are present. In the regular MS pattern, the morphology of each MCE is curved or oval, showing homogeneous shapes, symmetrical distribution, and regular arrangement. In the irregular MS pattern, the morphology of each MCE is curved or oval, or villous on rare occasions, showing heterogeneous shapes, an asymmetrical distribution, and an irregular arrangement. When no MS pattern including MCE is observable, the case is judged as absent MS pattern. In such cases, the MV pattern is necessarily well visualized; therefore, the features of the MV pattern, instead of the MS pattern, are used as indicators for magnifying endoscopic diagnosis (1).
It has also been shown in a multicenter prospective study that some lesions are difficult to observe by magnifying endoscopy using the VS classification system (5). Such lesions are discolored, flat undifferentiated-type cancer lesions. Taking into account clinical limitations, we have proposed a clinical strategy as shown in Figure 3.
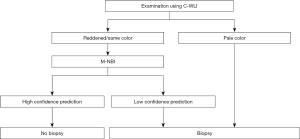
Specifically, we have suggested that biopsy is necessary for discolored, flat mucosal lesions, whereas magnifying endoscopy of the stomach can be used as an optical biopsy in cases of other lesions as long as there is high-confidence prediction. The magnifying endoscopy simple diagnostic algorithm for gastric cancer (MESDA-G) described below should also be applied according to this clinical strategy. Figure 4 shows discolored, flat undifferentiated-type gastric cancer, which is difficult to diagnose by magnifying endoscopy using the VS classification system.
MESDA-G using the VS classification system
Although numerous diagnostic systems aimed at magnifying endoscopic diagnosis of early gastric cancer have been proposed in Japan and elsewhere, no consensus had been reached on an established and standardized set of diagnostic criteria or diagnostic system. Against this background, the Japanese Gastroenterological Association Guidelines Subcommittee performed a systematic review and selected the terms and diagnostic system to be used for magnifying endoscopic diagnosis of the stomach in evidence-based medicine. Consequently, the VS classification system was adopted as a diagnostic system, together with the anatomical terms used for this system. On this basis, MESDA-G, was proposed (3). This algorithm was endorsed by the Japanese Gastroenterological Association, Japan Gastroenterological Endoscopy Society, Japanese Gastric Cancer Association, and World Endoscopy Organization, and a consensus was reached on its status as a standardized algorithm. Figure 5 shows the algorithm. First, white-light magnifying endoscopy is used to observe the inside of the stomach according to the systematic screening protocol for the stomach (15) to perform examinations for any lesions suggestive of early gastric cancer. If such a suspicious lesion is detected, magnifying endoscopy should be performed to differentiate between cancerous and non-cancerous lesions. More specifically, the presence/absence of a demarcation line should be determined using magnifying endoscopy (Figure 5). If no demarcation line is identified, the lesion should be diagnosed as a non-cancerous one. If there is a demarcation line, the MV and MS patterns inside the demarcation line should be separately examined regarding whether they are regular, irregular, or absent. If the irregular MV pattern and/or irregular MS pattern is identified, the lesion is diagnosed as cancer, whereas if these patterns are not identified, the lesion is diagnosed as a non-cancerous one. This is a truly simple and clear algorithm. Figures 6-8 provide actual endoscopic findings to show the procedures performed for diagnosis based on MESDA-G.
Characteristic findings of the gastric mucosa on image-enhanced magnifying endoscopy
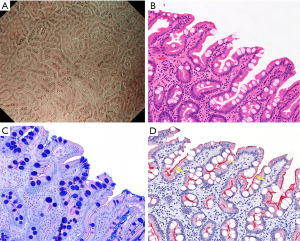
WOS
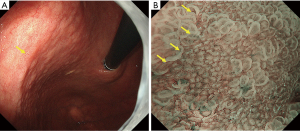
WOS is a white substance that obscured subepithelial MVs present in the superficial layer of the mucosa clearly visualized on magnifying endoscopy with narrow-band imaging (NBI). The presence of WOS in the gastric mucosa was first reported by Yao et al. in 2008. This finding is often obtained in intestinal metaplasia in the mucosa of chronic gastritis and in gastric epithelial tumors (adenoma or cancer) with intestinal phenotype (2,16,17). The nature of WOS is a phenomenon recognized as white substance observed through the strong scattering or reflection of the projected light from the endoscopy on minute fat droplets accumulated in and under the epithelium (18,19). When WOS is present, the projected light cannot reach subepithelial MVs, resulting in poor visibility of the vessels. Differences in the morphological features of WOS serve as useful indicators in the differential diagnosis of cancer and adenoma. More specifically, cancerous lesions have irregular WOS, whereas low-grade adenomatous lesions have regular WOS (2). In addition, it has been reported that WOS-positive gastric epithelial tumors have a mucous character of some intestinal phenotype (intestinal type or gastrointestinal phenotype) (18) and that WOS serves as an indicator of differentiated-type cancers because there is no WOS in undifferentiated-type cancers (20). Figures 9 and 10 show actual endoscopic findings.
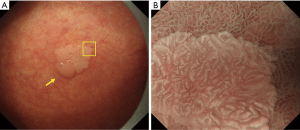
LBC
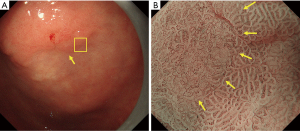
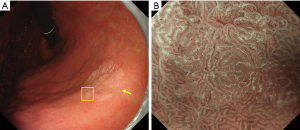
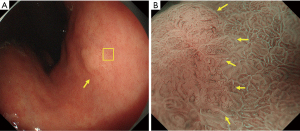
It has been shown that magnifying endoscopy with NBI is useful for the diagnosis of intestinal metaplasia (21-23). A comparison of magnifying endoscopy with NBI and white-light magnifying endoscopy has revealed the superiority of the former over the latter for correctly diagnosis of intestinal metaplasia (24). The LBC sign was first reported by Uedo et al. in 2006 (25). According to their report, LBC is defined as blue-white lines of light observed at the edge of MCE on magnifying endoscopy with NBI (Figure 11). The LBC sign is considered to be a phenomenon that occurs when the brush border of the absorptive epithelial surface of histopathologically CD10-positive intestinal metaplasia reflects narrow-band light having a central wavelength of 415 nm. Regarding the diagnostic ability of LBC for histopathological intestinal metaplasia, the reported sensitivity and specificity are 89% and 93%, respectively (25). In addition, Kanemitsu et al. used a combination of LBC and WOS for the diagnosis of intestinal metaplasia, and reported a sensitivity and specificity of 87.5% and 93.8%, respectively (17). Moreover, meta-analyses including studies from outside of Japan also confirmed the excellent diagnostic ability of LBC in the diagnosis of intestinal metaplasia (26-28). The LBC sign is also useful for determining the tumor border; the borderline devoid of LBC often matches the tumor borderline (Figure 12).
White globe appearance (WGA)
WGA, if present inside the demarcation line, is a highly specific finding on magnifying endoscopy that allows differential diagnosis between differentiated-type cancerous and non-cancerous lesions. WGA is defined as a small (measuring ≤1 mm) white globular appearance just beneath the epithelium found on magnifying endoscopy with NBI. WGA was first reported by Doyama et al. in 2015 (29). It corresponds to so-called intraglandular necrotic debris, which is a markedly dilated tumor gland duct containing retained eosinophilic necrotic material observed on close histopathological examination. WGA is a highly specific cancer marker for magnifying endoscopy. In other words, the presence of WGA allows differential diagnosis between differentiated-type cancerous and non-cancerous lesions, such as low-grade adenoma and gastritis, with high specificity (Figure 13).
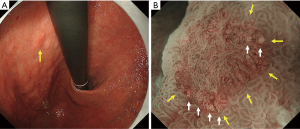
Vessels within epithelial circle (VEC) pattern
It has been reported that pathological examination shows higher biological malignancy of papillary adenocarcinoma than of tubular adenocarcinoma. However, using conventional endoscopy, it was not possible to diagnose papillary adenocarcinoma. Kanemitsu et al. observed early gastric cancer by magnifying endoscopy and noticed the specific finding that blood vessels were present under the superficial epithelium of intervening part surrounded by circular MCE. They named this the “vessels within epithelial circle (VEC) pattern” and reported that this pattern is characteristic of a differentiated-type cancer showing histologically a papillary structure (30) (Figure 14). In addition, they reported the coexistence of undifferentiated-type cancer and/or submucosal invasion in about one-quarter of resected histological specimens of VEC pattern-positive early gastric cancer and suggested the possibility that the VEC pattern observed on magnifying endoscopy could be a useful marker for predicting high malignancy prior to surgery (30).
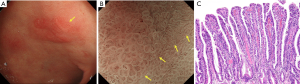
In summary, a diagnostic system for image-enhanced magnifying endoscopy for early gastric cancer and other representative findings have been described in this paper. Although details have not been mentioned here, it should be emphasized that a standard technique to observe the gastric mucosa at maximum magnification is indispensable for accurate diagnosis using magnifying endoscopy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009;41:462-7. [Crossref] [PubMed]
- Yao K, Iwashita A, Tanabe H, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc 2008;68:574-80. [Crossref] [PubMed]
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016;28:379-93. [Crossref] [PubMed]
- Ezoe Y, Muto M, Uedo N, et al. Magnifying narrow band imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology 2011;141:2017-25.e3. [Crossref] [PubMed]
- Yao K, Doyama H, Gotoda T, et al. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer 2014;17:669-79. [Crossref] [PubMed]
- Yamada S, Doyama H, Yao K, et al. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging:a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc 2014;79:55-63. [Crossref] [PubMed]
- Maki S, Yao K, Nagahama T, et al. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer 2013;16:140-6. [Crossref] [PubMed]
- Miwa K, Doyama H, Ito R, et al. Can magnifying endoscopy with narrow band imaging be useful for low grade adenomas in preoperative biopsy specimens? Gastric Cancer 2012;15:170-8. [Crossref] [PubMed]
- Fujiwara S, Yao K, Nagahama T, et al. Can we accurately diagnose minute gastric cancers (≤5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI). Gastric Cancer 2015;18:590-6. [Crossref] [PubMed]
- Zhang Q, Wang F, Chen ZY, et al. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer 2016;19:543-52. [Crossref] [PubMed]
- Dohi O, Yagi N, Majima A, et al. Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: a prospective study. Gastric Cancer 2017;20:297-303. [Crossref] [PubMed]
- Dohi O, Yagi N, Yoshida S, et al. Magnifying Blue Laser Imaging versus Magnifying Narrow-Band Imaging for the Diagnosis of Early Gastric Cancer: A Prospective, Multicenter, Comparative Study. Digestion 2017;96:127-34. [Crossref] [PubMed]
- Yoshimizu S, Yamamoto Y, Horiuchi Y, et al. Diagnostic performance of routine esophagogastroduodenoscopy using magnifying endoscope with narrow-band imaging for gastric cancer. Dig Endosc 2018;30:71-8. [Crossref] [PubMed]
- Yao K. Zoom gastroscopy: magnifying endoscopy in the stomach. Tokyo: Springer; 2013:66-7.
- Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11-22. [PubMed]
- Matsushita M, Mori S, Uchida K, et al. “White opaque substance” and “light blue crest” within gastric flat tumors or intestinal metaplasia: same or different signs? Gastrointest Endosc 2009;70:402-3. [Crossref] [PubMed]
- Kanemitsu T, Yao K, Nagahama T, et al. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: the white opaque substance marker. Endoscopy 2017;49:529-35. [Crossref] [PubMed]
- Yao K, Iwashita A, Nambu M, et al. Nature of white opaque substance in gastric epithelial neoplasia as visualized by magnifying endoscopy with narrow-band imaging. Dig Endosc 2012;24:419-25. [Crossref] [PubMed]
- Ueo T, Yonemasu H, Yada N, et al. White opaque substance represents an intracytoplasmic accumulation of lipid droplets: immunohistochemical and immunoelectron microscopic investigation of 26 cases. Dig Endosc 2013;25:147-55. [Crossref] [PubMed]
- Ueo T, Yonemasu H, Yao K, et al. Histologic differentiation and mucin phenotype in white opaque substance-positive gastric neoplasias. Endosc Int Open 2015;3:E597-604. [Crossref] [PubMed]
- Song J, Zhang J, Wang J, et al. Meta-analysis: narrow band imaging for diagnosis of gastric intestinal metaplasia. PLoS One 2014;9:e94869. [Crossref] [PubMed]
- Ang TL, Fock KM, Teo EK, et al. The diagnostic utility of narrow band imaging magnifying endoscopy in clinical practice in a population with intermediate gastric cancer risk. Eur J Gastroenterol Hepatol 2012;24:362-7. [PubMed]
- Lage J, Pimentel-Nunes P, Figueiredo PC, et al. Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: do we still need biopsies? Scand J Gastroenterol 2016;51:501-6. [Crossref] [PubMed]
- Dutta AK, Sajith KG, Pulimood AB, et al. Narrow band imaging versus white light gastroscopy in detecting potentially premalignant gastric lesions: a randomized prospective crossover study. Indian J Gastroenterol 2013;32:37-42. [Crossref] [PubMed]
- Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy 2006;38:819-824. [Crossref] [PubMed]
- Savarino E, Corbo M, Dulbecco P, et al. Narrow-band imaging with magnifying endoscopy is accurate for detecting gastric intestinal metaplasia. World J Gastroenterol 2013;19:2668-75. [Crossref] [PubMed]
- Rerknimitr R, Imraporn B, Klaikeaw N, et al. Non-sequential narrow band imaging for targeted biopsy and monitoring of gastric intestinal metaplasia. World J Gastroenterol 2011;17:1336-42. [Crossref] [PubMed]
- Wang L, Huang W, Du J, et al. Diagnostic yield of the light blue crest sign in gastric intestinal metaplasia: a meta-analysis. PLoS One 2014;9:e92874. [Crossref] [PubMed]
- Doyama H, Yoshida N, Tsuyama S, et al. The “white globe appearance” (WGA): a novel marker for a correct diagnosis of early gastric cancer by magnifying endoscopy with narrow-band imaging (M-NBI). Endosc Int Open 2015;3:E120-4. [Crossref] [PubMed]
- Kanemitsu T, Yao K, Nagahama T, et al. The vessels within epithelial circle (VEC) pattern as visualized by magnifying endoscopy with narrow-band imaging (ME-NBI) is a useful marker for the diagnosis of papillary adenocarcinoma: a case-controlled study. Gastric Cancer 2014;17:469-77. [Crossref] [PubMed]
Cite this article as: Miyaoka M, Yao K, Tanabe H, Kanemitsu T, Otsu K, Imamura K, Ono Y, Ishikawa S, Yasaka T, Ueki T, Ota A, Haraoka S, Iwashita A. Diagnosis of early gastric cancer using image enhanced endoscopy: a systematic approach. Transl Gastroenterol Hepatol 2020;5:50.


